Evaluation of the Use of Propolis and Sodium Hypochlorite as Methods to Control the Contamination of Free-Range Eggs †
Abstract
1. Introduction
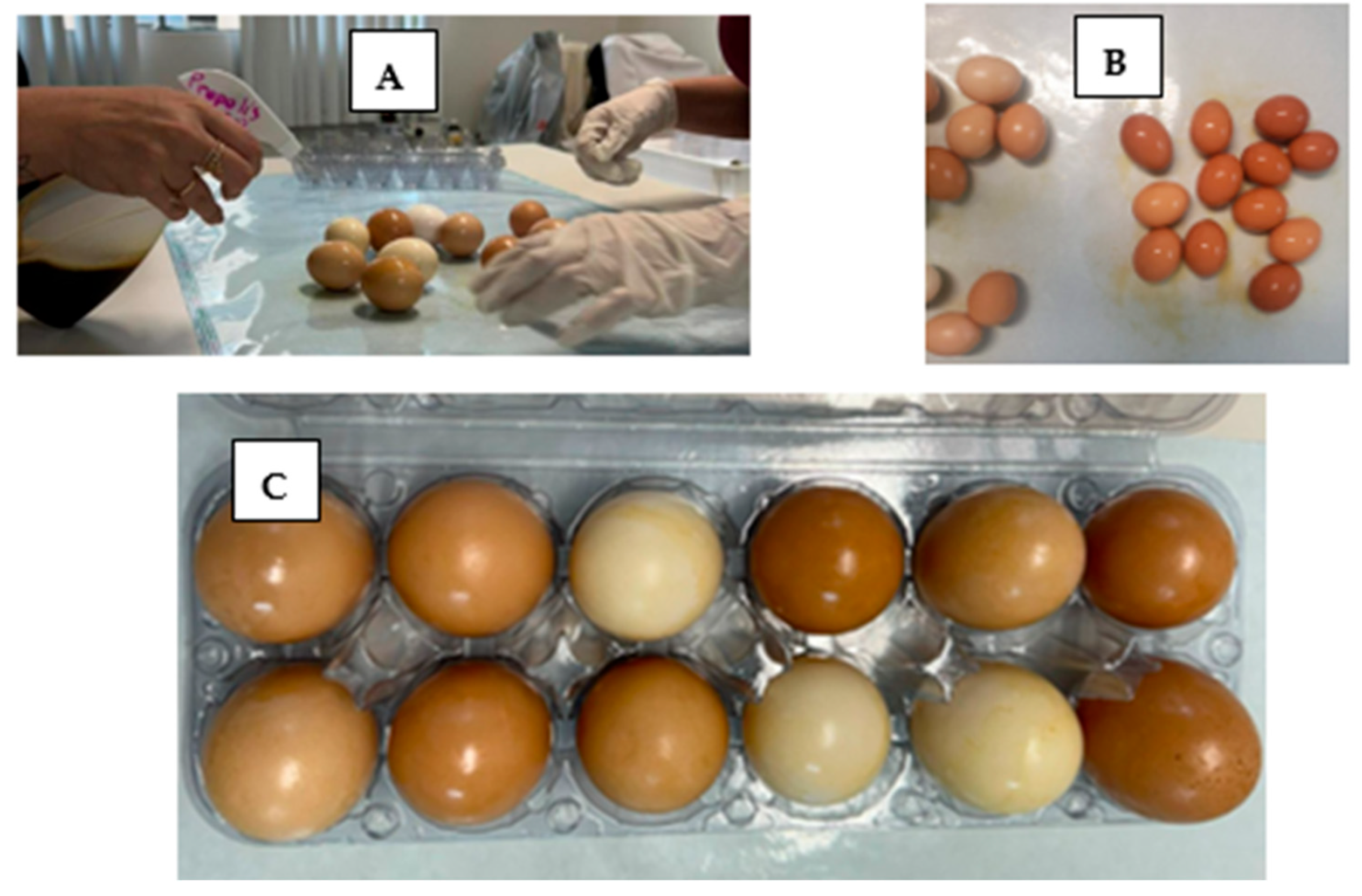
2. Materials and Methods
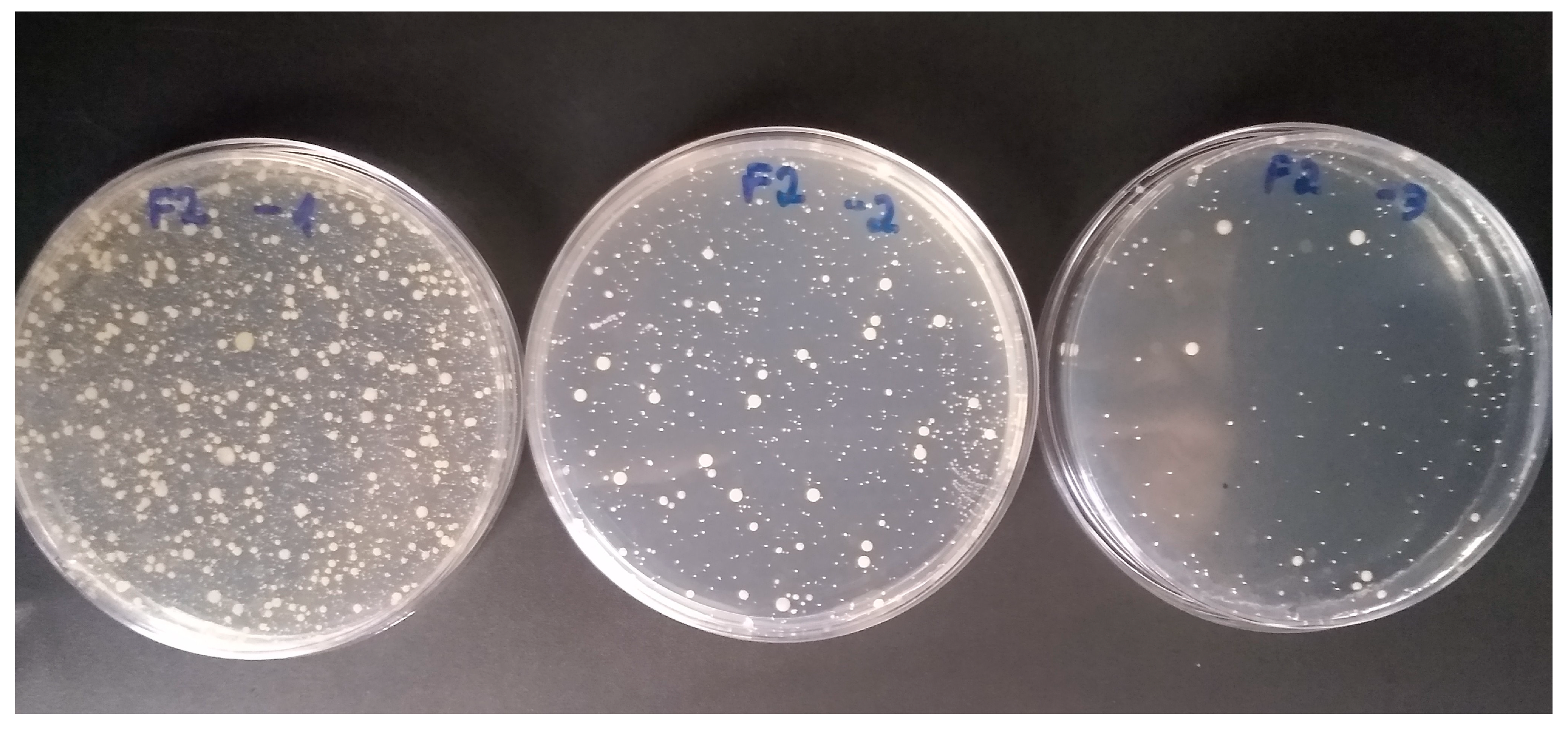
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McWhorter, A.R.; Chousalkar, K.K. From hatch to egg grading: Monitoring of Salmonella shedding in free-range egg production systems. Vet. Res. 2019, 50, 1–9. [Google Scholar] [CrossRef]
- Galvão, J.A.; Ribeiro, S.C.A.; Amaral, L.A.; Schmidt, V.; Souza, R.A.L.; Paulillo, A.C.; Rossi, D.A. Microbiological Vulnerability of Eggs and Environmental Conditions in Conventional and Free-Range Housing Systems. Semin. Ciênc. Agrár. 2018, 39, 133–142. [Google Scholar] [CrossRef]
- Alkan, S.; Ertürk, Ö.; Türker, İ. Determination of Microbial Activity and Quality Traits of Eggs Coated with Propolis. Turk. J. Agric. Food Sci. Technol. 2020, 8, 1380–1384. [Google Scholar] [CrossRef]
- Alves, G.P.; Eyng, C.; Garcia, R.G.; Orrico Junior, M.A.P.; Silva, T.S. Qualidade Microbiológica da Casca de Ovos de Poedeiras Comerciais Revestidos com Própolis e Armazenados por Diferentes Períodos. Agroecol 2016. Available online: https://www.cpao.embrapa.br/cds/agroecol2016/PDF's/Trabalhos/Qualidade%20microbiol%C3%B3gica%20da%20casca%20de%20ovos%20de%20poedeiras%20comerciais%20revestidos%20com%20pr%C3%B3polis%20e%20armazenados.pdf (accessed on 15 December 2024).
- Soares, A.C.B.; Brito, D.A.P.; Soares, S.C.P.; Gomes, K.S.; Saldanha, M.S.; Soares, V.S. Maintenance of Quality of Eggs Submitted to Treatment with Propolis Extract and Sanitizers. Acta Sci. Anim. Sci. 2022, 44, e53584. [Google Scholar] [CrossRef]
- Downes, F.P.; Ito, K. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; American Public Health Association (APHA): Washington, DC, USA, 2001; p. 676. [Google Scholar]
- Shahein, E.H.; Sedeek, E. Role of Spraying Hatching Eggs with Natural Disinfectants on Hatching Characteristics. Egypt. Poult. Sci. J. 2014, 34, 213–230. [Google Scholar] [CrossRef]
- Al-Sakhawy, M.; Salama, A.; Mohamed, S.A.A. Propolis Applications in Food Industries and Packaging. Biomass Conv. Bioref. 2024, 14, 13731–13746. [Google Scholar] [CrossRef]
- Chan, H.Y.; Meor Hussin, A.S.; Ahmad, N.H.; Rukayadi, Y.; Farouk, A.E. Effectiveness of Quaternary Ammonium in Reducing Microbial Load on Eggs. Molecules 2021, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.H.; Breslin, M. Investigations into possible alter-native decontamination methods for Salmonella Enteritidis on the surface of table eggs. J. Vet. Med. 2003, B50, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Herianto, S.; Syu, S.M.; Song, C.H.; Chen, H.L.; Hou, C.Y. Applying a large-scale device using non-thermal plasma for microbial decontamination on shell eggs and its effects on the sensory characteristics. LWT 2021, 142, 11106. [Google Scholar] [CrossRef]
- Gomes, J.L.F.; Santos, T.F.F.; de Macedo, B.F.; Machado, D.C.; Oliveira, M.C. Tratamentos na Casca de Ovos de Codornas e Qualidade Interna do Ovo Durante Armazenamento. Anais do CICURV 2023, 17. Available online: http://revistas.unirv.edu.br/index.php/cicurv/article/view/252 (accessed on 11 December 2024).
- Genc, M.; Ozenturk, U.; Atasever, M. Can Propolis, the Natural Disinfectant of Bees, Be Used as an Effective and Healthy Disinfectant for Hatching Eggs? Indian J. Anim. Res. 2020, 54, 775–780. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, G.S.; Philipp, J.U.; Julek, E.; Voltolin, G.C.; de Albuquerque, G.S.C.; Galvão, J.A. Evaluation of the Use of Propolis and Sodium Hypochlorite as Methods to Control the Contamination of Free-Range Eggs. Biol. Life Sci. Forum 2024, 40, 49. https://doi.org/10.3390/blsf2024040049
de Souza GS, Philipp JU, Julek E, Voltolin GC, de Albuquerque GSC, Galvão JA. Evaluation of the Use of Propolis and Sodium Hypochlorite as Methods to Control the Contamination of Free-Range Eggs. Biology and Life Sciences Forum. 2024; 40(1):49. https://doi.org/10.3390/blsf2024040049
Chicago/Turabian Stylede Souza, Giovana Scuissiatto, Julia Unicki Philipp, Elisana Julek, Gabriela Campi Voltolin, Guilherme Souza Cavalcanti de Albuquerque, and Julia Arantes Galvão. 2024. "Evaluation of the Use of Propolis and Sodium Hypochlorite as Methods to Control the Contamination of Free-Range Eggs" Biology and Life Sciences Forum 40, no. 1: 49. https://doi.org/10.3390/blsf2024040049
APA Stylede Souza, G. S., Philipp, J. U., Julek, E., Voltolin, G. C., de Albuquerque, G. S. C., & Galvão, J. A. (2024). Evaluation of the Use of Propolis and Sodium Hypochlorite as Methods to Control the Contamination of Free-Range Eggs. Biology and Life Sciences Forum, 40(1), 49. https://doi.org/10.3390/blsf2024040049






