Potential Sustainable Antagonistic Biocontrol Strategy Against Xanthomonas vesicatoria †
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation
2.2. Antagonistic Activity In Vitro
2.3. Antagonistic Activity Ex Vivo
2.4. Production of Hydrolytic Enzymes and Fixation of Nitrogen
3. Results
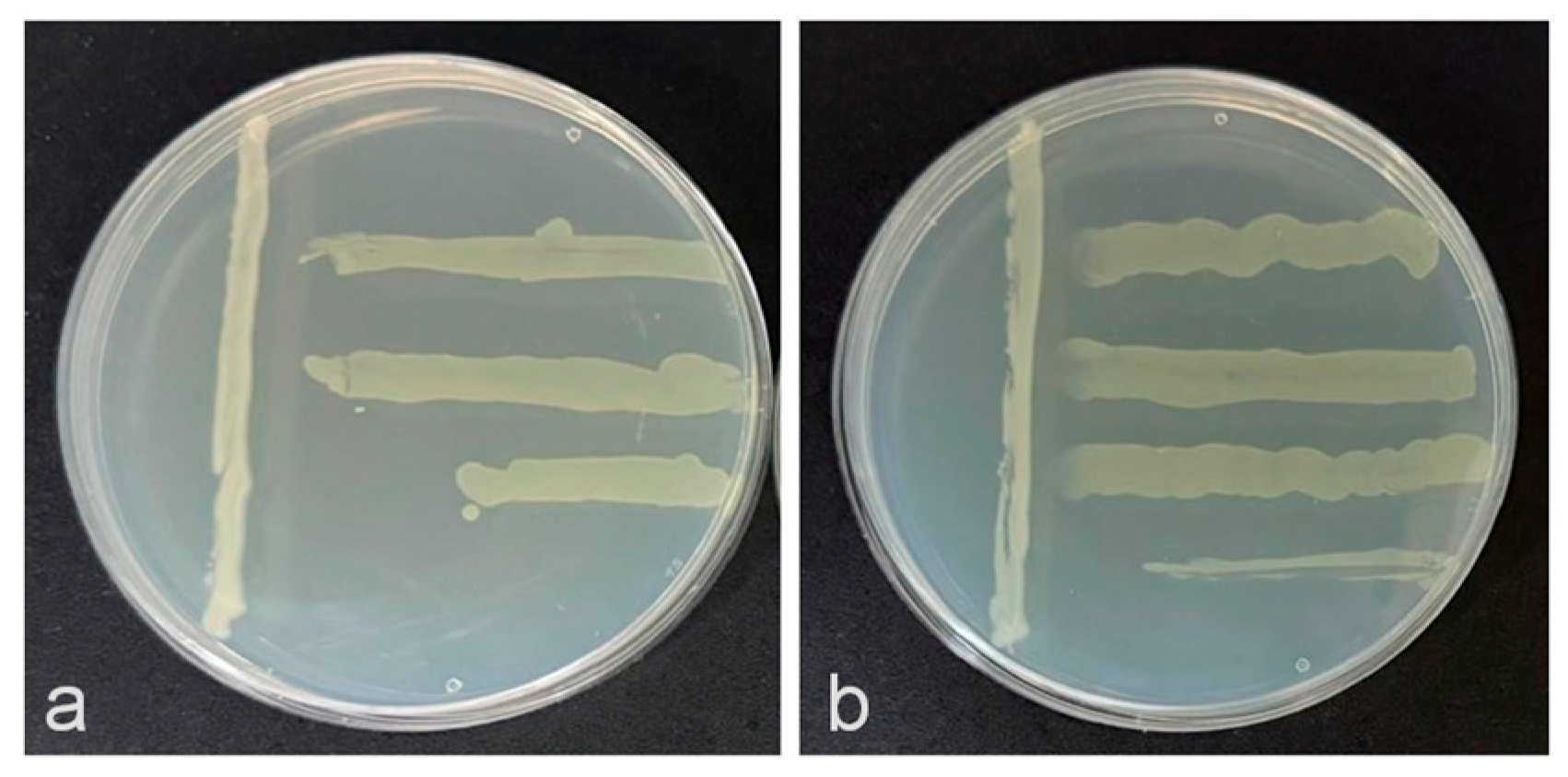
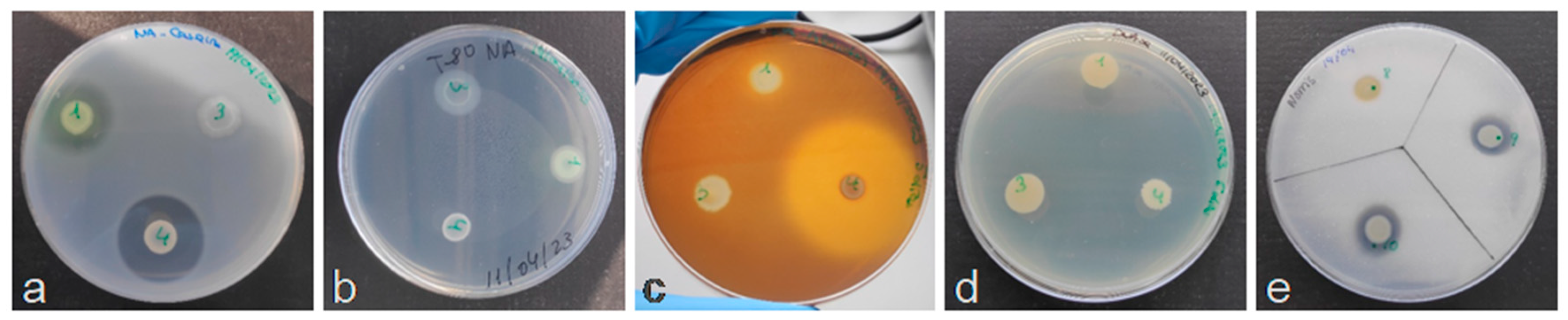
3.1. Antagonistic Activity In Vitro
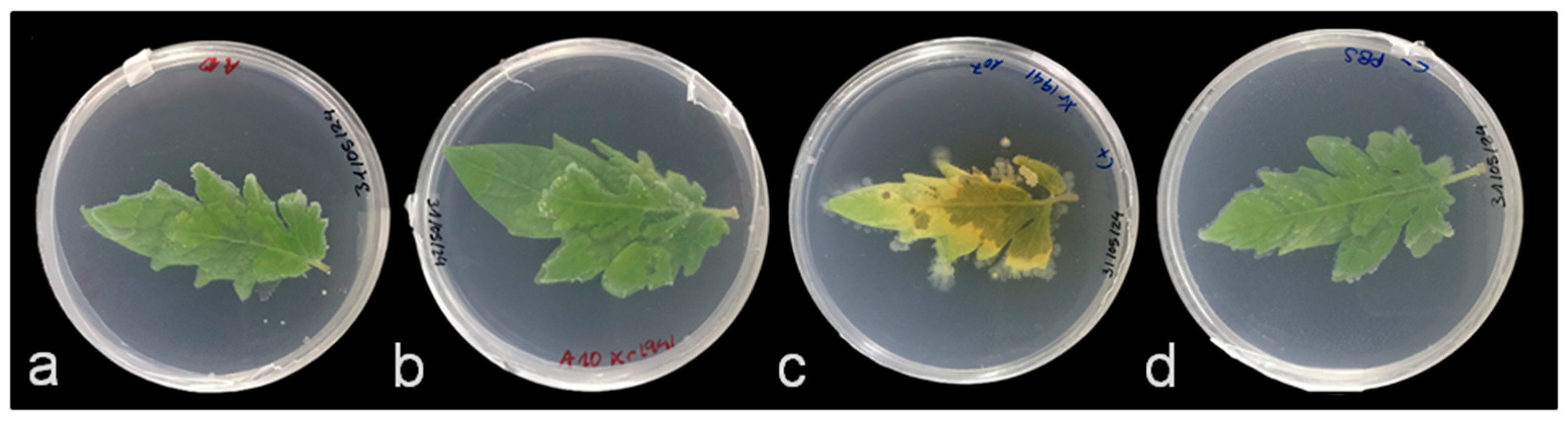
3.2. Antagonistic Activity Ex Vivo
3.3. Production of Hydrolytic Enzymes and Fixation of Nitrogen
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osdaghi, E.; Jones, J.B.; Sharma, A.; Goss, E.M.; Abrahamian, P.; Newberry, E.A.; Potnis, N.; Carvalho, R.; Choudhary, M.; Paret, M.L.; et al. A Centenary for Bacterial Spot of Tomato and Pepper. Mol. Plant Pathol. 2021, 22, 1500–1519. [Google Scholar] [CrossRef] [PubMed]
- EPPO. PM 7/110 (2) Xanthomonas spp. (Xanthomonas euvesicatoria pv. euvesicatoria, Xanthomonas hortorum pv. gardneri, Xanthomonas euvesicatoria pv. perforans, Xanthomonas vesicatoria) Causing Bacterial Spot of Tomato and Sweet Pepper. EPPO Bull. 2023, 53, 558–579. [Google Scholar] [CrossRef]
- An, S.Q.; Potnis, N.; Dow, M.; Vorhölter, F.J.; He, Y.Q.; Becker, A.; Teper, D.; Li, Y.; Wang, N.; Bleris, L.; et al. Mechanistic Insights Into Host Adaptation, Virulence and Epidemiology of the Phytopathogen Xanthomonas. FEMS Microbiol. Rev. 2020, 44, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Felipe, V.; Romero, A.M.; Montecchia, M.S.; Vojnov, A.A.; Bianco, M.I.; Yaryura, P.M. Xanthomonas vesicatoria Virulence Factors Involved in Early Stages of Bacterial Spot Development in Tomato. Plant Pathol. 2018, 67, 1936–1943. [Google Scholar] [CrossRef]
- Esteban-Herrero, G.; Álvarez, B.; Santander, R.D.; Biosca, E.G. Screening for Novel Beneficial Environmental Bacteria for an Antagonism-Based Erwinia amylovora Biological Control. Microorganisms 2023, 11, 1795. [Google Scholar] [CrossRef] [PubMed]
- Licciardello, G.; Caruso, P.; Bella, P.; Boyer, C.; Smith, M.W.; Pruvost, O.; Robene, I.; Cubero, J.; Catara, V. Pathotyping Citrus Ornamental Relatives with Xanthomonas citri pv. citri and X. citri pv. aurantifolii Refines Our Understanding of Their Susceptibility to These Pathogens. Microorganisms 2022, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Meza-Manzaneque, B.; Pérez-Díaz, M.; Biosca, E.G.; Álvarez, B. Isolation and Characterization of Agricultural Soil Bacteria with Biotechnological and Biological Control Potential Applications. Biol. Life Sci. Forum 2024, 31, 28. [Google Scholar] [CrossRef]
- Villavicencio-Vásquez, M.; Espinoza-Lozano, F.; Espinoza-Lozano, L.; Coronel-León, J. Biological Control Agents: Mechanisms of Action, Selection, Formulation and Challenges in Agriculture. Front. Agron. 2025, 7, 1578915. [Google Scholar] [CrossRef]
- Singh, N.V.; Sharma, J.; Dongare, M.D.; Gharate, R.; Chinchure, S.; Nanjundappa, M.; Parashuram, S.; Patil, P.G.; Babu, K.D.; Mundewadikar, D.M.; et al. In Vitro and In Planta Antagonistic Effect of Endophytic Bacteria on Blight Causing Xanthomonas axonopodis pv. punicae: A Destructive Pathogen of Pomegranate. Microorganisms 2023, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Salerno, C.M.; Sagardoy, M.A. Antagonistic Activity by Bacillus subtilis Against Xanthomonas campestris pv. glycines Under Controlled Conditions. Span. J. Agric. Res. 2003, 1, 55–58. [Google Scholar] [CrossRef]
- Negi, R.; Sharma, B.; Kaur, S.; Kaur, T.; Khan, S.S.; Kumar, S.; Ramniwas, S.; Rustagi, S.; Singh, S.; Rai, A.K.; et al. Microbial Antagonists: Diversity, Formulation and Applications for Management of Pest-Pathogens. Egypt. J. Biol. Pest Control 2023, 33, 105. [Google Scholar] [CrossRef]
- Giovanardi, D.; Biondi, E.; Biondo, N.; Quiroga, N.; Modica, F.; Puopolo, G.; Pérez Fuentealba, S. Sustainable and Innovative Biological Control Strategies Against Pseudomonas syringae pv. tomato, Pseudomonas savastanoi pv. phaseolicola and Xanthomonas spp. Affecting Vegetable Crops: A Review. Front. Plant Sci. 2025, 16, 1536152. [Google Scholar] [CrossRef] [PubMed]
| Biological Test | Global Isolates (%) |
|---|---|
| Proteolytic activity | 100 |
| Lipolytic activity | 60 |
| Amylolytic activity | 40 |
| DNase activity | 60 |
| Nitrogen fixation | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, B.; Salas, I.; Castellón, T.; Palacio-Bielsa, A.; Biosca, E.G. Potential Sustainable Antagonistic Biocontrol Strategy Against Xanthomonas vesicatoria. Biol. Life Sci. Forum 2025, 46, 5. https://doi.org/10.3390/blsf2025046005
Álvarez B, Salas I, Castellón T, Palacio-Bielsa A, Biosca EG. Potential Sustainable Antagonistic Biocontrol Strategy Against Xanthomonas vesicatoria. Biology and Life Sciences Forum. 2025; 46(1):5. https://doi.org/10.3390/blsf2025046005
Chicago/Turabian StyleÁlvarez, Belén, Isabel Salas, Thais Castellón, Ana Palacio-Bielsa, and Elena G. Biosca. 2025. "Potential Sustainable Antagonistic Biocontrol Strategy Against Xanthomonas vesicatoria" Biology and Life Sciences Forum 46, no. 1: 5. https://doi.org/10.3390/blsf2025046005
APA StyleÁlvarez, B., Salas, I., Castellón, T., Palacio-Bielsa, A., & Biosca, E. G. (2025). Potential Sustainable Antagonistic Biocontrol Strategy Against Xanthomonas vesicatoria. Biology and Life Sciences Forum, 46(1), 5. https://doi.org/10.3390/blsf2025046005






