Abstract
The positive effects of probiotic strains on human health are well documented, with growing evidence that interactions between different strains enhance these benefits. Lactoplantibacillus spp. and Bifidobacterium spp., key members of the gut microbiota, have been extensively studied for their probiotic potential. This study presents preliminary findings on the bioactive effects of cell-free supernatants from Lactiplantibacillus plantarum strains on bifidobacteria. Using the Gompertz equation, the impact on microbial growth kinetics was analyzed, revealing strain- and species-dependent stimulation or inhibition during the growth and death phases. These findings suggest potential prebiotic activity and represent novel insights into L. plantarum supernatants’ bioactivity on bifidobacteria.
1. Introduction
The human gastrointestinal tract harbors a highly diverse microbial ecosystem comprising hundreds of bacterial species, whose composition and activity significantly impact host health. This complex microbiota evolves along the length of the gastrointestinal tract, from the stomach, characterized by acid-tolerant species, to the colon, which contains the densest microbial population, reaching up to 1012 CFU/g and encompassing over 500 species [1,2,3].
Beneficial microorganisms, such as Lactoplantibacillus and Bifidobacterium species, are prominent members of the gut microbiota throughout life, playing crucial roles in maintaining gastrointestinal and overall health [4].
These genera have been extensively studied for their probiotic potential, which includes modulating gut flora, enhancing immune responses, and producing bioactive compounds. However, their ability to colonize and proliferate in the gastrointestinal environment depends on several factors, including the availability of bifidogenic factors and interactions with dietary components [5,6].
Recent advancements in microbiology have shifted attention towards non-viable derivatives of probiotics, such as paraprobiotics and postbiotics.
Paraprobiotics are non-viable microbial cells, either intact or lysed, that confer health benefits, while postbiotics are metabolic byproducts or secreted compounds from probiotics [7,8]. These substances offer advantages over live probiotics, including enhanced stability, ease of production, and reduced risks associated with bacterial translocation or antibiotic resistance. Postbiotics encompass a wide range of bioactive molecules, such as enzymes, peptides, short-chain fatty acids, and vitamins, which have shown promising pharmacological and functional applications in improving gut health and selectively stimulating beneficial microbiota [9].
Both lactobacilli and bifidobacteria are key contributors to this emerging field. Lactobacilli, commonly found in fermented foods, not only aid in food processing but also secrete bioactive compounds capable of influencing the growth and survival of other beneficial bacteria [10,11,12].
Similarly, bifidobacteria, predominant in the colon, are recognized for their bifidogenic responses to dietary prebiotics and postbiotics, enhancing gut health and microbiota balance.
The bifidogenic growth stimulator (BGS), a compound produced by Propionibacterium freudenreichii, exemplifies the potential of cell-free extracts to act as prebiotic or postbiotic agents, stimulating the growth of bifidobacteria through bioactive molecules such as 1,4-dihydroxy-2-naphthoic acid (DHNA) and 2-amino-3-carboxy-1,4-naphthoquinone (ACQN) [5,6,7,8,9,10,11,12,13,14].
The oral administration of probiotics and dietary prebiotics represents a primary strategy for modulating the gut microbiota. Probiotics, defined as live microorganisms that confer health benefits when administered in adequate amounts, directly influence gut flora by introducing beneficial species [15].
Prebiotics, on the other hand, are non-digestible dietary components that selectively stimulate the growth or activity of beneficial gut microorganisms [16]. These approaches aim to enhance gut health by promoting a favorable microbiota composition and suppressing potentially harmful bacteria such as clostridia and Bacteroidaceae [17].
Building on these insights, this study investigates the bioactivity of cell-free supernatants (CFSs) derived from probiotic Lactiplantibacillus plantarum strains on the growth and survival of probiotic Bifidobacterium spp.
The use of advanced modeling techniques, such as the Gompertz equation, allows for a detailed analysis of interaction dynamics during the microbial growth and death phases. The findings aim to elucidate the potential of these supernatants as functional ingredients to promote synergistic bacterial interactions within complex microbial ecosystems. By focusing on both the growth-stimulating and inhibitory effects of CFSs, this work contributes to a deeper understanding of the mechanisms underlying microbial interactions and their implications for gut health and functional food development.
2. Material and Methods
2.1. Microorganisms
In this study, the bioactivity of CFSs from Lactiplantibacillus plantarum strains c3, c4, and c15 was evaluated for their effects on bifidobacteria.
These strains were originally isolated from Italian table olives of the “Bella di Cerignola” variety [18], subsequently identified and characterized [19], and preserved at −80 °C in suitable media.
The bifidobacteria strains tested for their response to L. plantarum CFSs included Bifidobacterium animalis DSM 10140, Bifidobacterium subtile DSM 20096, and Bifidobacterium breve DSM 20213.
Bifidobacterium strains were obtained from the DSMZ collection (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig Science Campus, Braunschweig-Süd, Germany) and stored at −80 °C under the conditions recommended by the supplier (www.dsmz.de accessed on 1 September 2024).
2.2. Preparation of Biological CFSs
L. plantarum strains were cultured in MRS broth (Oxoid, Milan, Italy) at 30 °C for 48 h, while Bifidobacterium spp. were cultivated in MRS broth supplemented with 0.05% (w/v) cysteine (cMRS) (Sigma-Aldrich, Milan, Italy) at 37 °C for 48 h.
After incubation, cells were separated from the culture media by centrifugation at 3000× g for 15 min at 5 °C and subsequently washed twice using a sterile saline solution (NaCl 9 g/L). The resulting supernatants were passed through a 0.22 µm Millipore filter (Whatman, Dassel, Germany) to obtain cell-free hydrosoluble fractions [5].
2.3. Assay of Bioactive Effects of CFSs on Bifidobacterium Strains
Thawed frozen cultures of B. animalis, B. subtile, and B. breve were pre-cultured in cMRS broth.
The experiments were conducted in 250 mL Erlenmeyer flasks containing 100 mL of cMRS medium. CFSs from lactobacilli were incorporated into the medium at a concentration of 1% (v/v).
The samples were inoculated with each strain of Bifidobacterium (102 CFU/mL). Aliquots of cMRS, inoculated with the target strains but not containing CFSs, were used as controls.
The flasks were incubated anaerobically at 37 °C, and the viable bifidobacterial populations were periodically assessed by plating on cMRS agar and incubating at 37 °C for 48 h.
2.4. Modeling
The viable cell count for each strain, both in the presence and absence of CFSs, was analyzed using two distinct modeling approaches. For the growth phase (encompassing the lag phase, exponential growth, and steady state), a positive Gompertz equation was applied. Conversely, the decline phase was modeled using a negative Gompertz equation, where the initial growth phase was considered to be a shoulder length, followed by a linear death phase and, ultimately, a tail.
To describe the growth dynamics, the Gompertz equation reparametrized by Corbo et al. [20] (Equation (1)) was utilized, expressed as follows:
where [log (CFU)]max is the standard limit for the microbial population (7 log CFU/mL); is the maximum increase in bacterial load attained in the stationary phase; is the maximal growth rate [Δlog (CFU/mL)/h]; is the lag time (hours); and t* is t − 7log, that is the verge time (hours) after which the cell load attained a value higher than 107 CFU/mL.
Log (CFU) = [log (CFU)]max − A × exp{−exp{[(µmax × 2.7182) × (λ − t − 7log)/A] + 1}}
+ A × exp{−exp{[(µmax × 2.7182) × (λ − t)/A] + 1}
+ A × exp{−exp{[(µmax × 2.7182) × (λ − t)/A] + 1}
For the negative part of the growth curve, the Gompertz equation (Equation (2)) was cast as follows:
where [log (CFU)]max is the standard limit for the microbial population (7 log CFU/mL), is the maximum decrease in bacterial load attained in the death phase; is the maximal death rate [Δlog (CFU/mL)/h]; is the shoulder length, after which the decrease in cell load started (hours); is the time; and t − 7log* is the time limit, after which the cell load attained a value lower than 107 CFU/mL (h).
Log (CFU) = [log (CFU)]max − {−Δ × exp{−exp{[(dmax × 2.7182) × (λ – t − 7log*)/Δ] + 1}}
+ Δ × exp{−exp{[(dmax × 2.7182) × (α − t)/Δ] + 1}}}
+ Δ × exp{−exp{[(dmax × 2.7182) × (α − t)/Δ] + 1}}}
Statistic for Windows (ver. 7.0, Statsfot, Tulsa, OK, USA) was used to fit the Gompertz equations both in the growth and death phases.
The experiment was run twice on two different batches. All the analyses were carried out in triplicate, and the data shown are the average of all repetitions.
The experimental data were analyzed by a One-way ANOVA and Tukey’s test (p < 0.05) (Statistic for Windows ver. 7.0, Statsoft, Tulsa, OK, USA) in order to point out significant differences.
3. Results and Discussion
L. plantarum is an important species in the fermentation of various food products, and its probiotic attribute was evaluated in several studies [11,12].
In this work, the bioactivity of cell-free extracts obtained by Lactoplantibacillus plantarum strains, both in terms of the growth and survival of probiotic bifidobacteria, was investigated. These extracellular filtrates were used as ingredients in the growth medium (1% v/v) in order to evaluate their effects.
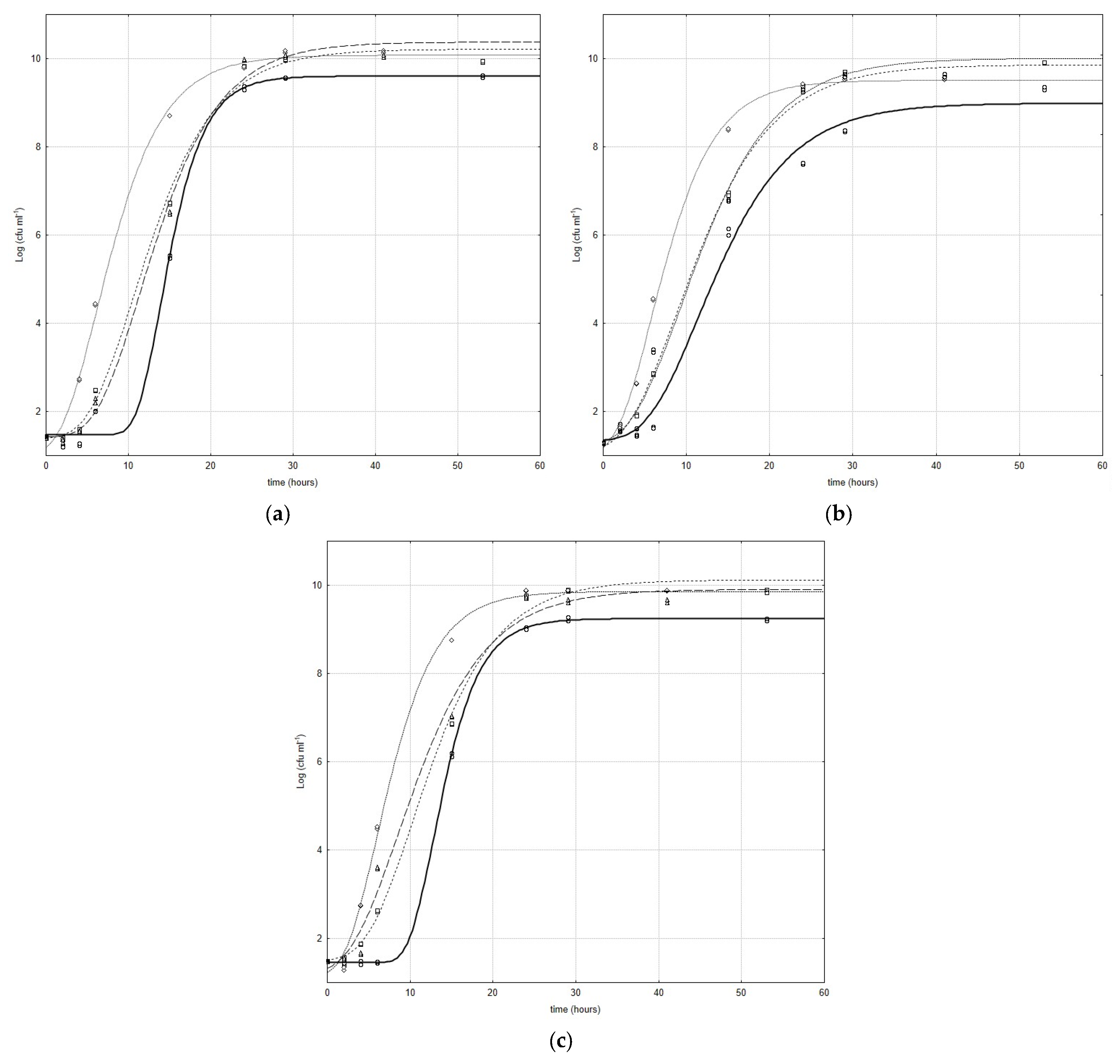
Figure 1a–c show the growth curves of Bifidobacterium species inoculated with and without CFSs obtained by L. plantarum strains; the curves represent the best fit of the model (Equation (1)) to the experimental data. In all combinations, a stimulant effect was registered, and the CFSs significantly influenced all kinetic parameters in the growth phase of the microbial targets.
Figure 1.
Evolution of B. animalis (a), B. subtile (b), and B. breve (c) in growth phase with and without CFSs obtained by L. plantarum strains (Lc). Curves represent best fit of model to experimental data (○ Bifidobacterium strains □ CFS Lc3 ◊ CFS Lc4 ∆ CFS Lc15).
In particular, for each microbial target, the cell-free extracts showed a greatly positive effect on the lag phase. An evident lowering of the values of this parameter was registered. The CFS obtained by Lc4 was the most effective in the lag phase; in fact, highly significant differences (p < 0.001) were recorded for all bifidobacteria/CFS from Lc4 combinations (Figure 1a–c).
In Figure 1a, the results that refer to the combination B. animalis/CFS from lactobacilli are represented; as can be inferred in B. animalis/CFS from the Lc3 combination and control, an evident, but not significant, difference was recorded, whereas slight effects were registered for B. animalis under CFSs from Lc15 and B. animalis under CFSs from Lc4. For all B. subtile/CFS from lactobacilli combinations, highly significant effects (p < 0.001) were recorded between the samples and control, while no significant difference was registered between B. subtile under CFSs from Lc3 and B. subtile under CFSs from Lc15 (Figure 1b). The influence of L. plantarum cell-free extracts on the B. breve lag phase was significantly different (p < 0.001) with respect to the strains (Figure 1c).
Additionally, an enhancement in the maximum increase in bacterial load attained in the stationary phase was recorded; in fact, a significant difference (p < 0.01) was recorded between the control and active samples. Considering all combinations (significance p < 0.01), the highest value of the same parameter was registered for the following: (i) B. animalis/CFS from Lc1 (Figure 1a); (ii) B. subtile/CFS from Lc15 (Figure 1b); and (iii) B. breve/CFS from Lc4 (Figure 1c). For this parameter, all the observed differences were significant (p < 0.01).
The data registered for all combinations also suggest that the lower the maximum growth rate, the higher the maximum cell load attained in the stationary phase. Only for B. animalis/CFS from lactobacilli combinations (Figure 1c) was the contrary observed. A highly significant increase in the maximum growth rate (p < 0.001) was recorded only for the combination obtained with the Lc4 cell-free extract (0.73 [Δlog (CFU/mL)/h] and 0.45 [Δlog (CFU/mL)/h], for the sample and control, respectively), while no significant differences were recorded for the other combinations (Figure 1a). For B. subtile/CFS from lactobacilli combinations, no significant differences were recorded only for the combination obtained with the CFS from Lc4 (Figure 1b). Significant differences (p < 0.1), instead, were registered for the other combinations (Figure 1b). In Figure 1c, the results that refer to the combination B. breve/CFS from lactobacilli are reported, and regarding the highest value of the results, a strain-dependent response could be hypothesized to be the cause. In fact, highly significant differences (p < 0.001) were recorded between samples and controls. In particular, values like 0.57 [Δlog (CFU/mL)/h], 0.81 [Δlog (CFU/mL)/h], 0.54 [Δlog (CFU/mL)/h], and 0.93 [Δlog (CFU/mL)/hours] were recorded for B. breve/CFS from Lc3, B. breve/CFS from Lc4, B. breve/CFS from Lc15 combinations, and the control, respectively (Figure 1c).
Considering these results, in the growth phase, the strain dependence of the response with respect to stimulant bacteria could be hypothesized.
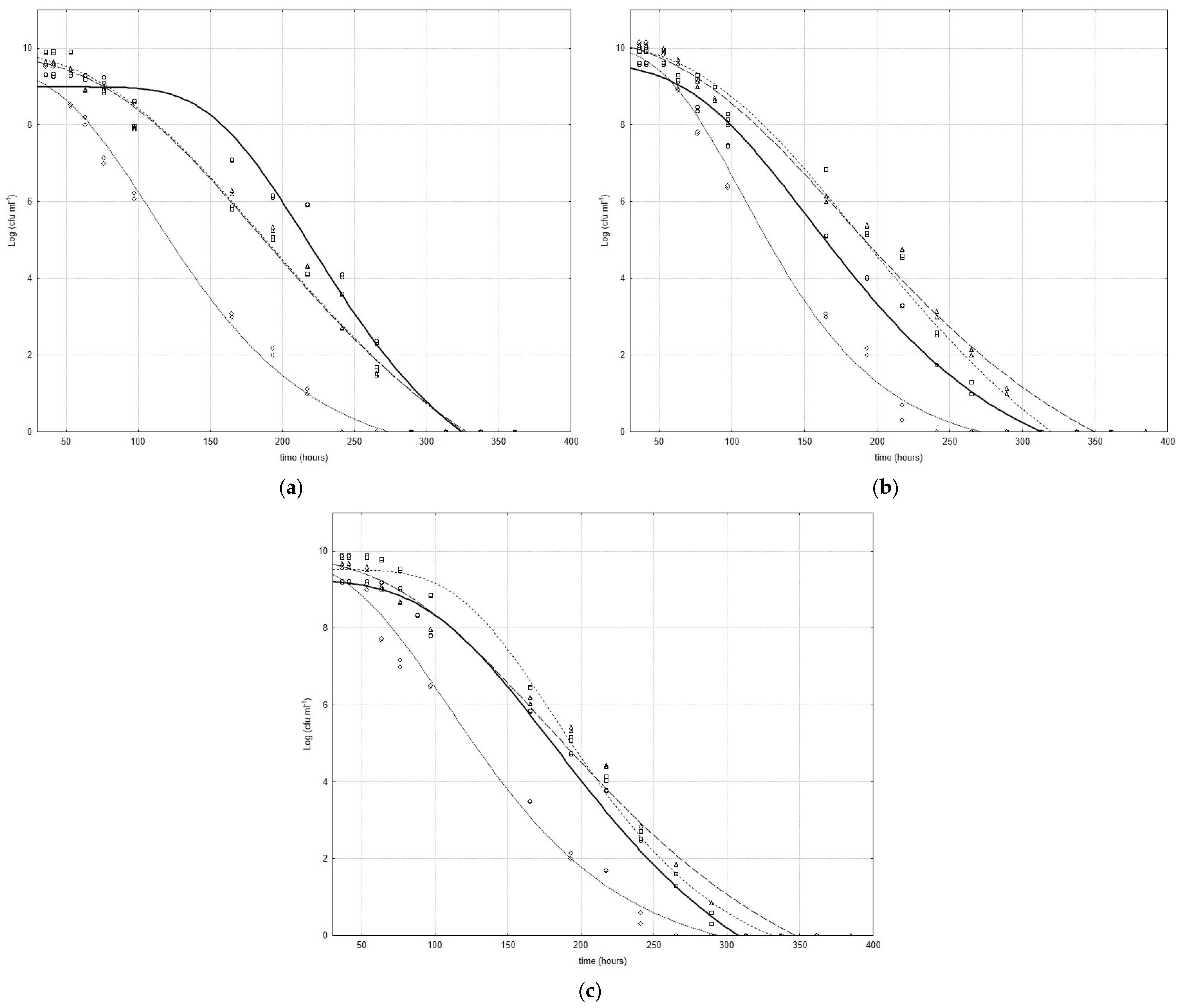
Figure 2a–c show the death curves for Bifidobacterium species inoculated with and without cell-free extracts obtained by L. plantarum strains; the curves represent the best fit of the model (Equation (2)) to the experimental data.
Figure 2.
Evolution of B. animalis (a), B. subtile (b), and B. breve (c) in death phase with and without CFSs obtained by L. plantarum strains (Lc). Curves represent best fit of model to experimental data (○ Bifidobacterium strains □ CFS Lc3 ◊ CFS Lc4 ∆ CFS Lc15).
The cell-free extract obtained by Lc4 was more effective in the growth phase, but in the death phase, it caused a faster death and a shorter stability time, after which a decrease in cell number was observed (α, life-time of microbial population) (Figure 2a–c). For bifidobacteria under the Lc4 CFS, a highly significant rise (p < 0.001) in the maximum death rate, with respect to the control, was recorded, and the life-time of the bifidobacterial microbial population was shorter than that of the controls [44.23 h, 51.49 h, and 150.23 h, 71.15 h, for B. animalis/Lc4 (Figure 2a), B. subtile/Lc4 (Figure 2b), samples and controls, respectively]. However, the differences observed were always highly significant (p < 0.001).
The cell-free extracts negatively influenced the stability time after which a decrease in cell number was observed (α) for all B. animalis strains under CFSs from lactobacilli (Figure 2a); in fact, a highly significant reduction (p < 0.001) was evident for all samples. The effects of cell-free extracts were also negative in terms of the stability time for B. subtile under lactobacilli CFSs, and only for B. subtile under the Lc3 CFS was an increase in this parameter recorded (78.34 h and 71.15 h for the sample and control, respectively), but the differences were not significant (Figure 2b). The same behavior was also observed for B. breve under CFSs from lactobacilli, and the increase observed for B. breve under the Lc4 CFS was the most evident and significant (p < 0.01) (115.58 h and 94.63 h for the sample and control, respectively) (Figure 2c).
In the death phase, L. plantarum’s strain dependence and Bifidobacterium’s species dependence were emphasized in terms of the maximum death rate. An increase in this parameter was observed for B. subtile and B. breve under the Lc4 CFS (Figure 2b,c) and for B. breve under the Lc3 CFS, whereas a decrease in the same parameter was recorded for B. animalis under the Lc3 and Lc15 CFSs (Figure 2a), for B. subtile under the Lc15 CFS (Figure 2b), and for B. breve under the Lc15 CFS (Figure 2c); in these cases, the differences observed were highly significant (p < 0.001), while no differences were recorded for the other combinations.
This result strengthens the hypothesis of the prebiotic activity of these cell-free extracts, and it relates to the hypothesis of L. plantarum’s strain dependence and Bifidobacterium’s species dependence in our experimental conditions.
4. Conclusions
The findings of this study highlight the significant role of Lactobacillus and Bifidobacterium species as key components of the gastrointestinal microbiota, where interactions between these microbial groups are frequent and impactful. This research demonstrated that the cell-free extracts (CFSs) produced by L. plantarum strains exhibit notable bioactivity, influencing both the growth and survival of probiotic bifidobacteria.
The observed effects varied in a strain-dependent manner for lactobacilli and a species-dependent manner for bifidobacteria, emphasizing the specificity of these interactions. The bifidogenic and bioactive properties of the extracts were evident during both the growth phase, where stimulation occurred, and the death phase, where protective effects were noted. These results suggest the presence of bioactive factors within the CFSs, although further studies are needed to elucidate the exact composition of these extracts and the mechanisms underlying their effects. Variables such as the concentration of CFSs, environmental conditions (e.g., temperature and pH), and the nature of microbial interactions warrant further investigation.
This study provides valuable insights into the synergistic potential of lactobacilli and bifidobacteria within a postbiotic framework. By leveraging microbial metabolites, cell-derived molecules, and other bioactive components from inactivated probiotic cells, these extracts represent promising tools for enhancing probiotic functionality. The findings reinforce the concept that targeted interventions utilizing postbiotic strategies can positively modulate the gut microbiome, offering significant implications for improving host health.
Author Contributions
Conceptualization, A.F. and C.A.; methodology, A.F. and C.A.; software, A.F.; validation, A.F. and C.A.; formal analysis, A.F.; investigation, A.F.; resources, A.F.; data curation, A.F.; writing—original draft preparation, A.F.; writing—review and editing, A.F. and C.A.; visualization, A.F.; supervision, C.A.; project administration, A.F. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zoetendal, E.G.; Mackie, R.I. Molecular methods in microbial ecology. In Probiotics and Prebiotics: Scientific Aspects; Tannock, G.W., Ed.; Caistar Academic Press: Wymondham, UK, 2005; pp. 1–24. [Google Scholar]
- Holzapfel, W.H. Introduction to prebiotics and prebiotics. In Probiotics in Food Safety and Human Health; Goktepe, I., Junepa, V.K., Ahmedna, M., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–4. [Google Scholar]
- Drasar, B.S.; Hill, J.M. Human Intestinal Flora; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Salminen, S.; Isolauri, E.; Onnela, T. Gut microflora in health and disease. Chemotherapy 1995, 41 (Suppl. S1), 5–15. [Google Scholar] [PubMed]
- Kaneko, T.; Mori, H.; Iwata, M.; Meguro, S. Growth stimulator for bifidobacteria produced by Propionibacterium freudenreichii and several intestinal bacteria. J. Dairy Sci. 1994, 77, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Modler, H.W.; McKellar, R.C.; Yaguchi, M. Bifidobacteria and bifidogenic factors. Can. Inst. Food Sci. Technol. J. 1990, 23, 29–40. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international Scientifc Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [PubMed]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Kanawjia, S.K.; Nageswara Rao, K.; Singh, S.; Sabikhi, L. Role of Lactobacilli in cheese. Ind. J. Dairy Sci. 1993, 46, 187–197. [Google Scholar]
- Wouters, J.T.M.; Ayad, E.H.E.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109. [Google Scholar]
- Kieronczyk, A.; Skeie, S.; Langsrud, T.; Yvon, M. Cooperation between Lactococcus lactis and non-starter lactobacilli in the formation of cheese aroma from amino acids. Appl. Environ. Microbiol. 2003, 69, 734–739. [Google Scholar] [PubMed]
- Mori, H.; Sato, Y.; Taketomo, N.; Kamiyama, T.; Yoshiyama, Y.; Meguro, S.; Sato, H.; Kaneko, T. Isolation and structural identification of bifidogenic growth stimulator produced by Propionibacterium freudenreichii. J. Dairy Sci. 1997, 80, 1959–1964. [Google Scholar] [PubMed]
- Furuichi, K.; Hojo, K.; Katakura, Y.; Ninomiya, K.; Shioya, S. Aerobic culture of Propionibacterium freudenreichii ET-3 can increase producton ratio of 1,4-dihydroxy-2-naphthoic acid to menaquinone. J. Biosci. Bioeng. 2006, 101, 464–470. [Google Scholar] [PubMed]
- Filippone, A.; Sinigaglia, M.; Altieri, C. Study on bioactivity of cell-free filtrates from dairy propionibacteria. Anaerobe 2014, 30, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.L.; Vallance, B.A. Intestinal microbiota are transiently altered during Salmonella-induced gastroenteritis. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 525–529. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell. Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Campaniello, D.; Bevilacqua, A.; D’Amato, D.; Corbo, M.R.; Altieri, C.; Sinigaglia, M. Microbial characterization of table olives processed accordino to spanish and natural style. Food Technol. Biotechnol. 2005, 43, 289–294. [Google Scholar]
- Bevilacqua, A.; Altieri, C.; Corbo, M.R.; Sinigaglia, M.; Ouoba, L.I.I. Characterization of Lactic Acid Bacteria Isolated from Italian Bella di Cerignola Table Olives: Selection of Potential Multifunctional Starter Cultures. J. Food Sci. 2010, 75, M536–M544. [Google Scholar] [PubMed]
- Corbo, M.R.; Del Nobile, M.A.; Sinigaglia, M. A novel approach for calculating shelf life of minimally processed vegetables. Int. J. Food Microb. 2006, 106, 69–73. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).