Bioaccessibility and Intestinal Permeability from Andean Blackberry (Rubus glaucus Benth) Powders Encapsulated with OSA-Modified FHIA-21 Banana Starch †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blackberry Powder Encapsulation
2.2. In Vitro Gastrointestinal Digestion
2.3. Total Phenolic Compound Quantification and Identification of Individual Phenolic Compounds
2.4. Bioaccessibility (% B) and Apparent Permeability Coefficient (Papp) Determination
2.5. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolics and Bioaccessibility of Individual Phenolic Compounds from Capsules
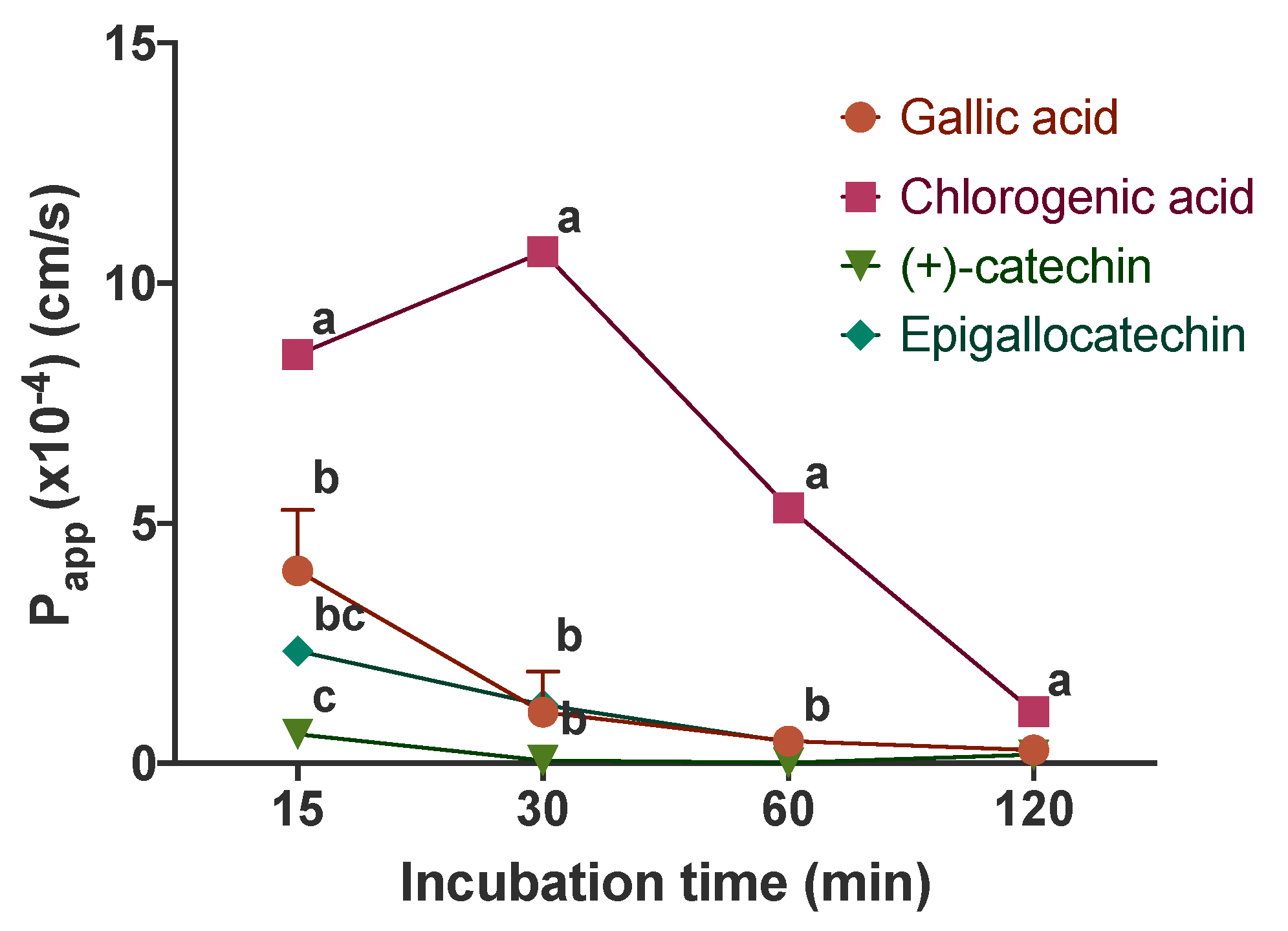
3.2. Apparent Permeability Coefficients
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moraes, D.P.; Lozano-Sánchez, J.; Machado, M.L.; Vizzotto, M.; Lazzaretti, M.; Leyva-Jimenez, F.J.J.; da Silveira, T.L.; Ries, E.F.; Barcia, M.T. Characterization of a New Blackberry Cultivar BRS Xingu: Chemical Composition, Phenolic Compounds, and Antioxidant Capacity In Vitro and In Vivo. Food Chem. 2020, 322, 126783. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Chan, Y.H.; Rathinasabapathy, T.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Enhanced Stability of Berry Pomace Polyphenols Delivered in Protein-Polyphenol Aggregate Particles to an In Vitro Gastrointestinal Digestion Model. Food Chem. 2020, 331, 127279. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Castaño, V.D.; Vasco-Leal, J.F.; Cuellar-Nuñez, M.; Luzardo-Ocampo, I.; Castellanos-Galeano, F.; Álvarez-Barreto, C.; Bello-Pérez, L.A.; Cortés-Rodriguez, M.; Quintero-Castaño, V.D.; Vasco-Leal, J.F.; et al. Novel OSA-Modified Starch from Gros Michel Banana for Encapsulation of Andean Blackberry Concentrate: Production and Storage Stability. Starch-Stärke 2021, 73, 2000180. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of Wall Materials on the Spray Drying Efficiency, Powder Properties and Stability of Bioactive Compounds in Tamarillo Juice Microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced Material Applications of Starch and Its Derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Wurzburg, O.B. Modified Starches. In Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 88–113. ISBN 978-0-8247-5922-3. [Google Scholar]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and Physicochemical Properties of Octenyl Succinic Anhydride Modified Starches: A Review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef] [PubMed]
- FHIA. Plátano FHIA-21. 2012. Available online: http://www.fhia.org.hn/descargas/Programa_de_Banano_y_Platano/hibridos-FHIA/fhia-21.pdf (accessed on 10 October 2021).
- Luzardo-Ocampo, I.; Campos-Vega, R.; Gaytán-Martínez, M.; Preciado-Ortiz, R.; Mendoza, S.; Loarca-Piña, G.; Preciado-Ortíz, R.; Mendoza, S.; Loarca-Piña, G. Bioaccessibility and Antioxidant Activity of Free Phenolic Compounds and Oligosaccharides from Corn (Zea mays L.) and Common Bean (Phaseolus vulgaris L.) Chips during In Vitro Gastrointestinal Digestion and Simulated Colonic Fermentation. Food Res. Int. 2017, 100, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated Gastrointestinal Digestion and In Vitro Colonic Fermentation of Spent Coffee (Coffea arabica L.): Bioaccessibility and Intestinal Permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Aguillón-Osma, J.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Maldonado-Celis, M.E.; Loango-Chamorro, N.; Campos-Vega, R. Impact of In Vitro Gastrointestinal Digestion on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Passion Fruit (Passiflora edulis) Leaves and Juice Extracts. J. Food Biochem. 2019, 43, e12879. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Lopez, L.H.L.H.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.L.; Campos-Vega, R.; Mendoza, S.; Loarca-Piña, G. Effect of the In Vitro Gastrointestinal Digestion on Free-Phenolic Compounds and Mono/Oligosaccharides from Moringa oleifera Leaves: Bioaccessibility, Intestinal Permeability and Antioxidant Capacity. Food Res. Int. 2019, 120, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Reynoso-Camacho, R.; Mendoza-Díaz, S.; Loarca-Piña, G. Functional and Technological Potential of Dehydrated Phaseolus vulgaris L. Flours. Food Chem. 2014, 161, 254–260. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from Artichoke Heads (Cynara cardunculus (L.) Subsp. Scolymus Hayek): In Vitro Bio-Accessibility, Intestinal Uptake and Bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, M.A.; Khemiss, F.; Sfar, S. Comparative Study of Two In Vitro Methods for Assessing Drug Absorption: Sartorius SM 16750 Apparatus versus Everted Gut Sac. J. Pharm. Pharm. Sci. 2011, 14, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.D.; Luzardo-Ocampo, I.; Campos-Vega, R.; Loarca-Piña, G.; Maldonado-Celis, M.E. Bioaccessibility during In Vitro Digestion and Antiproliferative Effect of Bioactive Compounds from Andean Berry (Vaccinium meridionale Swartz) Juice. J. Agric. Food Chem. 2018, 66, 7358–7366. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In Vitro Gastrointestinal Digestion Impact on Stability, Bioaccessibility and Antioxidant Activity of Polyphenols from Wild and Commercial Blackberries (Rubus Spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of Drug Permeability and Prediction of Drug Absorption in Caco-2 Monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Quilaqueo, M.; Millao, S.; Luzardo-Ocampo, I.; Campos-Vega, R.; Acevedo, F.; Shene, C.; Rubilar, M. Inclusion of Piperine in β-Cyclodextrin Complexes Improves Their Bioaccessibility and In Vitro Antioxidant Capacity. Food Hydrocoll. 2019, 91, 143–152. [Google Scholar] [CrossRef]
| Sample/Stage | TPC (mg GAE/g Sample) | Individual Phenolic Compounds (HPLC-DAD) (% B) | |||
|---|---|---|---|---|---|
| Gallic Acid | Chlorogenic Acid | (+)-Catechin | Epigallocatechin Gallate | ||
| ME | 909.64 ± 3.67 a | - | - | - | - |
| Oral | 49.20 ± 4.23 c | 4.20 ± 1.18 | 6.17 ± 0.72 | 2.91 ± 0.14 | 0.97 ± 0.19 |
| Gastric | 57.58 ± 0.50 b | 1.59 ± 0.18 | 51.02 ± 4.04 | 5.70 ± 0.54 | 15.59 ± 0.75 |
| Small intestine (DF) | |||||
| 15 min | 2.83 ± 0.33 e | 4.19 ± 0.37 | 0.62 ± 0.08 | n. d. | n. d. |
| 30 min | 3.54 ± 0.11 e | 11.43 ± 1.23 | 1.24 ± 0.53 | n. d. | n. d. |
| 60 min | 2.49 ± 0.94 e | 10.47 ± 1.18 | 1.71 ± 0.47 | 0.69 ± 0.01 | 1.15 ± 0.17 |
| 120 min | 2.91 ± 0.36 e | 17.52 ± 0.69 | 0.57 ± 0.08 | 0.11 ± 0.00 | 1.76 ± 0.04 |
| Small intestine (NDF) | |||||
| 15 min | 24.08 ± 1.18 d | 20.47 ± 3.34 | 3.07 ± 0.02 | 4.01 ± 0.33 | 4.48 ± 0.16 |
| 30 min | 21.20 ± 2.35 d | 17.39 ± 2.82 | 19.15 ± 0.74 | 8.05 ± 0.71 | 4.66 ± 0.05 |
| 60 min | 21.33 ± 1.46 d | 13.80 ± 0.99 | 19.47 ± 0.64 | 1.08 ± 0.16 | 4.53 ± 0.07 |
| 120 min | 21.72 ± 2.17 d | 24.09 ± 3.81 | 8.70 ± 0.37 | 10.06 ± 0.28 | 4.29 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Castaño, V.D.; Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Vasco-Leal, J.F.; Castellanos-Galeano, F.J.; Álvarez-Barreto, C.I.; Campos-Vega, R. Bioaccessibility and Intestinal Permeability from Andean Blackberry (Rubus glaucus Benth) Powders Encapsulated with OSA-Modified FHIA-21 Banana Starch. Biol. Life Sci. Forum 2021, 6, 111. https://doi.org/10.3390/Foods2021-10981
Quintero-Castaño VD, Cuellar-Nuñez ML, Luzardo-Ocampo I, Vasco-Leal JF, Castellanos-Galeano FJ, Álvarez-Barreto CI, Campos-Vega R. Bioaccessibility and Intestinal Permeability from Andean Blackberry (Rubus glaucus Benth) Powders Encapsulated with OSA-Modified FHIA-21 Banana Starch. Biology and Life Sciences Forum. 2021; 6(1):111. https://doi.org/10.3390/Foods2021-10981
Chicago/Turabian StyleQuintero-Castaño, Victor D., Mardey Liceth Cuellar-Nuñez, Ivan Luzardo-Ocampo, Jose F. Vasco-Leal, Francisco J. Castellanos-Galeano, Cristina I. Álvarez-Barreto, and Rocio Campos-Vega. 2021. "Bioaccessibility and Intestinal Permeability from Andean Blackberry (Rubus glaucus Benth) Powders Encapsulated with OSA-Modified FHIA-21 Banana Starch" Biology and Life Sciences Forum 6, no. 1: 111. https://doi.org/10.3390/Foods2021-10981
APA StyleQuintero-Castaño, V. D., Cuellar-Nuñez, M. L., Luzardo-Ocampo, I., Vasco-Leal, J. F., Castellanos-Galeano, F. J., Álvarez-Barreto, C. I., & Campos-Vega, R. (2021). Bioaccessibility and Intestinal Permeability from Andean Blackberry (Rubus glaucus Benth) Powders Encapsulated with OSA-Modified FHIA-21 Banana Starch. Biology and Life Sciences Forum, 6(1), 111. https://doi.org/10.3390/Foods2021-10981








