Evaluation of Analytical Methods to Determine Regulatory Compliance of Coffee Leaf Tea †
Abstract
:1. Introduction
2. Methods
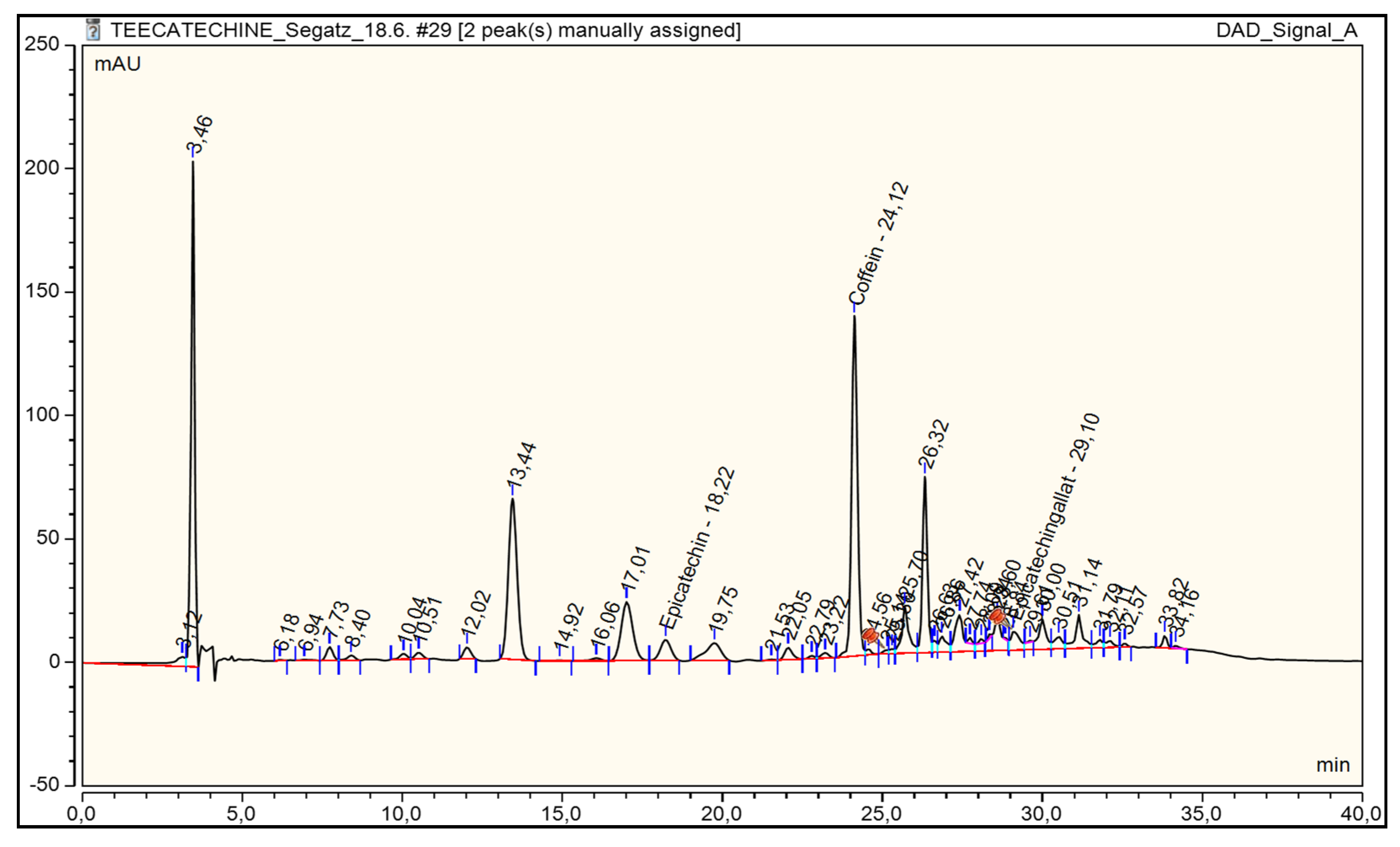
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Commission Implementing Regulation (EU) 2020/917. Off. J. Euro. Union 2020, L209, 10. [Google Scholar]
- ISO 14502-1:2005-03; Determination of Substances Characteristic of Green and Black Tea-Part 1: Content of Total Polyphenols in Tea-Colorimetric Method Using Folin-Ciocalteu Reagent. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 14502-2:2007-12; Determination of Substances Characteristic of Green and Black Tea-Part 2: Content of Catechins in Green tea-Method Using High-Performance Liquid Chromatography (ISO 14502-2:2005 + Corrigendum 1:2006). International Organization for Standardization: Geneva, Switzerland, 2005.
- Langer, T.; Winkler, G.; Lachenmeier, D.W. Untersuchungen zum Abkühlverhalten von Heißgetränken vor dem Hintergrund des temperaturbedingten Krebsrisikos. Deut. Lebensm. Rundsch. 2018, 114, 307–314. [Google Scholar] [CrossRef]
- Claassen, L.; Rinderknecht, M.; Porth, T.; Röhnisch, J.; Seren, H.Y.; Scharinger, A.; Gottstein, V.; Noack, D.; Schwarz, S.; Winkler, G.; et al. Cold brew coffee—Pilot studies on definition, extraction, consumer preference, chemical characterization and microbiological hazards. Foods 2021, 10, 865. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segatz, V.; Steger, M.C.; Blumenthal, P.; Gottstein, V.; Rigling, M.; Schwarz, S.; Zhang, Y.; Lachenmeier, D.W. Evaluation of Analytical Methods to Determine Regulatory Compliance of Coffee Leaf Tea. Biol. Life Sci. Forum 2021, 6, 45. https://doi.org/10.3390/Foods2021-10937
Segatz V, Steger MC, Blumenthal P, Gottstein V, Rigling M, Schwarz S, Zhang Y, Lachenmeier DW. Evaluation of Analytical Methods to Determine Regulatory Compliance of Coffee Leaf Tea. Biology and Life Sciences Forum. 2021; 6(1):45. https://doi.org/10.3390/Foods2021-10937
Chicago/Turabian StyleSegatz, Valerie, Marc C. Steger, Patrik Blumenthal, Vera Gottstein, Marina Rigling, Steffen Schwarz, Yanyan Zhang, and Dirk W. Lachenmeier. 2021. "Evaluation of Analytical Methods to Determine Regulatory Compliance of Coffee Leaf Tea" Biology and Life Sciences Forum 6, no. 1: 45. https://doi.org/10.3390/Foods2021-10937
APA StyleSegatz, V., Steger, M. C., Blumenthal, P., Gottstein, V., Rigling, M., Schwarz, S., Zhang, Y., & Lachenmeier, D. W. (2021). Evaluation of Analytical Methods to Determine Regulatory Compliance of Coffee Leaf Tea. Biology and Life Sciences Forum, 6(1), 45. https://doi.org/10.3390/Foods2021-10937










