Abstract
The aim of this work was to perform a comparative study of composition and antimicrobial properties in eleven cultivars of honeysuckle berries. Using spectrophotometric methods, we compared the content of total phenolic compounds (TPC), anthocyanins, and chromatic characteristics of berries, which were grown in the collection of Vytautas Magnus University Botanical Garden and collected at maturation stage. In addition, the content of ascorbic acid and saccharides were evaluated by HPLC using diode ray and light scattering detectors. Antimicrobial activity of ethanolic and water extracts of honeysuckle berry were evaluated by the agar well diffusion method. Bacterial tests identified antimicrobial properties of honeysuckle berries against undesirable in food products bacteria but without affecting Candida and Saccharomyces cerevisiae yeast. The cultivar ‘Morena’ had the highest anthocyanins (781 mg/100 g) and total phenolic compounds (799 mg/100 g), while the lowest anthocyanins (282 mg/100 g) and TPC (300 mg/100 g) content were detected in the ‘Vostorg’ cultivar. Cultivars ‘Pavlovskaja’ and ‘Pereselenka’ had high contents of ascorbic acid. The maximum glucose and fructose contents were detected in the ‘Leningradskaya’ cultivar.
1. Introduction
For a healthy diet it is recommended to consume at least 400 g of vegetables and fruit per day. According to the World Health Organization’s recommendations, consumption of sugar should be lowered and the amount of dietary fiber increased. Berries are a good source of dietary fiber, polyphenols, ascorbic acid, and other bioactive compounds [1]. The edible blue honeysuckle comes from Russia and in recent years has been planted considerably in some European countries, including Lithuania. Its interesting characteristics include its high resistance to cold, different soil acidities, pests, and various diseases [2]. The berries are rich in ascorbic acid and phenolic contents, which have nutritional and health-promoting properties for humans. In Japan, honeysuckle berries are used in traditional medicine for slowing the aging process, and preventing heart diseases and gastrointestinal dysfunction [3]. Anthocyanins, plant pigments responsible for red to blue color of fruits, are the biggest contributors to total phenolics in blue honeysuckle berries. High contents of cyanidin-3-glucoside suggest their good antioxidant, anti-inflammatory, antimicrobial, cardioprotective, and hepatoprotective activities [4,5]. In recent years, there has been increased consumer demand for the use of plant extracts as natural antimicrobial agents in food, instead of synthetic food additives. Some studies have shown the antimicrobial properties of honeysuckle berries. Phenolic acids present in honeysuckle berries can act as natural antimicrobial agents to control Candida parapsilosis, Enterococcus faecalis, Escherichia coli, Staphylococcus epidermidis, or Streptococcus mutant [6]. The antibacterial effects of ethanol and buffered water infusions obtained from freeze-dried fruits of L. caerulea were studied on Gram-positive bacteria (Listeria monocytogenes, Kocuria rhizophila, Bacillus subtilis), Gram-negative food-borne pathogenic bacteria (Escherichia coli, Campylobacter jejuni), and Gram-positive bacteria selected as probiotic bacterial species often used in dairy products (Bifidobacterium bifidum, Lactobacillus acidophilus). It was found that some blue honeysuckle infusions can elicit high antimicrobial activity against food-borne pathogens without strong inhibition towards probiotics. Consequently, tested probiotic bacteria with honeyberry extract can be used in the food processing industry as potential antimicrobials and functional components [7].
Studies on the bioactivity of honeysuckle berries have only started recently, and therefore the data are limited. It is well known that the composition of bioactive compounds depends on genotype, climatic conditions, and agronomic practices. This is a preliminary report on honeysuckle berries planted in Lithuania. The aim of this paper was to perform a comparative study of the composition and antimicrobial properties in eleven cultivars of honeysuckle berries grown in Lithuania.
2. Materials and Methods
Berries of eleven Lonicera caerulea L. cultivars were grown in collection of Vytautas Magnus University Botanical Garden and collected at maturation stage. Fresh berries were homogenized using a blender (Bosch MSM16500, Ljublijana, Slovenia) and stored at 4 °C in the refrigerator.
The dry matter content was determined by drying crushed berries in the moisture analyzer MB 64M (Bel Engineering, Monza, Italy), and the pH of crushed berries was determined directly using a pH-meter (Denver Instrument Company, Bohemia, NY, USA). The soluble solids were determined using a refractometer Atago RX-5000CX (Fukaya, Japan). Color CIE L*a*b* characteristics were evaluated using a chroma meter CR-410 (Konica Minolta, Osaka, Japan).
Ethanolic extract. Two grams of crushed berries were extracted with 15 mL of 95% (v/v) food grade ethanol acidified with 0.1 N of HCl in an ultrasonic bath (Ultrasonix cleaner proclean 3.ODSP, Ulsonix, Zielona Gora, Poland) for 20 min. The obtained extract was decanted, and a new 15-mL portion of solvent was used. The extraction was repeated three times, with all three extracts combined and the volume adjusted to 50 mL with acidified ethanol. This extract was used for the evaluation of the total phenolics and total anthocyanins content.
Total anthocyanin content. The absorption of 1:10 diluted ethanolic extract was measured on a spectrophotometer Genesys-5 (Thermo Spectronic, Rochester, NY, USA) at 535 nm. The concentration of anthocyanins was determined from the calibration curve, which was constructed by measuring the absorption of cyanidin-3-glucoside (MW 449.4, ε = 26.900) reference solution.
Total phenolic content (TPC). The TPC was measured with a Folin–Ciocalteu reagent [8]. One milliliter of the sample was mixed with 5 mL of the 10-fold diluted (v/v) Folin–Ciocalteu reagent and 4 mL of 7.5% Na2CO3. After incubation for 30 min at room temperature in the dark, the absorbance was measured at 765 nm. The results were expressed in mg of gallic acid equivalents.
Vitamin C content. Extract for vitamin C determination was prepared according to a slightly modified method of Auzanneaau et al. [1]. Five grams of berry paste were extracted with 10 mL of oxalic acid solution (10 g/L) in an ultrasonic bath for 20 min. After centrifugation for 10 min at 5300 rpm (2700 g), the obtained supernatant was filtered through a paper filter and then through a 0.22-µm pore size membrane filter. The Shimadzu Prominence series (Shimadzu Corp., Kyoto, Japan) HPLC system with Atlantis dC18 5 μm 4.6 × 150 mm column (Waters, Ireland) was used for separation and quantification of vitamin C. Mobile phase A was 0.1% TFA in H2O, and B was 0.1% TFA in ACN. Time program: B Conc. 0% → 3% (0.0–5.0 min) → 15% (6.0 min) → 20 % (10.0 min) → 100% (12.0 min) → 100% (25.0 min). The flow rate was 1.4 mL/min, column temperature 30 °C, and injection volume 20 μL. Vitamin C was recorded at 210 nm using an SPD-M20A diode array detector (Shimadzu Corp., Kyoto, Japan). Quantification of vitamin content was done using calibration curve of standard solutions.
Determination of saccharides. An aqueous extract was prepared from 2 g of berry paste [9]. Samples were extracted with 60 mL of water in a water bath at 60 °C for 30 min (GFL No-1092, Burgwedel, Germany), then clarified with Carrez I and Carrez II solution, diluted to 100 mL with water and filtered through a paper filter followed by a 0.22 µm pore size membrane filter. Obtained filtrate was used for HPLC analysis. Separation conditions were as follows: The eluent was a mixture of 75 parts by volume of ACN and 25 parts by volume water; the flow rate was 1.2 mL/min, and 20 μL was injected. The YMC-Pack Polyamine II 250 × 4.6 mm, 5 μm (YMC Co., Ltd., Kyoto, Japan) column was used with a temperature of 28 °C. Detection was performed using an Evaporative Light Scattering Detector ELSD-LTII (Shimadzu Corp., Kyoto, Japan). Calibration curves of fructose, glucose, and sucrose were used for the quantification.
Antimicrobial activity was evaluated using the agar well diffusion method. Different extracts were prepared from homogenized berries to analysis. Two grams of berry paste were extracted with 8 mL of water in an ultrasonic bath (Ultrasonix cleaner proclean 3.ODSP, Ulsonix, Zielona Gora, Poland) for 30 min. It was then centrifuged for 10 min at 5300 rpm (Labofuge 200 Heraeus Thermo Scientific, Waltham, MA, USA Rotor 3760, 2700 g). Ethanolic extract was prepared from 3 g of berry cakes and 10 mL of ethanol. Extraction and centrifugation conditions were the same. Obtained supernatants were used for evaluation of antimicrobial activity. The yeasts and bacteria were undesirable in food products, and were used in the test cultures. Bacteria were grown in a peptone-soy bouillon. Yeasts were grown on a slant potato dextrose agar. Eight-millimeter-diameter wells were pushed in the agar and filled with 50 µL of sample. The plates were incubated overnight at 37 °C. After incubation the inhibition zones were measured, and the effect was calculated as a mean of three replicate tests.
3. Results
3.1. pH, Soluble Solids and Dry Matter Amount of Honeysuckle Berries
Eleven cultivars of Lonicera caerulea L. were investigated in this study. Berries were grown in the Vytautas Magnus University Botanical Garden collection. Only fruits at maturity stage, based on the color and texture, were collected. After crushing the berries, the pH, total soluble solids, and dry matter amount were evaluated (Table 1).

Table 1.
pH, TTS, and dry matter content in fresh honeysuckle berries. Dry matter content and TSS are expressed as means of triplicate analysis; pH was measured in one uniform sample.
pH measured in fresh berries ranged from 2.99 (‘Morena’ and ‘Kalinka’ cultivars) to 3.37 (‘Leningradskaya’ and ‘Balalaika’ cultivars).
Dry matter content was around 15%. The highest values of total soluble solids (16.09 °Brix) and dry matter content (18.41%) were obtained from the ‘Balalaika’ cultivar. The lowest dry matter content (11.70%) was detected in ‘Čelnočnaja’.
On average, TSS was 11.74 °Brix. Other authors reported that TSS values in the same studied cultivars depended on year: For 2016 it was 10.8 °Brix, for 2015 and 2014, they were 14.8 and 14.6, respectively [1].
3.2. Qualitative and Quantitative Composition of Saccharides
Fructose and glucose were the predominant free sugars in honeysuckle berries. Only in ‘Čelnočnaja’, ‘Pavlovskaja’, and ‘Pereselenka’ cultivars was sucrose found (Figure 1).
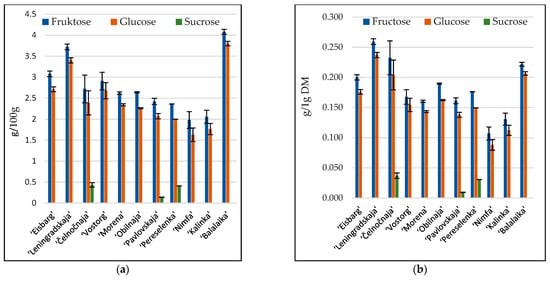
Figure 1.
Glucose, fructose, and sucrose content in eleven cultivars of honeysuckle berries: (a) g/100 g; (b) g/g DM. Data are mean of triplicate analysis with standard deviation.
Differences in saccharide content between cultivars were observed. Glucose content in samples ranged from 3.80 (‘Balalaika’) to 1.62 g/100 g (‘Nimfa’), and from 0.24 (‘Leningradskaya’) to 0.09 g/g DM (‘Nimfa’). Fructose content was higher and ranged from 4.08 (‘Balalaika’) to 1.98 g/100 g (‘Nimfa’), and 0.26 (‘Leningradskaya’) to 0.11 g/g DM (‘Nimfa’). ‘Nimfa’ seemed to be the cultivar with the lowest content of sacharides. The highest content of sucrose (0.43 g/100 g) was found in ‘Čelnočnaja’ cultivar, while less was found in ‘Pereselenka’ (0.41 g/100 g) and ‘Pavlovskaja’ (0.14 g/100 g) cultivars. Glucose content in berries grown in Switzeland in the 3-year period was between 80.0 and 327 mg/g DM, and fructose content was in the range of 140–337 mg/g DM [1]. The content of saccharides is an important parameter of berries, because it determines the taste and has a great influence on consumer acceptibility.
3.3. Ascorbic Acid Content
Another important parameter in the composition of berries is the amount of ascorbic acid. It was on average 3.3 mg/g DM. Unusually large amounts of vitamin C were found in ‘Pereselenka’ (10.5 mg/g DM) and ‘Pavlovskaja’ (7.6 mg/g DM) cultivars (Figure 2). After excluding the maximum values, the average values would be 34.3 g/100 g and 2.28 mg/g DM. Vitamin C content was the lowest in the ‘Nimfa’ cultivar—13.46 g/100 g and 0.73 g/g DM. Ascorbic acid content reported for honeysuckle berries grown in Switzerland was 1.78–4.21 mg/g DM [1], while that of those grown in Slovenia was 17.75–25.77 mg/100 g [2]. The differences could be the result of different extraction techniques and growing conditions.
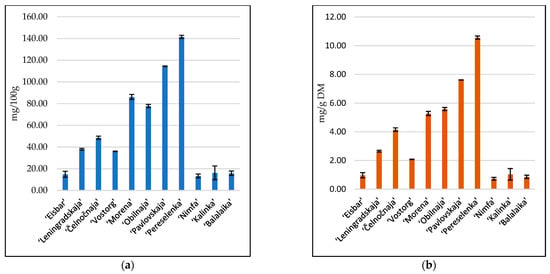
Figure 2.
Content of ascorbic acid in eleven cultivars of honeysuckle berries: (a) g/100 g; (b) g/g DM. Data are mean of duplicate analysis with standard deviation.
3.4. Chromatic Properties of Honeysuckle Berries
Chromatic properties of honeysuckle berries were presented in Table 2. The L* values of the Lonicera caerulea L. cultivars were not variable and ranged from 23.34 to 26.44. The cultivar ‘Pereselenka’ was characterized by high a* (13.15) and b* (5.33) values, and the chroma C (14.18) and hue angle h° (0.38) for this cultivar were also the highest. The lowest values of C and h° were determined in ‘Balalaika’ cultivar—6.90 and 0.18, respectively.

Table 2.
Color characteristics of eleven cultivars fresh honeysuckle berries. Results are expressed as means of triplicate analysis and standard deviation.
3.5. Total Phenolic Compounds and Anthocyanins Content
The total phenolic compounds and anthocyanin content are presented in Figure 3. The average contents of total phenolic compounds expressed in mg of gallic acid equivalents were 492.1 mg/100 g and 31.6 mg/g DM; total anthocyanins was 451.5 mg of cyanidin-3-glucoside equivalents/100 g and 29.2 mg of cyanidin-3-glucoside equivalents/g DM. Literature refers to values between 8.4 and 65 mg cyanidin-3-glucoside equivalents/g DM and from 7.0 to 57.1 mg gallic acid equivalents/g DM [1].
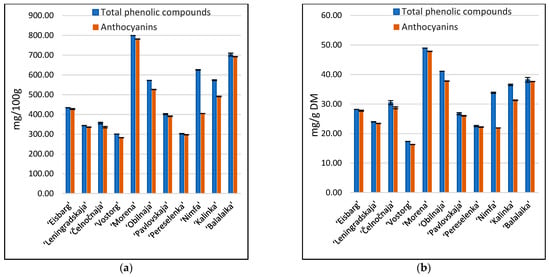
Figure 3.
Content of total phenolic compounds and anthocyanins in eleven cultivars of honeysuckle berries: (a) g/100 g; (b) g/g DM. Data are mean of triplicate analysis with standard deviation.
The cultivar ‘Morena’ had the highest anthocyanins (781 mg/100 g) and total phenolic compounds (799 mg/100 g), while the lowest anthocyanins (282 mg/100 g) and TPC (300 mg/100 g) content were detected in the ‘Vostorg’ cultivar. The same tendency was observed after conversion to mg/g DM.
3.6. Antimicrobial Activity
Investigation of antimicrobial activity showed that aqueous extracts of honeysuckle berries weakly inhibited the growth of Gram-negative and Gram-positive test cultures (Table 3).

Table 3.
The antimicrobial influence of aqueous berry extracts on test cultures. Inhibition zone in mm, including the 8-mm hole. If the inhibition zone was not observed, results were presented as 0.
B. subtillis were the most sensitive, with zones of inhibition were 9.5–11.0 mm. S. typhimurium showed the largest resistance to aqueous berry extracts; only ‘Kalinka’ and ‘Balalaika’ cultivars showed 9.0- and 8.5-mm zones of inhibition. No inhibition zone was observed against yeast C. albicans and S. cerevisiae.
Berry cakes, a by-product of juice extraction, are of particular interest because they can be used as a cheap raw material for functional ingredient production. The investigation of antimicrobial activity of ethanolic extracts of berry cakes showed a greater inhibition effect compared to aqueous berry extracts on test cultures (Table 4). Only E. coli was resistant to the ‘Eisbar’ and ‘Leningradskaya’ cultivars. Again, no inhibition zone was observed against the yeasts C. albicans and S. cerevisiae. Pure ethanol, used as a control, did not show any inhibition effect on test cultures.

Table 4.
The antimicrobial influence of ethanolic berry cakes extracts on test cultures. Inhibition zone in mm, including the 8-mm hole. If inhibition zone was not observed, results were presented as 0.
4. Conclusions
Eleven cultivars of Lonicera caerulea berries were investigated for their composition and antimicrobial properties. This study showed that ‘Morena’ cultivar had the highest anthocyanins (781 mg/100 g) and total phenolic compounds (799 mg/100 g), the lowest anthocyanins (282 mg/100 g) and polyphenols (300 mg/100 g) content was detected in ‘Vostorg’ cultivar. Cultivars ‘Pavlovskaja’ and ‘Pereselenka’ had high content of ascorbic acid. The maximum glucose and fructose content were detected in ‘Leningradskaya’ and ‘Balalaika’ cultivars. Bacterial tests have identified antimicrobial properties of honeysuckle berries against undesirable in food products bacteria but without affecting Candida and Saccharomyces cerevisiae yeast. Results indicate that, due to the amount of bioactive substances and beneficial health properties, honeysuckle berries are valuable component to the diet.
Supplementary Materials
The poster presentation is available online at https://www.mdpi.com/article/10.3390/IECPS2020-08626/s1.
Author Contributions
I.J. conceived and designed the experiments, performed HPLC analysis, analyzed the data, and wrote the paper. A.P. performed preparation of sample, spectrophotometric assay, and other measurements. A.Š. performed antimicrobial activity experiment and analyzed the data. L.Č. provided berry material and contributed substantially in preparing the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPLC | High-performance liquid chromatography |
| TPC | Total phenolic compounds |
| TFA | Trifluoroacetic acid |
| ACN | Acetonitrile |
| DM | Dry matter |
References
- Auzanneau, N.; Weber, P.; Kosińska-Cagnazzo, A.; Andlauer, W. Bioactive compounds and antioxidant capacity of Lonicera caerulea berries: Comparison of seven cultivars over three harvesting years. J. Food Compos. Anal. 2018, 66, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue honeysuckle (Lonicera cearulea L. subs. edulis) berry; a rich source of some nutrients and their differences among four different cultivars. Sci. Hortic. 2018, 238, 215–221. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Haskap berries (Lonicera caerulea L.)—A critical review of antioxidant capacity and health-related studies for potential valueadded products. Food Bioprocess Technol. 2014, 7, 1541–1554. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Wu, S.; He, X.; Wu, X.; Qin, S.; He, J.; Zhang, S.; Hou, D.-X. Inhibitory effects of blue honeysuckle (Lonicera caerulea L.) on adjuvant-induced arthritis in rats: Crosstalk of anti-inflammatory and antioxidant effects. J. Funct. Foods 2015, 17, 514–523. [Google Scholar] [CrossRef]
- Palíková, I.; Heinrich, J.; Bednář, P.; Marhol, P.; Křen, V.; Cvak, L.; Valentová, K.; Růžička, F.; Holá, V.; Kolář, M.; et al. Constituents and antimicrobial properties of blue honeysuckle: A novel source for phenolic antioxidants. J. Agric. Food Chem. 2008, 56, 11883–11889. [Google Scholar] [CrossRef]
- Raudsepp, P.; Anton, D.; Roasto, M.; Meremäe, K.; Pedastsaar, P.; Mäesaar, M.; Raal, A.; Laikoja, K.; Püssa, T. The antioxidative and antimicrobial properties of the blue honeysuckle (Lonicera caerulea L.), Siberian rhubarb (Rheum rhaponticum L.) and some other plants, compared to ascorbic acid and sodium nitrite. Food Control 2013, 31, 129–135. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Česonienė, L.; Daubaras, R.; Jasutienė, I.; Miliauskienė, I.; Zych, M. Investigations of anthocyanins, organic acids, and sugars show great variability in nutritional and medicinal value of European cranberry (Vaccinium oxycoccos) fruit. J. Appl. Bot. Food Qual. 2015, 88, 295–299. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).