Evaluation of Technologies for the Co-Extraction of Phenolic Compounds and Proteinaceous Material from Olive-Derived Biomasses †
Abstract
:1. Introduction
2. Material and Methods
2.1. Samples and Reagents
2.2. Extraction Technologies
2.3. Determination of the Total Phenolic Content and Phenolic Profile
2.4. Determination of the Protein Content
3. Results and Discussion
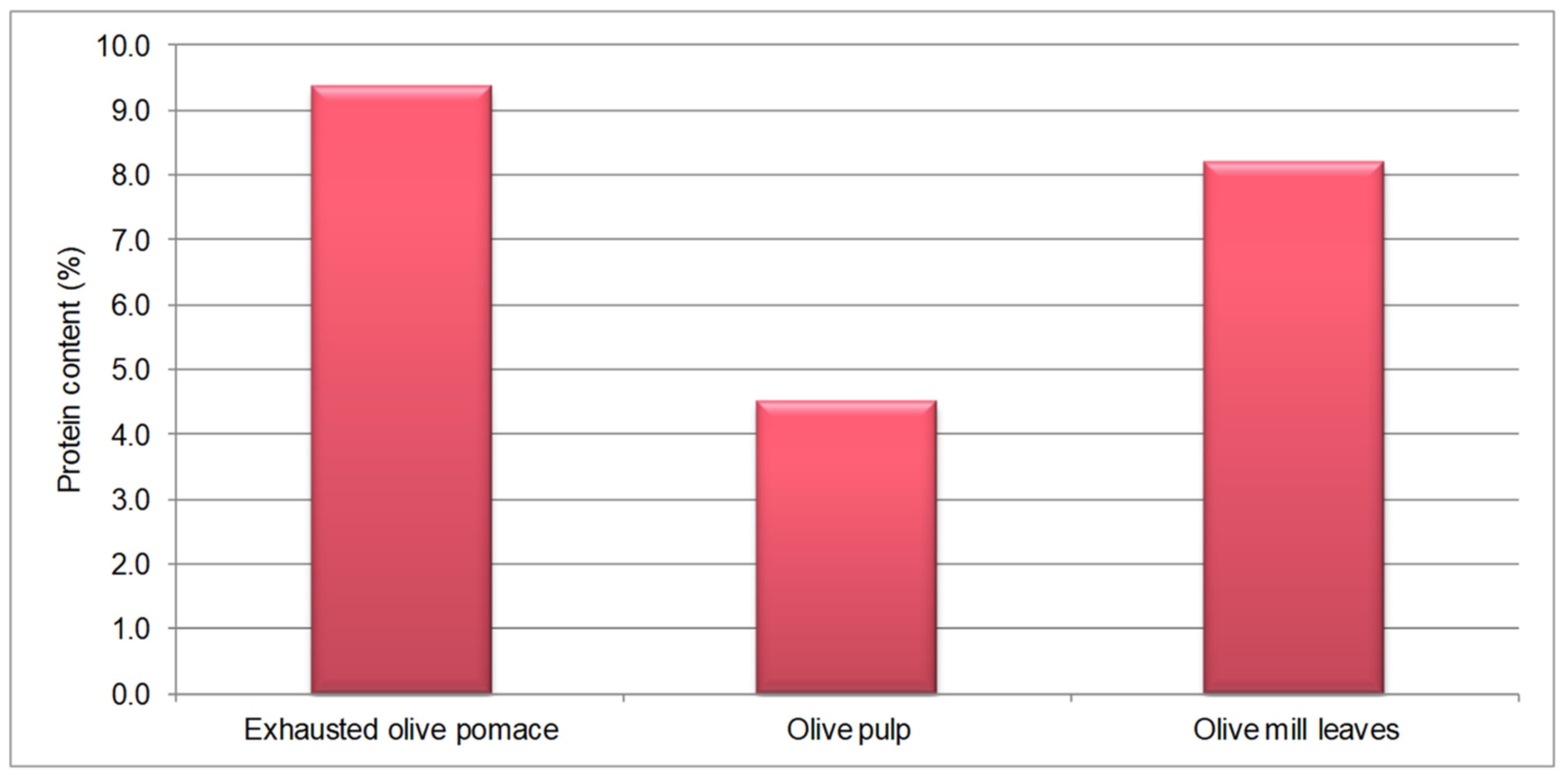
3.1. Protein Content of Olive Biomasses
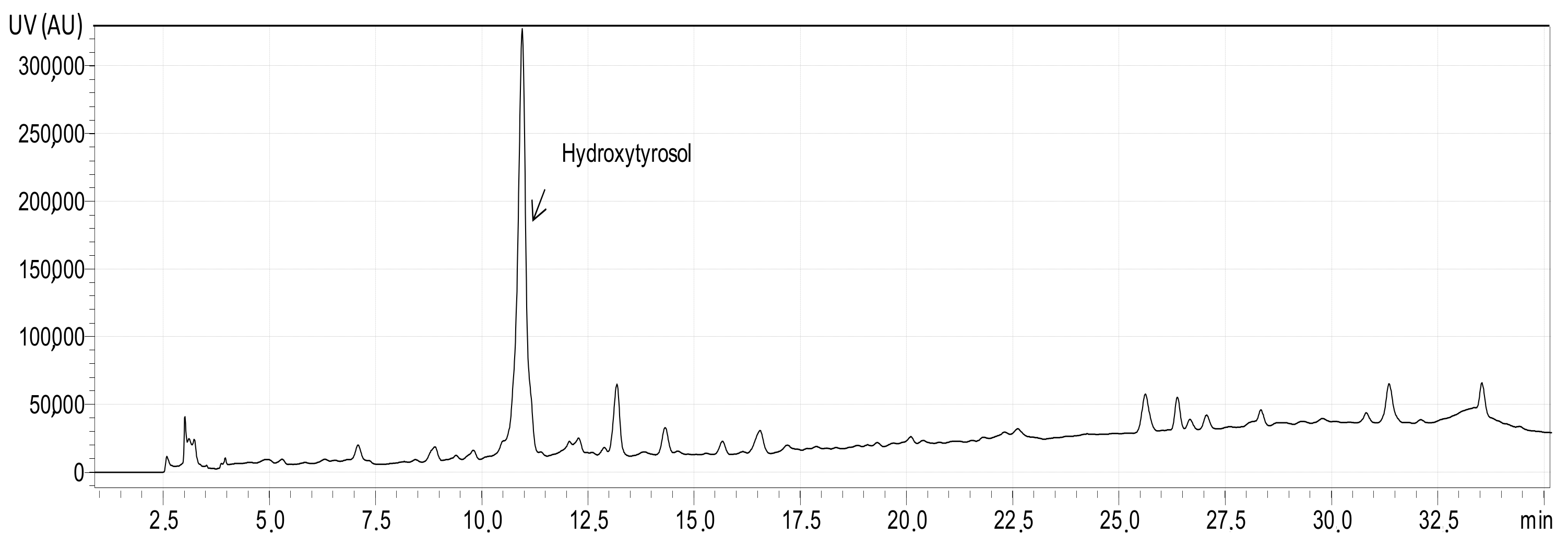
3.2. Total Phenolic Content of the Extracts
3.3. Protein Solubilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Olive biophenols as new antioxidant additives in food and beverage. ChemistrySelect 2017, 2, 1360–1365. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Romero, I.; Moya, M.; Castro, E. Olive-derived biomass as a renewable source of value-added products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Gómez-Cruz, I.; Romero, I.; Castro, E. Olive pomace-derived biomasses fractionation through a two-step extraction based on the use of ultrasounds: Chemical Characteristics. Foods 2021, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an extract rich in phenolic compounds from olive pomace by pressurized liquid extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medfai, W.; Contreras, M.d.M.; Lama-Muñoz, A.; Mhamdi, R.; Oueslati, I.; Castro, E. How cultivar and extraction conditions affect antioxidants type and extractability for olive leaves valorization. ACS Sustain. Chem. Eng. 2020, 8, 5107–5118. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Pérez-Sánchez, A.; Herrero, M.; Ibañez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A.; et al. Use of advanced techniques for the extraction of phenolic compounds from Tunisian olive leaves: Phenolic composition and cytotoxicity against human breast cancer cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, M.d.M.; Lama-Muñoz, A.; Manuel Gutiérrez-Pérez, J.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.W.; Mulder, W.J.; Sanders, J.P.M.; Bruins, M.E. Towards plant protein refinery: Review on protein extraction using alkali and potential enzymatic assistance. Biotechnol. J. 2015, 10, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of exhausted olive pomace by an eco-friendly solvent extraction process of natural antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, M.d.M.; Gómez-Cruz, I.; Romero, I.; Castro, E. Evaluation of Technologies for the Co-Extraction of Phenolic Compounds and Proteinaceous Material from Olive-Derived Biomasses. Biol. Life Sci. Forum 2021, 6, 60. https://doi.org/10.3390/Foods2021-10968
Contreras MdM, Gómez-Cruz I, Romero I, Castro E. Evaluation of Technologies for the Co-Extraction of Phenolic Compounds and Proteinaceous Material from Olive-Derived Biomasses. Biology and Life Sciences Forum. 2021; 6(1):60. https://doi.org/10.3390/Foods2021-10968
Chicago/Turabian StyleContreras, María del Mar, Irene Gómez-Cruz, Inmaculada Romero, and Eulogio Castro. 2021. "Evaluation of Technologies for the Co-Extraction of Phenolic Compounds and Proteinaceous Material from Olive-Derived Biomasses" Biology and Life Sciences Forum 6, no. 1: 60. https://doi.org/10.3390/Foods2021-10968
APA StyleContreras, M. d. M., Gómez-Cruz, I., Romero, I., & Castro, E. (2021). Evaluation of Technologies for the Co-Extraction of Phenolic Compounds and Proteinaceous Material from Olive-Derived Biomasses. Biology and Life Sciences Forum, 6(1), 60. https://doi.org/10.3390/Foods2021-10968







