Correlations between Capsaicin, Dihydrocapsaicin and Phenolic Content in Habanero Chillies †
Abstract
:1. Introduction
2. Methods
2.1. Sample Procurement
2.2. Extraction Protocol and Measurement of TP Content and FRAP
2.3. Measurement of Capsaicinoids by HPLC
2.4. Data Analysis
3. Results and Discussion
3.1. Total Phenolic Content and Antioxidant Activity
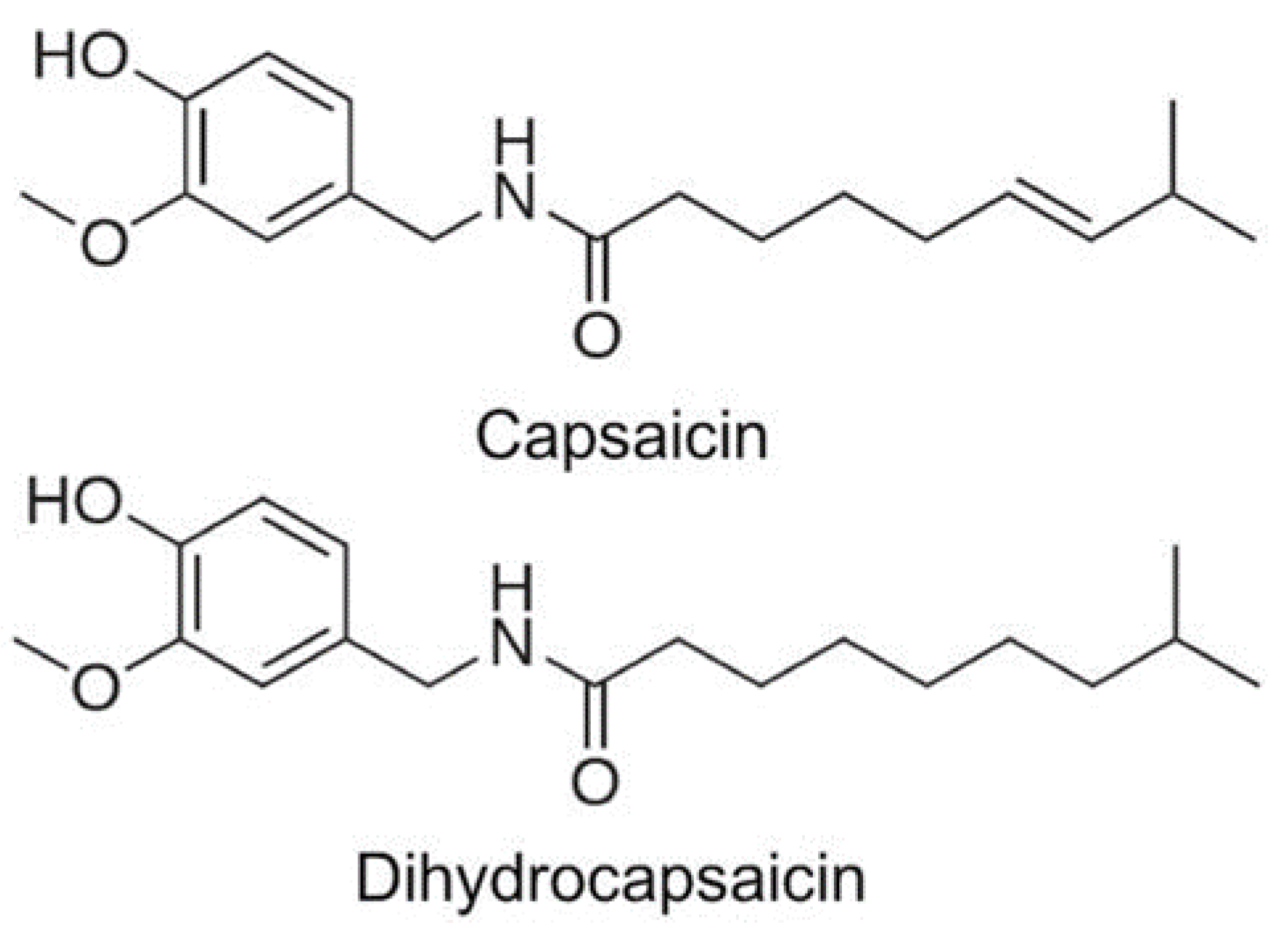
3.2. Capsaicinoids
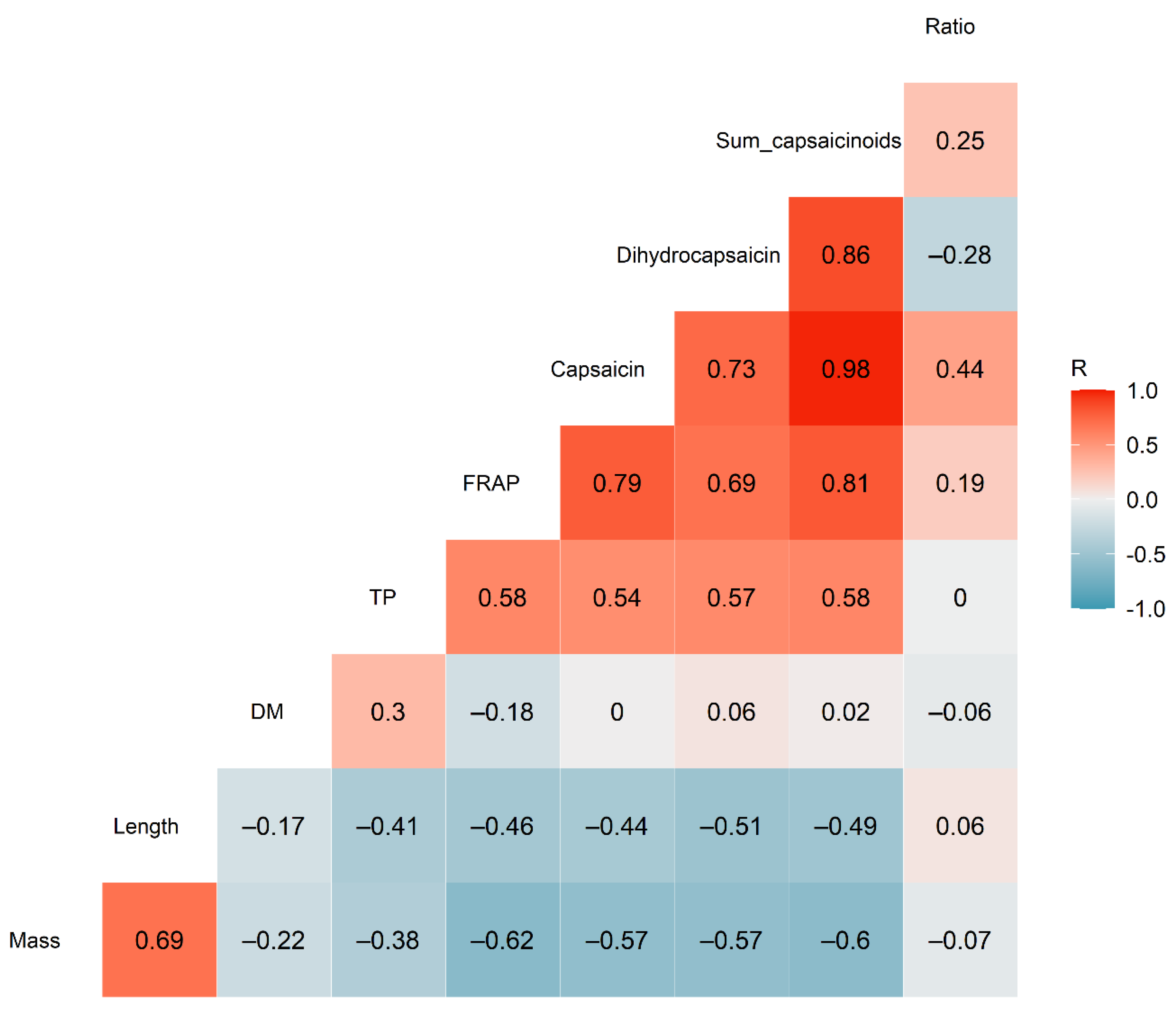
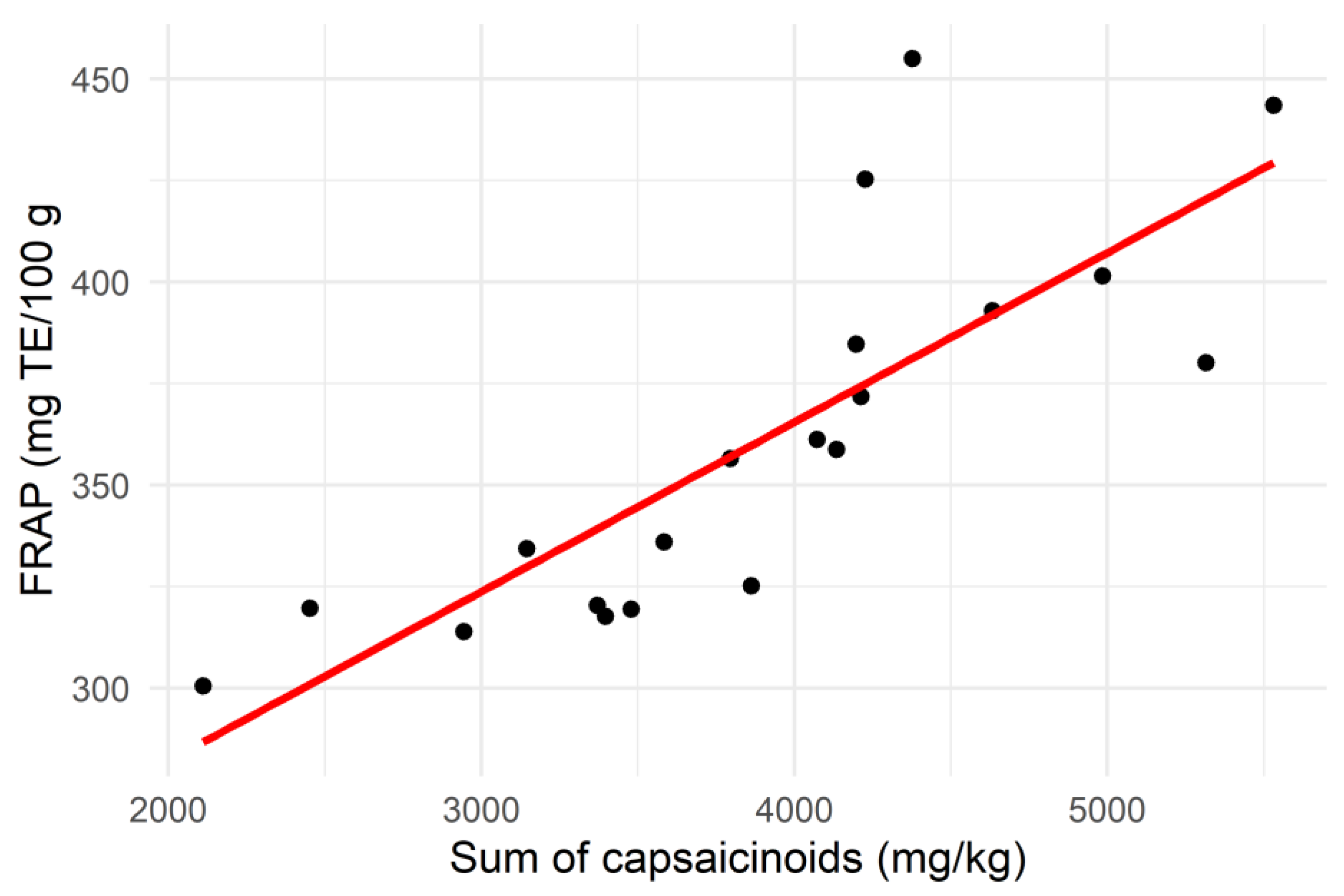
3.3. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, M.; Higuera, I.; Goycoolea, F.M. Capsaicinoids: Occurrence, Chemistry, Biosynthesis, and Biological Effects. In Fruit and Vegetable Phytochemicals, 2nd ed.; Yahia, E.M., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 499–514. [Google Scholar]
- Ofori-Asenso, R.; Mohsenpour, M.A.; Nouri, M.; Faghih, S.; Liew, D.; Mazidi, M. Association of Spicy Chilli Food Consumption With Cardiovascular and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. Angiology 2021, 72, 625–632. [Google Scholar] [CrossRef]
- Guzmán, I.; Bosland, P.W. A Matter of Taste: Capsaicinoid Diversity in Chile Peppers and the Importance to Human Food Preference. In Capsaicin and Its Human Therapeutic Development; Mozsik, G., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Canto-Flick, A.; Balam-Uc, E.; Bello-Bello, J.J.; Lecona-Guzmán, C.; Solís-Marroquín, D.; Avilés-Viñas, S.; Gómez-Uc, E.; López-Puc, G.; Santana-Buzzy, N.; Iglesias-Andreu, L.G. Capsaicinoids Content in Habanero Pepper (Capsicum chinense Jacq.): Hottest Known Cultivars. HortScience 2008, 43, 1344. [Google Scholar] [CrossRef] [Green Version]
- Naves, E.R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Peres, L.E.P.; Zsögön, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef]
- AusVeg. Veggie Stats: Chillies; AusVeg: Melbourne, Australia, 2016; pp. 19–21. [Google Scholar]
- Johnson, J.; Collins, T.; Skylas, D.; Quail, K.; Blanchard, C.; Naiker, M. Profiling the varietal antioxidative content and macrochemical composition in Australian faba beans (Vicia faba L.). Legume Sci. 2020, 2, e28. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.B.; Walsh, K.B.; Bhattarai, S.P.; Naiker, M. Partitioning of nutritional and bioactive compounds between the kernel, hull and husk of five new chickpea genotypes grown in Australia. Future Foods 2021, 4, 100065. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; Skylas, D.; Walsh, K.B.; Bhattarai, S.P.; Naiker, M. Phenolic profiles and nutritional quality of four new mungbean lines grown in northern Australia. Legume Sci. 2020, 3, e70. [Google Scholar] [CrossRef]
- Waite, M.S.; Aubin, A.J. A Modular HPLC System for Routine Analysis of Capsaicin from Hot Sauces; Waters Corporation: Milford, MA, USA, 2008; p. 5. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Shaha, R.K.; Rahman, S.; Asrul, A. Bioactive compounds in chilli peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Ann. Biol. Res. 2013, 4, 27–34. [Google Scholar]
- Sricharoen, P.; Lamaiphan, N.; Patthawaro, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Phytochemicals in Capsicum oleoresin from different varieties of hot chilli peppers with their antidiabetic and antioxidant activities due to some phenolic compounds. Ultrason. Sonochem. 2017, 38, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Singh, S.B.; Saha, S.; Akoijam, R.S.; Boopathi, T.; Banerjee, A.; Lungmuana; Vanlalhmangaiha; Roy, S. Diversity in Bird’s Eye Chilli (Capsicum frutescens L.) Landraces of North-East India in Terms of Antioxidant Activities. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 1317–1326. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Menichini, F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007, 102, 1096–1104. [Google Scholar] [CrossRef]
- Jansasithorn, R.; East, A.R.; Hewett, E.W.; Molan, A.L.; Heyes, J.A.; Mawson, A.J. Harvest maturity influences the antioxidant activity in Jalapeño chili. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Emerging Health Topics in Fruits and Vegetables, Lisbon, Portugal, 2012; pp. 379–384. [Google Scholar]
- Juangsamoot, J.; Ruangviriyachai, C.; Techawongstien, S.; Chanthai, S. Determination of capsaicin and dihydrocapsaicin in some hot chilli varieties by RP-HPLC-PDA after magnetic stirring extraction and clean up with C18 cartridge. Int. Food Res. J. 2012, 19, 1217–1226. [Google Scholar]
- Garcés-Claver, A.; Arnedo-Andrés, M.S.; Abadía, J.; Gil-Ortega, R.; Álvarez-Fernández, A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruits by Liquid Chromatography−Electrospray/Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 9303–9311. [Google Scholar] [CrossRef] [Green Version]
- Weaver, K.M.; Luker, R.G.; Neale, M.E. Rapid quality control procedure for the determination of Scoville heat units and the detection of chillies in black pepper, via high-performance liquid chromatography. J. Chromatogr. A 1984, 301, 288–291. [Google Scholar] [CrossRef]
- Tainter, D.R.; Grenis, A.T. Spices and Seasonings: A Food Technology Handbook, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Othman, Z.A.A.; Ahmed, Y.B.H.; Habila, M.A.; Ghafar, A.A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruit Samples using High Performance Liquid Chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.C.N.; Gururaj, H.B.; Kumar, V.; Giridhar, P.; Ravishankar, G.A. Valine Pathway Is More Crucial than Phenyl Propanoid Pathway in Regulating Capsaicin Biosynthesis in Capsicum frutescens Mill. J. Agric. Food Chem. 2006, 54, 6660–6666. [Google Scholar] [CrossRef]
- Blum, E.; Mazourek, M.; O’Connell, M.; Curry, J.; Thorup, T.; Liu, K.; Jahn, M.; Paran, I. Molecular mapping of capsaicinoid biosynthesis genes and quantitative trait loci analysis for capsaicinoid content in Capsicum. Theor. Appl. Genet. 2003, 108, 79–86. [Google Scholar] [CrossRef]
- Sricharoen, P.; Techawongstein, S.; Chanthai, S. A high correlation indicating for an evaluation of antioxidant activity and total phenolics content of various chilli varieties. J. Food Sci. Technol. 2015, 52, 8077–8085. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Sharma, M.; Sharma, A. Genetic Variation, Association of Characters, and Their Direct and Indirect Contributions for Improvement in Chilli Peppers. Int. J. Veg. Sci. 2009, 15, 340–368. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, J.B.; Mani, J.S.; Naiker, M. Correlations between Capsaicin, Dihydrocapsaicin and Phenolic Content in Habanero Chillies. Biol. Life Sci. Forum 2021, 6, 30. https://doi.org/10.3390/Foods2021-11066
Johnson JB, Mani JS, Naiker M. Correlations between Capsaicin, Dihydrocapsaicin and Phenolic Content in Habanero Chillies. Biology and Life Sciences Forum. 2021; 6(1):30. https://doi.org/10.3390/Foods2021-11066
Chicago/Turabian StyleJohnson, Joel B., Janice S. Mani, and Mani Naiker. 2021. "Correlations between Capsaicin, Dihydrocapsaicin and Phenolic Content in Habanero Chillies" Biology and Life Sciences Forum 6, no. 1: 30. https://doi.org/10.3390/Foods2021-11066
APA StyleJohnson, J. B., Mani, J. S., & Naiker, M. (2021). Correlations between Capsaicin, Dihydrocapsaicin and Phenolic Content in Habanero Chillies. Biology and Life Sciences Forum, 6(1), 30. https://doi.org/10.3390/Foods2021-11066







