Abstract
Background: Crude extracts are easily available and considered safe and cost-effective in comparison with synthetic extracts and are more accessible compared with purified compounds, making them suitable for initial screening and exploratory studies in drug discovery. Introduction: Cucumis sativus and Cucurbita pepo are medicinal plants that belong to the Cucurbitaceae family, commonly known as cucumber and pumpkin, comprising a series of phytochemicals such as chlorophylls, carotenoids, oleanolic acid, saponin, and triterpenoids. Materials and Methods: In this study, an ethanol extract of Cucumis sativus and Cucurbita pepo whole plants was used to assess their hypoglycemic effects in a fasted, fed, glucose-loaded and streptozotocin-induced diabetes model of albino rats followed by Molecular Spectroscopic (FTIR and UV-Vis) analysis. Blood sugar levels were determined from samples collected at different intervals (0, 1, 3, and 4 h). Results and Conclusions: A significant blood glucose reduction was observed as a result of both plants’ extracts, while the greatest reduction was shown by Cucumis sativus. The UV-Vis profile showed several absorption bands ranging from 200 to 800 nm, showing the presence of flavonoids, phenolic compounds, terpenoids, carotenoids, and chlorophyll. The FTIR spectra reveal the presence of carbohydrates, proteins, lipids, and phenolic compounds, which contribute to the extracts’ nutritional and biological value. Further research is needed to determine the active agents and the likely mechanism of action of both the plants regarding their hypoglycemic effects.
1. Introduction
Diabetes mellitus is a severe metabolic disorder, characterized by the presence of excessive sugar in the blood (hyperglycemia) due to the absence of insulin or an insensitivity of the insulin receptor in the body, and has become a major global health problem [1]. Both type 1 and type 2 diabetes are commonly associated with impaired insulin secretion or resistance, leading to poor blood sugar control and long-term complications such as cardiovascular diseases, neuropathy, and kidney damage [2]. Current therapeutic strategies, including insulin therapy and oral synthetic antidiabetic agents, are often associated with several side effects, prompting a growing interest in alternative therapies, particularly those derived from natural sources [3].
Crude plant extracts, which are often cheaper and more readily available than purified compounds, are increasingly being studied for their therapeutic potential and are thought to work more biologically than synthetic alternatives since phytoconstituents are a part of the physiological processes of living flora, enhancing their suitability for the human body [4]. Cucumis sativus (cucumber) and Cucurbita pepo (pumpkin) are two such plants from the Cucurbitaceae family that are traditionally used for various medicinal purposes, including the treatment of diabetes. Both plants contain a variety of bioactive compounds, including flavonoids, phenolic acids, carotenoids, oleanolic acid, saponins, and triterpenoids, which may contribute to their antidiabetic effects [5].
While individual compounds such as cucurbitacins from pumpkin have been studied for their biological activity, there is limited research directly comparing the hypoglycemic potential of the whole plant extracts of Cucumis sativus (CS) and Cucurbita pepo (CP). The current study was designed to evaluate and compare the hypoglycemic effects of ethanol extracts from CS and CP whole plant in normal and streptozotocin (STZ)-induced diabetic rats and to determine the chemical profiles of these extracts using UV-Vis and FTIR spectroscopy.
2. Materials and Methods
2.1. Collection and Preparation of Extract
The whole plant samples of members of the Cucurbitaceae family, namely Cucumis sativus and Cucubita pepo, were collected in May-June 2022 from the Amaushi (Coordinates—26°44′ N, 80°52′ E) and Dilawar nagar Malihabad (Coordinates—26°91′ N, 80°70′ E) region of Lucknow district, Uttar Pradesh, India. The plants were first verified in World Flora Online http://www.worldfloraonline.org/ (accessed on 22 January 2023) (ID: wfo-0000628992, ID: wfo-0000629123), further validated by the database http://www.theplantlist.org (accessed on 24 January 2023), and then identified by the botanist Expert Prof. Alka Kumari, Department of Botany, University of Lucknow—U.P., India. The collected plants were thoroughly washed and kept in the shade at room temperature for drying and the preparation of the crude extract. The fully dried plants were then stored separately in an airtight container. Coarse dried Cucumis sativus and Cucurbita pepo powders were extracted with 95% ethanol in a Soxhlet extractor. The semi-solid, alcohol-free material extract was obtained using a rotary evaporator. For further study, these dry extracts were stored in an airtight container [6].
2.2. Animals
Healthy male albino rats (Rattus norvegicus) of the Sprague Dawley (SD) strain (120–150 g) were used and purchased from the National Laboratory Animal Facility (NLAF), Central Drug Research, Lucknow (CDRI), India. The animal husbandry and animal ethics guidelines were strictly followed, conducted as per the ARRIVE (Animal Research: Reporting of In Vivo Experiments) and CCSEA (Committee for Control and Supervision of Experiments on Animals) guidelines, and the protocol was approved by the Institutional Animal Ethical Committee (IAEC) of the Department of Zoology, University of Lucknow, Lucknow, India, with Reference no. 14/I/2023/IAEC/LU. The animals were maintained under controlled conditions: temperature 25–26 °C, relative humidity 60–70%, and 12/12 h light/dark cycle. The rats were divided into an experimental group and a control group of six rats each. Water was allowed ad libitum. One week before this study, animals were acclimatized to the laboratory environment in accordance with OECD (Organisation for Economic Co-operation and Development) guidelines.
2.3. Experimental Design
This study was designed to investigate the hypoglycemic potential of Cucumis sativus and Cucurbita pepo ethanol whole plant extracts in following Fasted, Fed, Glucose-loaded and Streptozotocin-induced diabetic rat models. A schematic overview of the experimental design is presented in Figure 1 below.
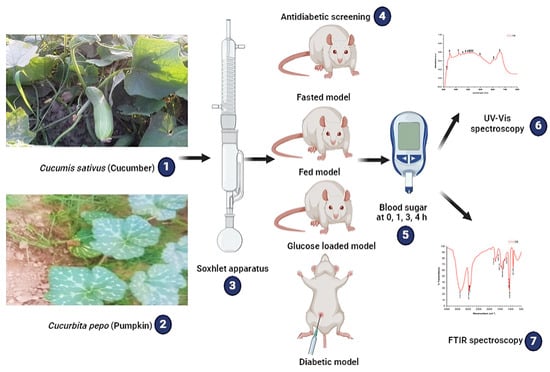
Figure 1.
Schematic overview of the experimental design: 1 and 2 represent the tested plants (Cucumis sativus and Cucurbita pepo), 3 represents the method used for the plant extraction, 4 and 5 represent the antidiabetic screening models and blood sugar determination up to the 4 h time interval, and 6 and 7 represent the techniques used for the phytochemical profiling (UV-Vis and FTIR spectroscopy).
- Fasted model: Animals were fasted overnight (18 h), and blood was collected at 0 h from the tail vein; afterwards, the tested plant extracts were administered at a dose of 250 mg/kg body weight. Blood samples were collected at different time intervals (1, 3, and 4 h). Blood glucose concentration was measured using the standard glucometer (Accu-Chek Active Glucometer; Roche, Germany);
- Fed model: excess pellets were kept in the cages on the previous evening, leaving some pellets left over the next morning. For blood glucose estimation, blood was collected before (at 0 h) and after administration of the extracts at a dose of 250 mg/kg body weight at time intervals of 1, 3, and 4 h;
- Diabetic model: Diabetes was induced in rats by streptozotocin (35 mg/kg body weight) dissolved in freshly prepared, chilled citrate-phosphate buffer (pH 4.5) by intraperitoneal (i.p.) injection. Before injection, the rats were fasted for 18 h. After streptozotocin injection, all rats were returned to their cages and given free access to food and water. To avoid transit hypoglycemia, all rats were administered a 10% glucose solution for 24 h. After 3 days of i.p. administration, the blood glucose concentration of the rats was checked. Rats with fasting blood glucose levels over 200 mg/dL were considered diabetic rats and were used for the experiment. Then, the same protocol that we had carried out in the fasted group was followed and the blood glucose concentration of the rats in this group was measured at different time intervals;
- Glucose-loaded model: animals were fasted for 18 h, and blood was collected (at 0 h) for glucose estimation. Then, the plant extract was administered, and half an hour after the feeding extract, a glucose (1.5 gm/kg body weight orally) solution was administered using an oral cannula (18G × 50 mm), and blood samples were taken for glucose estimation at 1/2, 1, and 3 h post administration.
2.4. UV-Vis Spectroscopy
The tested extracts (CS and CP) were centrifuged (3000 rpm) for 10 min and filtered through Whatman No. 1 filter paper [7]. The spectra were recorded at a speed of 20 nm s−1 in a quartz cuvette with a resolution of 1 nm. Scanning between the 200 and 800 nm wavelengths was performed using a dual-beam spectrophotometer (Shimadzu UV-1601 Tokyo, Japan). The UV-Vis peaks were found and analyzed.
2.5. FTIR Spectroscopy
Fourier transform infrared spectroscopy (FTIR) is an analytical technique commonly used for identifying the functional groups of the active components based on the peak value in the infrared spectrum of absorption or emission of organic (and in some cases inorganic) materials. The extracts (1 g) were suspended in 1 mL of the same solvent in 1 mL cryovials. A vortex was used to allow for dissolution of the extract, and the tube was used to allow for further solubilization. The tested extracts (CS and CP) were then analyzed using ATR-IR FTIR (Nicole 6700, Thermo-Scientific, Waltham, MA, USA). To obtain the IR spectra, the extracts were analyzed using the standard procedure for scanning the wavenumbers ranging from 4000 to 400 cm−1 with a resolution of 4 cm−1 [8].
2.6. Statistical Analysis of Data
Results are expressed as the mean ± standard deviation (SD) (n = 6). All results were analyzed using a one-way ANOVA followed by a post hoc Tukey multiple comparison test, and Graph Pad Prism version 7.0 was used for statistical analysis. OriginPro 2024b (64-bit) 10.1.5.132 (Learning Edition) was used for the plotting of UV-Vis and FTIR diagrams. The difference between control and experimental groups was considered significant at p < 0.05, p < 0.01, p < 0.001, and p < 0.0001.
3. Results and Discussion
3.1. Antidiabetic Effect
The comparative analysis of the (Table 1) Cucumis sativus (CS) and Cucurbita pepo (CP) ethanolic extracts demonstrate a range of blood glucose-lowering effects on different (fasted, fed, glucose-loaded, and diabetic) models, where CS consistently resulted in the greatest lowering of blood glucose in all the models, justifying its traditional role in treating diabetes and its associated complications. In the fasted state, both CS and CP (9.52% and 4.35%) lower glucose level. Under fed conditions, these extracts significantly lowered blood glucose levels, whereas CS again demonstrated the greatest reduction (15.54%) compared with CP (7.23%). For the glucose-loaded condition (Table 2), the decrease in blood glucose indicates the postprandial efficacy, where CS (9.68%) again demonstrated the greatest lowering when compared with CP (1.68%). Finally, CS caused the highest and most significant blood glucose lowering in the STZ-induced diabetic (20.4%) model compared with CP (10.49%). Of all the four experimental groups, the CS extract showed promising results indicating its ability to reduce blood glucose levels under different metabolic conditions and highlighting its potential as a natural agent for glucose diabetes management.

Table 1.
Effect of 95% ethanol extracts of Cucumis sativus and Cucurbita pepo plant at a single dose of 250 mg/kg body weight on blood glucose levels in fasted, fed, and streptozotocin-induced diabetic male albino rats.

Table 2.
Effect of 95% ethanol extracts of Cucumis sativus and Cucurbita pepo plant at a single dose of 250 mg/kg body weight on blood glucose levels in glucose-loaded model.
3.2. UV-Vis Analysis
The UV-Vis profile (Figure 2) revealed the presence of 9 peaks of CP at 224, 230, 238, 249, 274, 320, 321, 339, and 562 nm, with absorption ranging from 0.01 to 0.32 a.u., and 10 peaks of CS at 224, 238, 249, 321, 504, 575, 624, 647, 668, and 714 nm, with absorption ranging from 0.21 to 0.60 a.u. The UV−Vis profiles of CP and CS suggested the presence of various phytochemicals, including flavonoids, tannins, phenolics, and carotenoids compounds [9,10].
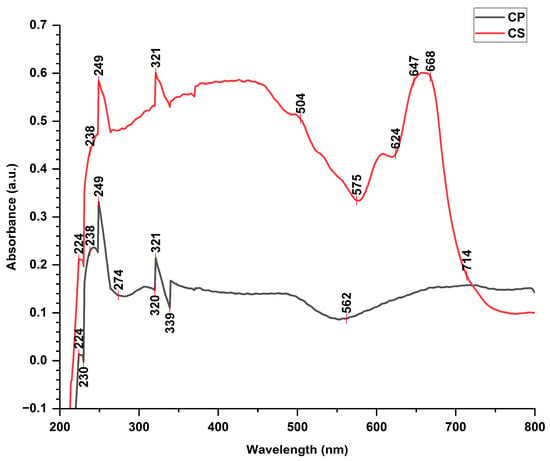
Figure 2.
UV–visible spectra of Cucumis sativus and Cucurbita pepo extract from 200 to 800 nm.
3.3. FTIR Analysis
FTIR analysis revealed the presence of various functional groups in the CS and CP ethanolic extracts. The different peaks (Figure 3) determine the different functional groups that have potential therapeutic activities. Alkanes and alkenes are used in drug delivery systems and have strong anticancer and anti-inflammatory properties. The FTIR range displays band intensities in different regions, and the peaks that correspond to the presence of phenolic and alcoholic compounds indicate their role in antioxidant and antimicrobial activities. A variety of biological processes, including the formulation of drugs, depend on carboxylic acid, aromatic compounds, ester groups, and bromo alkanes. The mentioned peaks are mainly attributed to alkaloids, flavonoids, saponins, terpenoids, and phenols [11,12,13].
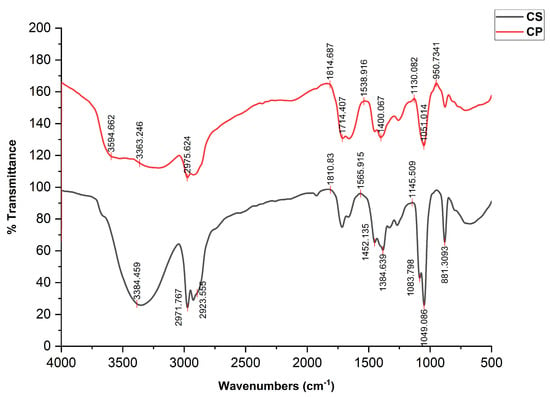
Figure 3.
FTIR spectra of the Cucumis sativus and Cucurbita pepo extract from 500 to 4000 nm.
From many years, plant-derived products have been the primary source for drug discovery and development and have played a key role in the global healthcare system. The testing of glucose lowering in different models (fasted, fed, glucose-loaded, and diabetic) offers a time saving approach and a better understanding of plant extracts’ role in managing blood glucose level [14]. The results indicate promising therapeutic potential, but several key limitations need to be addressed to have a broader view of diabetes drug screening and its management. The glucose lowering of tested plants at different time intervals (0, 1, 3, and 4 h) limits our understanding of the long-term regulation of blood glucose. However, there is a need to further explore the therapeutic potential of Cucumis sativus and Cucurbita pepo to identify the active principles and the mechanisms of their medicinal effects.
4. Conclusions
Our observations through this experiment suggest that 95% ethanolic extracts of Cucumis sativus and Cucurbita pepo possess hypoglycemic activity in normal and diabetic male rats and can improve oral glucose tolerance probably by stimulating insulin release from beta cells or through insulin-like action. Additionally, these plants are affordable and widely accessible, which makes them a viable alternative to antidiabetic medications. Future research should focus on the identification, purification, and isolation of the compound responsible for the therapeutic potential. Furthermore, although the streptozotocin (STZ)-induced diabetic rat model is widely accepted, it does not fully replicate human diabetes; so, further validation in human clinical trials is needed to confirm the efficacy of Cucumis sativus and Cucurbita pepo in diabetes management. Lastly, a long-term safety profile along with toxicity considerations of these plant extracts are necessary for establishing their suitability as sustainable natural agents for diabetes.
Author Contributions
Conceptualization, A.M.S. and V.G.; methodology, A.M.S.; software, V.G.; validation, V.G.; formal analysis, A.M.S.; investigation, V.G.; resources, A.M.S. and V.G.; data curation, V.G.; writing—original draft preparation, V.G.; writing—review and editing, V.G. and A.M.S.; visualization, V.G. and A.M.S.; supervision, A.M.S.; project administration, A.M.S.; funding acquisition, A.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal husbandry and animal ethics guidelines were strictly followed, conducted as per ARRIVE (Animal Research: Reporting In Vivo Experiments) and CCSEA (Committee for Control and Supervision of Experiments on Animals) guidelines, and the protocol was approved by the Institutional Animal Ethical Committee (IAEC) of the Department of Zoology, University of Lucknow, Lucknow, India, with reference no. 14/I/2023/IAEC/LU.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
The authors acknowledge Mohammad Sirajuddin, Department of Zoology, University of Lucknow, Lucknow, for providing access to central instrumentation facilities to carry out this work. We also want to thank Mohammad Amir, Department of Botany, University of Lucknow, Lucknow, for providing the UV−Vis Spectroscopy facility, and BBAU Lucknow, for providing the FTIR spectroscopy facility.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. 1), S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, N.; Alzahrani, A.R.; Ibrahim, I.A.A.; Shahid, I.; Alanazi, I.M.; Falemban, A.H.; Imam, M.T.; Mohsin, N.; Azlina, M.F.N.; Arulselvan, P. Therapeutic strategy of biological macromolecules based natural bioactive compounds of diabetes mellitus and future perspectives: A systematic review. Heliyon 2024, 10, e24207. [Google Scholar] [CrossRef] [PubMed]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, K.; Gupta, A.; Sharma, D.; Gill, N.; Goyal, A. A review on the medicinally important plants of the family cucurbitaceae. Asian J. Clin. Nutr. 2012, 4, 16–26. [Google Scholar] [CrossRef]
- Suryavanshi, A.; Gautam, V.; Saxena, A.; Panjwani, D.; Kumar, S. Evaluation of the Anti-diabetic Potential of Rumex vesicarius L. in normal and Streptozotocin induced Diabetic Rats. Res. J. Biotechnol. 2022, 17, 63–67. [Google Scholar] [CrossRef]
- Makita, C.; Chimuka, L.; Steenkamp, P.; Cukrowska, E.; Madala, E. Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. S. Afr. J. Bot. 2016, 105, 116–122. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Mudalip, S.K.A.; Olalere, O.A. Characterization and effect of extraction solvents on the yield and total phenolic content from Vernonia amygdalina leaves. J. Food Meas. Charact. 2018, 12, 311–316. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef]
- Saxena, M.; Saxena, J. Evalution of phytoconstituents of Acorus calamus by FTIR and UV-VIS spectroscopic analysis. Int. J. Biol. Pharm. Res. 2012, 3, 498–501. [Google Scholar]
- Gautam, A.K.; Pandey, G. Green Synthesis of Pistia stratiotes Ag/AgCl Nanomaterials and Their Anti-Bacterial Activity. Chem. Proc. 2023, 15, 88. [Google Scholar]
- Gautam, N.; Singh, K.B.; Snigdha; Upadhyay, D.D.; Pandey, G. Structural and optical properties of silver supported α-Fe2O3nanocomposite fabricated by Saraca asoca leaf extract for the effective photo-degradation of cationic dye Azure B. RSC Adv. 2023, 13, 23181–23196. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, R.; Kumar, K.M.M.; Narayanan, N.S. Synthesis of bio-oil from waste Trichosanthes cucumerina seeds: A substitute for conventional fuel. Sci. Rep. 2020, 10, 17815. [Google Scholar] [CrossRef] [PubMed]
- Gautam, V.; Ranjan, A.; Bajpai, K.G.; Baqri, S.S.R.; Saxena, A.M. Exploring the Therapeutic Potential of Three Cucurbit Plants Involving In Vivo Diabetes Screening. Cureus 2025, 11, 17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).