Microbial Growth Kinetics of Fermenting Botanicals Used as Gluten-Free Flour Blends †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sourcing and Preparation of Samples
2.2. Fermentation of Samples
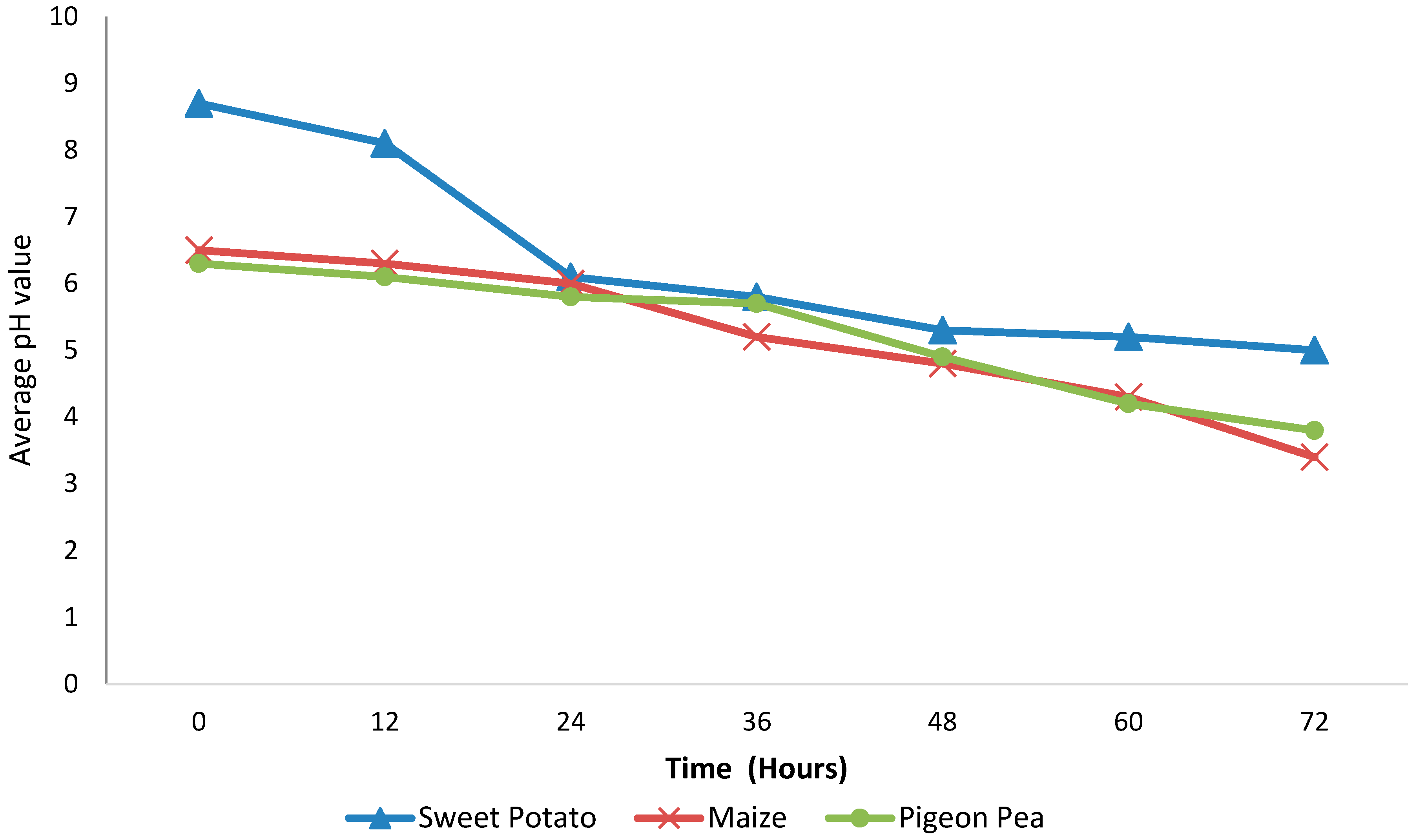
2.3. Determination of pH Variation with Fermentation Time
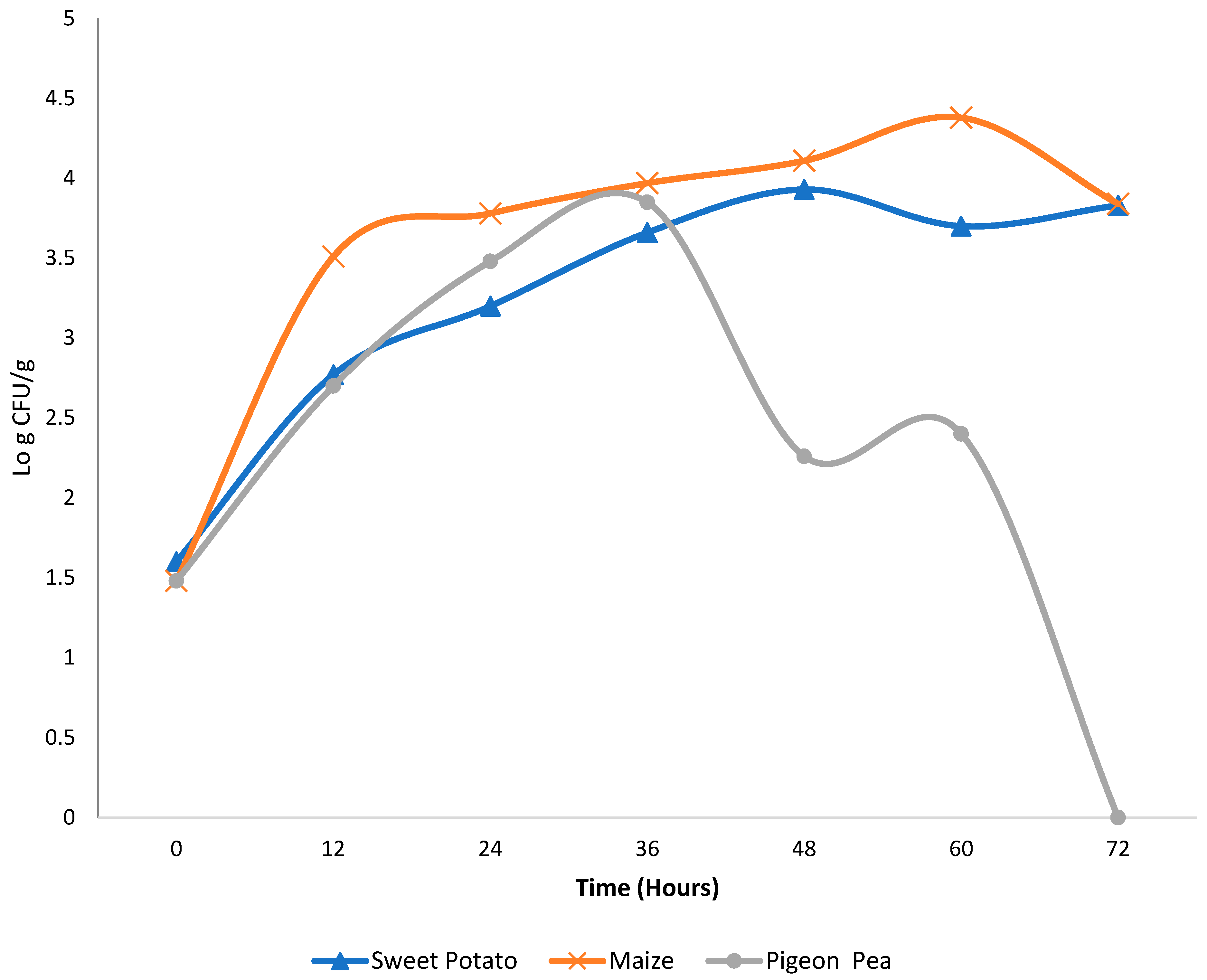
2.4. Microbiological Analysis
Isolation and Enumeration of Bacteria and Fungi
2.5. Determination of Microbial Kinetics Equation
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Li, Y.; Li, X.; Zhou, D.; Wang, Y.; Wen, X.; Wang, C.; Liu, X.; Feng, Y.; Li, B.; et al. Functional foods and intestinal homeostasis: The perspective of in vivo evidence. Trends Food Sci. Technol. 2021, 111, 475–482. [Google Scholar] [CrossRef]
- John, R.; Singla, A. Functional Foods: Components, health benefits, challenges, and major projects. DRC Sustainable Future. J. Environ. Agric. Energy 2021, 2, 61–72. [Google Scholar] [CrossRef]
- Nergiz, C.; Gokgoz, E. Effects of traditional cooking methods on some anti-nutrients and In-vitro protein digestibility of Dry Bean varieties (Phaseolus vulgancus L.) grown in Turkey. Int. J. Food Sci. Technol. 2007, 42, 868–873. [Google Scholar] [CrossRef]
- Oleghe, P.O.; Akharaiyi, F.C.; Ehis-Eriakha, C.B. Harnessing indigenous agro-processed flour blends as composite bioresources in functional food development. Food Res. 2024, 8, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Setta, M.C.; Matemu, A.; Mbega, E.R. Potential of probiotics from fermented cereal-based beverages in improving health of poor people in Africa. J. Food Sci. Technol. 2020, 57, 3935–3946. [Google Scholar] [CrossRef]
- Anaemene, D.I.; Fadupin, G.T. Effect of Fermentation, Germination and Combined Germination-Fermentation Processing Methods on the Nutrient and Anti-nutrient Contents of Quality Protein Maize (QPM) Seeds. J. Appl. Sci. Environ. Manag. 2020, 24, 1625–1630. [Google Scholar] [CrossRef]
- Al-Ansi, W.; Zhang, Y.; Alkawry, T.A.A.; Al-Adeeb, A.; Mahdi, A.A.; Al-Maqtari, Q.A.; Ahmed, A.; Mushtaq, B.S.; Fan, M.; Li, Y.; et al. Influence of germination on bread-making behaviors, functional and shelf-life properties, and overall quality of highland barley bread. LWT 2022, 159, 113200. [Google Scholar] [CrossRef]
- Schlörmann, W.; Zetzmann, S.; Wiege, B.; Haase, N.U.; Greiling, A.; Lorkowski, S.; Dawczynski, C.; Glei, M. Impact of different roasting conditions on sensory properties and health-related compounds of oat products. Food Chem. 2020, 307, 125548. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci. Biotechnol. 2018, 27, 333–342. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Huang, Q.; Lu, L.; Guo, Y.; Xu, H.; Zhou, J. Microbial diversity and community structure of traditional Chinese fermented foods. Food Res. Int. 2019, 116, 1226–1233. [Google Scholar]
- Hasmadi, M.; Noorfarahzilah, M.; Noraidah, H.; Zainol, M.K.; Jahurul, M.H.A. Functional properties of composite flour: A review. Food Res. 2020, 4, 1820–1831. [Google Scholar] [CrossRef]
- Shah, A.M.; Tarfeen, N.; Mohamed, H.; Song, Y. Fermented foods: Their health-promoting components and potential effects on gut microbiota. Fermentation 2023, 9, 118. [Google Scholar] [CrossRef]
- Kumari, M.; Platel, K. Impact of soaking, germination, fermentation, and thermal processing on the bioaccessibility of trace minerals from food grains. J. Food Process. Preserv. 2020, 44, e14752. [Google Scholar] [CrossRef]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef]
- Gopikrishna, T.; Suresh-Kumar, H.K.; Perumal, K.; Elangovan, E. Impact of Bacillus in fermented soybean foods on human health. Ann. Microbiol. 2021, 71, 30. [Google Scholar] [CrossRef]
- Oleghe, P.O.; Akharaiyi, F.C.; Ehis-Eriakha, C.B.; Oladebeye, A.A.; Johnson, D.O. Microbiological and Techno-functional Assessment of Unfermented and Fermented Gluten-Free Flour Mixes. Int. J. Life Sci. Res. 2023, 11, 33–48. [Google Scholar] [CrossRef]
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in grain, flour, amino acid composition, protein profiling, and proportion of total flour proteins of different wheat cultivars of North India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A major source of sustainable protein for health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef]
- Kiin-Kabari, D.B.; Giami, S.Y. Physicochemical properties and in-vitro protein digestibility of non-wheat cookies prepared from plantain flour and bambara groundnut protein concentrate. J. Food Res. 2015, 4, 78–86. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C. Plant proteins from legumes. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–32. [Google Scholar] [CrossRef]
- Tufan, B.; Sahin, S.; Sumnu, G. Utilization of legume flours in wafer sheets. Legume Sci. 2019, 2, e12. [Google Scholar] [CrossRef]
- Arif, A.R.; Natsir, H.; Rohani, H.; Karim, A. Effect of pH fermentation on production bioethanol from jackfruit seeds (Artocarpus heterophyllus) through separate fermentation hydrolysis method. J. Phys. Conf. Ser. 2018, 979, 012015. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Terefe, S.N.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef]
- Petrova, P.; Arsov, A.; Petrov, K. Cereal fermentation by LAB: From ancient to modern alimentation biotechnologies. In Lactic Acid Bacteria in Food Biotechnology: Innovations and Functional Aspects. Applied Biotechnology Reviews; Ray, R.C., Paramithiotis, S., Azevedo, V.A.C., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–26. [Google Scholar] [CrossRef]
- Hernández, A.; Larsson, C.U.; Sawicki, R.; van Niel, E.-W.J.; Roos, S.; Håkansson, S. Impact of the fermentation parameters pH and temperature on stress resilience of Lactobacillus reuteri DSM 17938. AMB Express 2019, 9, 66. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Anal, A. Quality Ingredients and Safety Concerns for Traditional Fermented Foods and Beverages from Asia: A Review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Aldawoud, T.M.S.; Rizou, M.; Rowan, N.; Ibrahim, S. Food Ingredients and Active Compounds against the Coronavirus Disease (COVID-19) Pandemic: A Comprehensive Review. Foods 2020, 9, 1701. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Friman, A.; Hyytiä, N. The Economic and Welfare Effects of Food Waste Reduction on a Food-Production-Driven Rural Region. Sustainability 2022, 14, 3632. [Google Scholar] [CrossRef]
- Salfinger, Y.; Tortorello, M.L. Compendium of Methods for the Microbiological Examination of Foods; American Public Health Association: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Oleghe, P.O.; Akharaiyi, F.C.; Ehis-Eriakha, C.B. Phylogenetic identification of microbes from fermented botanicals used in gluten-free composite flour mixes. Foods Raw Mater. 2025, 13, 82–93. [Google Scholar] [CrossRef]
- Abdalla, M.O.M.; Omer, H.E.A. Microbiological characteristics of white cheese (Gibna bayda) manufactured under traditional conditions. J. Adv. Microbiol. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Alsohaili, S.A.; Bani-Hasan, B.M. Morphological and Molecular Identification of Fungi Isolated from Different Environmental Sources in the Northern Eastern Desert of Jordan. Jordan J. Biol. Sci. 2018, 11, 329–337. [Google Scholar]
- Viccini, G.; Mitchell, D.A.; Boit, S.D.; Gern, J.C.; da Rosa, A.S.; Costa, R.M.; Dalsenter, F.D.H.; von Meien, O.F.; Krieger, N. Analysis of growth kinetic profilesin solid-state fermentation. Food Technol. Biotechnol. 2001, 39, 271–294. [Google Scholar]
- Omemu, A.M.; Okafor, U.I.; Obadina, A.O.; Bankole, M.O.; Adeyeye, S.A.O. Microbiological assessment of maize ogi co-fermented with pigeon pea. Food Sci. Nutr. 2018, 6, 1238–1253. [Google Scholar] [CrossRef]
- Adebo, O.A.; Oyedeji, A.B.; Adebiyi, J.A.; Chinma, C.E.; Oyeyinka, S.A.; Olatunde, O.O.; Green, E.; Njobeh, P.B.; Kondiah, K. Kinetics of phenolic compounds modification during maize flour fermentation. Molecules 2021, 26, 6702. [Google Scholar] [CrossRef]
- Oleghe, P.O.; Oshoma, C.; Agholor, K. Growth kinetics of bacteria isolated from laboratory prepared cheese made from different milk sources. Saudi J. Pathol. Microbiol. 2023, 8, 30–37. [Google Scholar] [CrossRef]
- Oyarekua, M.A. Biochemical and Microbiological changes during the production of fermented pigeon pea (Cajanus cajan) flour. Afr. J. Food Sci. Technol. 2011, 2, 223–231. [Google Scholar]
- Ajayi, O.I.; Ehiwuogu-Onyibe, J.; Oluwole, O.B.; Jegede, A.A.; Salami, T.A.; Asieba, G.O.; Chiedu, I.E.; Suberu, Y.L.; Aba, E.M.; Dike, E.N.; et al. Production of fermented sweet potato flour using indigenous starter cultures. Afr. J. Microbiol. Res. 2016, 10, 1746–1758. [Google Scholar] [CrossRef]
- Nurdini, A.L.; Nuraida, L.; Suwanto, A.; Suliantari. Microbial growth dynamics during tempe fermentation in two different home industries. Int. Food Res. J. 2015, 22, 1668–1674. [Google Scholar]
- Yun-Ting, H.; Chung-Yi, W. Microbial Shelf-Life, Starch Physicochemical Properties, and in-vitro Digestibility of Pigeon Pea Milk Altered by High Pressure Processing. Molecules 2020, 25, 2516. [Google Scholar] [CrossRef]
- Bello, B.K.; Ojokoh, A.O. Effect of fermentation on the microbial growth and proximate composition of water yam. Int. J. Biosci. Technol. 2013, 6, 9–13. [Google Scholar]
- Bintsis, T. Lactic acid bacteria: Their applications in foods (Review Article). J. Bacteriol. Mycol. Open Access 2018, 6, 89–94. [Google Scholar] [CrossRef]
- Ziarno, M.; Cichońska, P. Lactic Acid Bacteria-Fermentable Cereal- and Pseudocereal-Based Beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef]
- Botta, C.; Cocolin, L. Microbial dynamics and biodiversity in table olive fermentation: Culture-dependent and independent approaches. Front. Microbiol. 2012, 3, 245. [Google Scholar] [CrossRef]
- Yang, Q.; Yao, H.; Liu, S.; Mao, J. Interaction and Application of Molds and Yeasts in Chinese Fermented Foods. Front. Microbiol. 2022, 12, 664850. [Google Scholar] [CrossRef]
- Hippolyte, T.M.; Yadang, G.; Sibozo, O.G.; Kemadjou, R.E.; Kenfack, L.B.M. Development of Fermented Sweet Potato Flour (Ipomoea batatas L.) Supplemented with Mackerel (Scomber scombrus) Meal-Based Biscuits. Hindawi Int. J. Food Sci. 2022, 1, 8033978. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleghe, P.O.; Akharaiyi, F.C.; Ehis-Eriakha, C.B. Microbial Growth Kinetics of Fermenting Botanicals Used as Gluten-Free Flour Blends. Biol. Life Sci. Forum 2025, 41, 9. https://doi.org/10.3390/blsf2025041009
Oleghe PO, Akharaiyi FC, Ehis-Eriakha CB. Microbial Growth Kinetics of Fermenting Botanicals Used as Gluten-Free Flour Blends. Biology and Life Sciences Forum. 2025; 41(1):9. https://doi.org/10.3390/blsf2025041009
Chicago/Turabian StyleOleghe, Peace Omoikhudu, Fred Coolborn Akharaiyi, and Chioma Bertha Ehis-Eriakha. 2025. "Microbial Growth Kinetics of Fermenting Botanicals Used as Gluten-Free Flour Blends" Biology and Life Sciences Forum 41, no. 1: 9. https://doi.org/10.3390/blsf2025041009
APA StyleOleghe, P. O., Akharaiyi, F. C., & Ehis-Eriakha, C. B. (2025). Microbial Growth Kinetics of Fermenting Botanicals Used as Gluten-Free Flour Blends. Biology and Life Sciences Forum, 41(1), 9. https://doi.org/10.3390/blsf2025041009





