Abstract
Bioactive compounds derived from plants are secondary metabolites that can act through various bioactivities, namely as antioxidant, antimicrobial, anti-inflammatory, anti-hypertensive, and hypoglycemic agents. Combined with the pressure generated by consumers for more natural products with beneficial effects on health, these compounds may be suitable candidates to act as preservatives in food products. For this purpose, the extraction process becomes essential for the acquisition of a quality extract with efficiency and with the desired final properties. Therefore, the main objective of this work was to perform the optimization of the extraction yield of olive leaves (Olea europaea L.), by applying response surface methodology (RSM) and employing dynamic maceration as extraction technique. Three factors were analyzed: time (F1), temperature (F2), and solvent (F3), ranging from 5 to 120 min, 25 to 100 °C, and from 0 to 100% ethanol, respectively. The study used the Box Behnken design, relying on 17 individual randomized runs. The response was the dry weight of the extract (Y1), which ranged from 21.1 to 90.5 mg. The optimization studies pointed to the increase of yield with the increase of time and temperature, but inversely by applying higher time and lower temperature values and higher temperature and lower time values. The highest yield of the dry extract was achieved at 120 min (F1), 25 °C (F2), and 87% (F3) of ethanol:water. Future studies will be carried out to analyze the preservative effects of incorporating olive extract in foods, as well as analysis of other response for optimizing the best food preserving extract.
1. Introduction
Olea europaea L., a species of Oleaceae and known as olive tree, is one of the most popular plants in the Mediterranean region. Besides its use in the food industry, its leaves, essential oil, and extracts are important sources for the pharmaceutical and cosmetic industry [1]. Olive leaves represent a large part of agricultural waste generated. Thus, with the recovery of discarded leaves, extracts with high bioactive capacity can be obtained [2]. These bioactive compounds may be potential substitutes for synthetic preservatives, and this shift is largely driven by consumer demand for more natural and healthful products [3]. The antioxidant, antimicrobial, anti-inflammatory, anti-hypertensive, and hypoglycemic properties of olive leaf extracts have been reported by several authors, and these characteristics are attributed to the phenolic compounds present in the leaves of this species [2,4,5,6,7,8]. Previous studies carried out on olive leaf extracts revealed the significant presence of active phenolics, namely oleuropeosides (oleuropein and verbascoside); flavonoids (apigenin-7-O-glucoside, apigenin-7-O-rutinoside, luteolin-7-O-glucoside, luteolin-7-O-rutinoside, rutin, luteolin, apigenin, diosmetin), hydroxycinnamic acid derivatives, with a predominance of verbascoside and substituted phenols (hydroxytyrosol, tyrosol, caffeic and vanillic acids) [5,6,9]. In this scenario, the adequate extraction becomes important to acquire a quality extract with a good functionalization in the final product [10]. Several methods have been applied for the extraction of these compounds from O. europaea L. leaves, such as maceration [5,6,7,11,12], ultrasound-assisted extraction [7,13], and microwave-assisted extraction [7,14]. Although conventional extraction by maceration has some important disadvantages, such as long extraction times and high levels of energy consumption, it is still a widely used technique considering its simplicity and the possible application of green solvents to the process. Therefore, the main objective of this work was to perform the optimization of the extraction yield of olive leaves (Olea europaea L.), through dynamic maceration as extraction technique, by applying response surface methodology (RSM).
2. Materials and Methods
2.1. Reagents and Plant Material
Reagents with analytical grade were used and purchased from scientific suppliers. The olive leaves (Olea europaea L.) after being purchased were dried and ground to a powder of approximately 20 mesh using a grinder (Moulinex A320, Mayenne, France).
2.2. Response Surface Methodology (RSM)
Response surface methodology (RSM) was the technique applied for optimization with dynamic maceration as the extractive method and Box Behnken design as the model used. Three analysis factors were defined: time (F1) ranging from 5 to 120 min, temperature (F2) from 25 to 100 °C, and solvent (F3) from 0 to 100% ethanol. The response variable (Y1) was the dry weight, expressed in mg. Seventeen independent runs with distinct conditions were performed (Table 1), with each factor having minimum, medium, and maximum values based on the literature, that is, upper and lower limits and center point.

Table 1.
Experimental design applied for optimization.
2.3. Dynamic Maceration (DM)
The maceration was carried out in a thermostatic bath in which the temperature was controlled. Stirring was carried out with the aid of submersible magnetic stirrers (Micro Stirrers, Thermo Scientific Cimarec, Thermo Fisher Scientific, Waltham, MA, USA). The solid/liquid ratio was 30 g/L and constant for all runs applied. After the extraction process, the solution was filtered through Whatman paper filter #4 and then stored for the dry weight procedure. For dry weight, 5 mL of the extraction solution was added to adequately tared crucibles. The crucibles were placed in an oven and remained for four days for complete drying. At the end, the dry crucibles were weighed a second time and the dry weight of each sample was calculated.
3. Results and Discussion
Extraction Optimization Studies
The purpose of the extraction was to obtain the highest yield as a function of the dry weight. Table 2 presents the obtained values of Y1, with the response ranging from 21.1 to 90.5 mg/mL.

Table 2.
Olive tree leaf optimization responses (Y1) in dry weight.
The coded values were analysed using the Design Expert software, with no transformation, using a quadratic model. The model showed and adequate p-value and an insignificant lack of fit.
Figure 1 shows the optimal points with the highest extraction yield, ploting two factors in each graph, maintaining the third at the optimal point. Thus, it is clear that in Figure 1a, low temperature, and 120 min promote extraction yield, which is in acordance with Figure 1b, in which low temperature and a high percentage of ethanol also promotes the dry residue. Finally, in Figure 1c, long extraction time and a high amount of ethanol show higher yields.
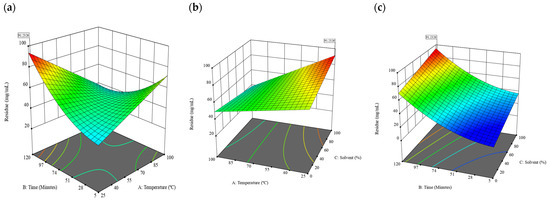
Figure 1.
3D plot of the different factors and respective variation in dry weight.
Using the maximize function, the optimum point was set at 25 °C (F1), 120 min (F2), and 87% ethanol (F3). The maceration extraction technique is a conventional and simple technique in which 120 min becomes time consuming, and thus other non-conventional techniques (e.g., ultrasound-assisted extraction) could be applied and may provide the same or higher yield in shorter times. The main advantage presented in the maceration optimization was in relation to the temperature factor, since at 25 °C the need for heating becomes minimal. In industrial applications, the absence of heating avoids additional costs for the extraction process, which is an attractive aspect.
Although a higher dry weight value might not indicate a higher amount of phenolic compounds and higher bioactivity of the extract due to the eventual presence of other substances, it may be an indication of a trend towards a higher presence of bioactive compounds as demonstrated in previous studies in the literature [15,16,17]. Therefore, the optimization performed serves as a basic study for further comparative analyses. Nevertheless, there is also a need for further studies involving the optimization and identification based on the phenolic content present in the sample, as well as antioxidant and antimicrobial analyses to associate with the bioactivity and obtain more concrete results for application.
4. Conclusions
The optimization performed by RSM indicated the optimal extractive point as a function of dry weight at 120 min (F1), 25 °C (F2) and 87% (F3) of ethanol:water. According to this study, the yield increases inversely with temperature. In addition, for industrial applications, the temperature value found for maceration becomes an attractive factor, considering that it avoids additional costs. Future studies will be conducted to optimize for specific phenolic compounds and by applying other extractive methods to analyze the potential of the extract as a natural preservative.
Author Contributions
Conceptualization, M.C. and S.H.; methodology, M.C.P. and L.L.; software, M.C.; validation, M.C.; formal analysis, M.C.; investigation, M.C.P. and L.L.; resources, L.B.; data curation, M.C.; writing—original draft preparation, M.C. and S.H.; writing—review and editing, M.C. and S.H.; visualization, M.C.; supervision, L.B. and I.C.F.R.F.; project administration, L.B.; funding acquisition, L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Science and Technology (FCT, Portugal) and the European Fund for Regional Development (Fundo Europeu de Desenvolvimento Regional (FEDER)) through the Programa Operacional Regional Norte 2020, within the “PlantCovid” project (NORTE-01-02B7-FEDER-054870).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES to the CIMO (UIDB/00690/2020). Acknowledgments to the Project financed by the European Fund for Regional Development (Fundo Europeu de Desenvolvimento Regional (FEDER)) through the Programa Operacional Regional Norte 2020, within the “PlantCovid” project, NORTE-01-02B7-FEDER-054870. M.C. Pedrosa thanks “PlantCovid” project for her scholarship. S. Heleno and M. Carocho thank FCT for their individual employment program-contract (CEECIND/00831/2018, CEECIND/03040/2017). L. Barros also thanks the national funding by FCT, through the institutional scientific employment program-contract for her contract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ray, N.B.; Lam, N.T.; Luc, R.; Bonvino, N.P.; Karagiannis, T.C. Cellular and molecular effects of bioactive phenolic compounds in olives and olive oil. In Olive and Olive Oil Bioactive Constituents; AOCS Press: Urbana, IL, USA, 2015; pp. 53–91. [Google Scholar]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Comp. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Makowska-Wąs, J.; Galanty, A.; Gdula-Argasińska, J.; Tyszka-Czochara, M.; Szewczyk, A.; Nunes, R.; Carvalho, I.S.; Michalik, M.; Paśko, P. Identification of Predominant Phytochemical Compounds and Cytotoxic Activity of Wild Olive Leaves (Olea europaea L. ssp. sylvestris) Harvested in South Portugal. Chem. Biodivers. 2017, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Mylonaki, S.; Kiassos, E.; Makris, D.P.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Elhussein, E.; Bilgin, M.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S. Effect of drying method on oleuropein, total phenolic content, flavonoid content, and antioxidant activity of olive (Olea europaea) leaf. J. Food Process. Preserv. 2018, 42, e13604. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of ultrasound-assisted extraction of flavonoids from olive (olea europaea) leaves, and evaluation of their antioxidant and anticancer activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kırbaşlar, Ş.İ.; Şahin, S. Recovery of bioactive ingredients from biowaste of olive tree (Olea europaea) using microwave-assisted extraction: A comparative study. Biomass Convers. Biorefinery 2021, 1–13. [Google Scholar] [CrossRef]
- Contrerasa, M.M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314, 126218. [Google Scholar] [CrossRef] [PubMed]
- Xynos, N.; Papaefstathiou, G.; Gikas, E.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A. Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology. Sep. Purif. Technol. 2014, 122, 323–330. [Google Scholar] [CrossRef]
- Şahin, S.; Şamlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).