Plants Biodiversity in Olive Orchards and Surrounding Landscapes from a Conservation Biological Control Approach †
Abstract
:1. Introduction
2. Experiments
2.1. Study Areas
2.2. Flowering Plant Inventories
2.3. Data Analysis
2.3.1. Alpha Diversity
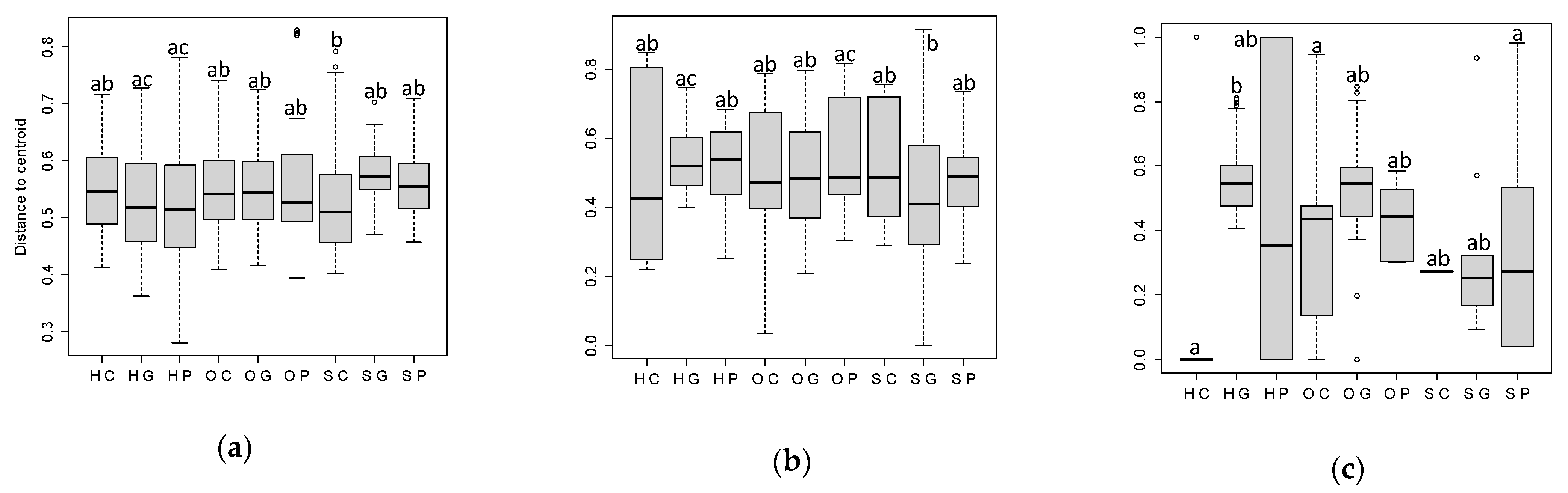
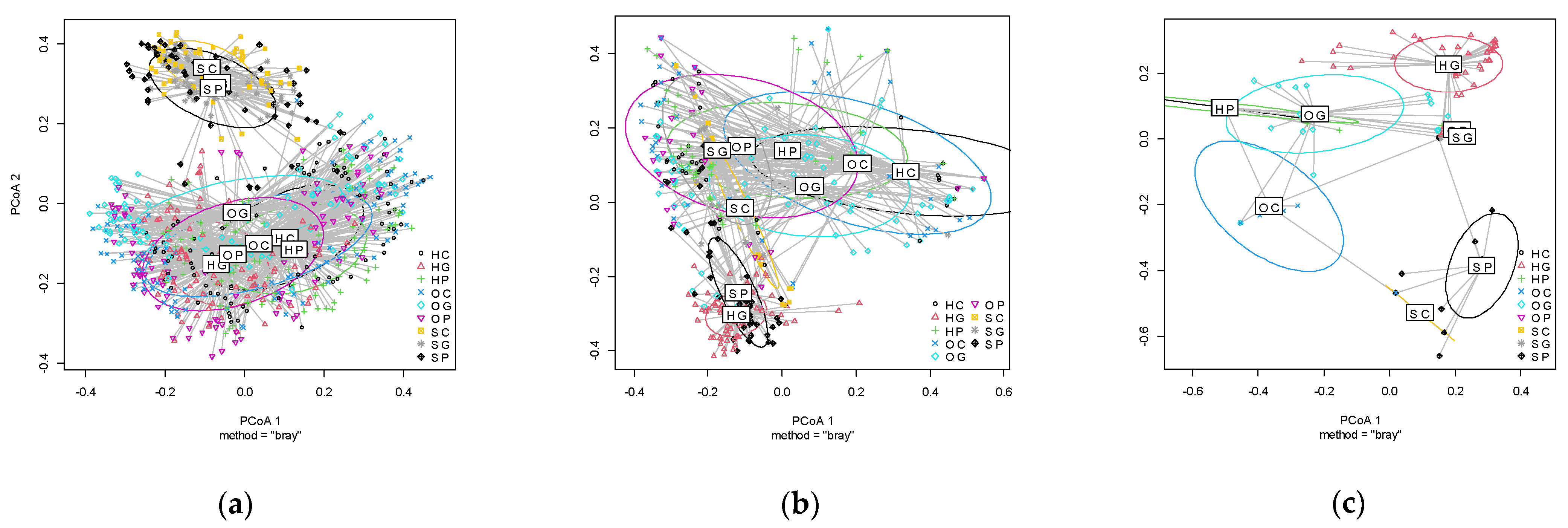
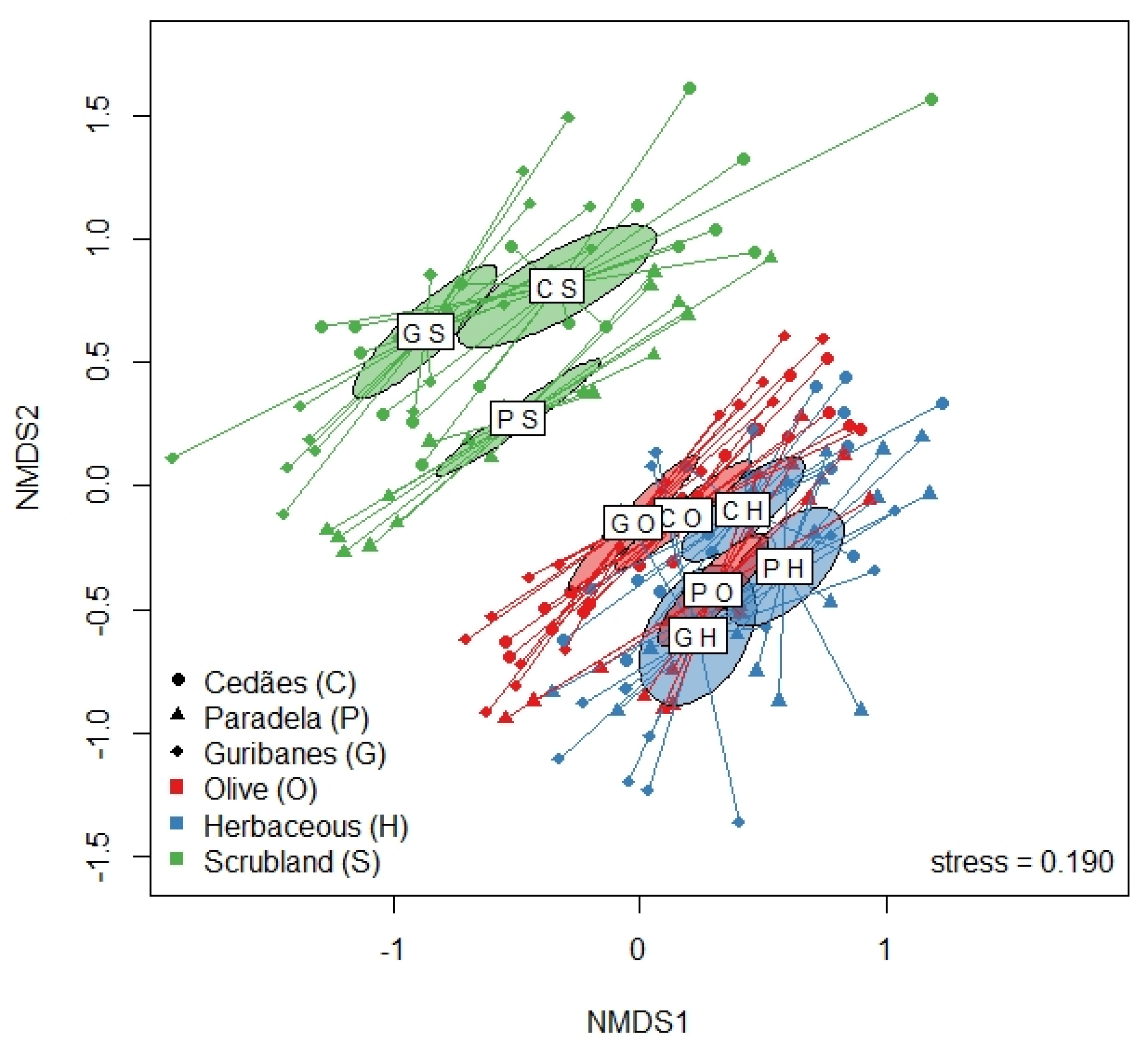
2.3.2. Beta Diversity
3. Results
3.1. Alpha Diversity: Diversity Incides
3.2. Plants Communities in the Spring, Summer, and Autumn by Land Use and Location
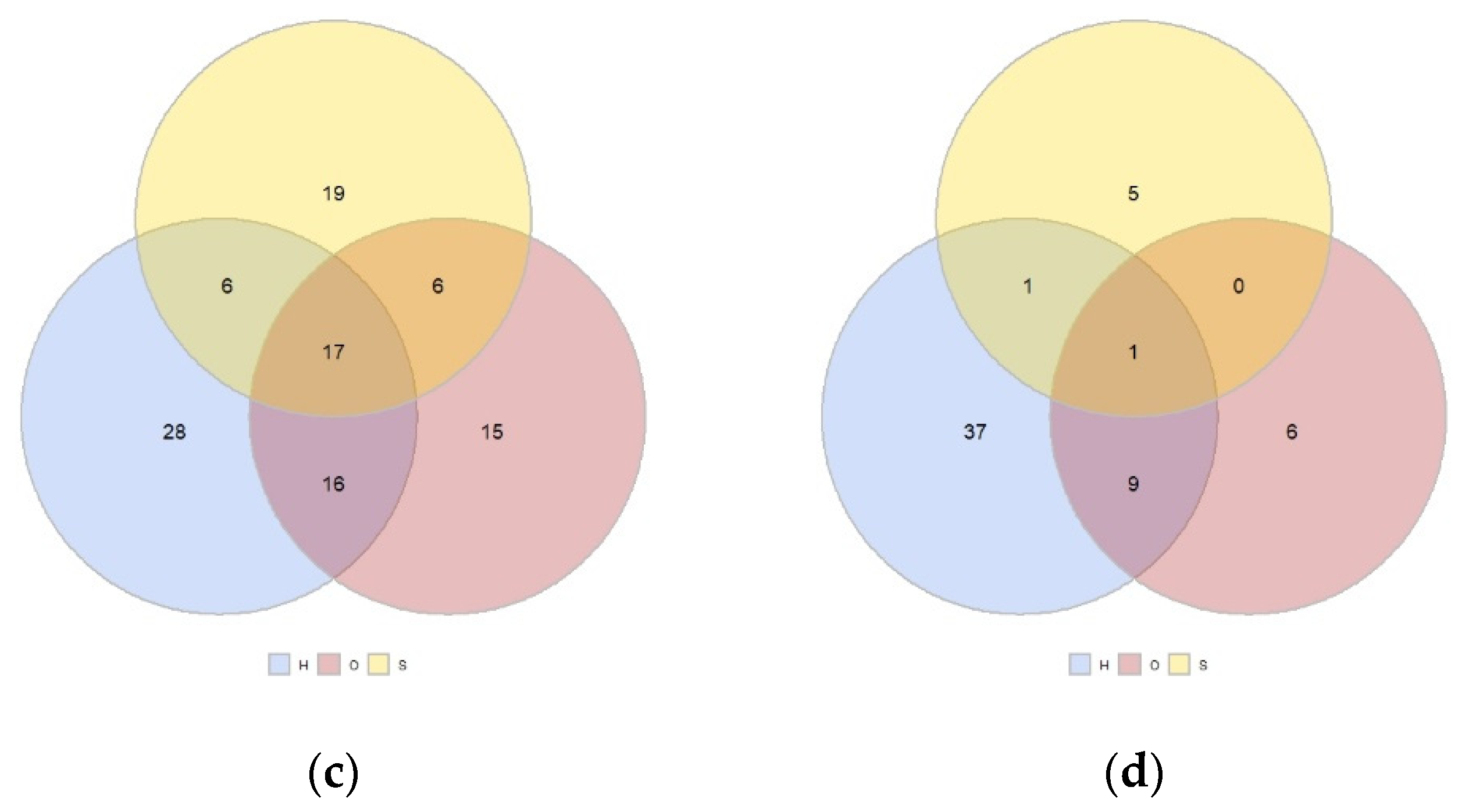
3.3. Simper Analysis: Species Divring the Differences among Land Uses and Sites and Most Abundant Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Landis, D.L.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: The role of native plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Wäckers, F.; van Rijn, P.C.J. Pick and mix: Selecting flowering plants to meet the requirements of target biological control insects. In Biodiversity and Insect Pests: Key Issues for Sustainable Management, 1st ed.; Gurr, G.M., Wratten, S.D., Snyder, W.E., Read, D.M.Y., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 139–165. [Google Scholar]
- Alcalá Herrera, R.; Ruano, F.; Gálvez Ramírez, C.; Frischie, S.; Campos, M. Attraction of green lacewings (Neuroptera: Chrysopidae) to native plants used as ground cover in woody Mediterranean agroecosystems. Biol. Control 2019, 139, 104066. [Google Scholar] [CrossRef] [Green Version]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A review of Bactrocera oleae (Rossi) impact in olive products: From the tree to the table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Villa, M.; Santos, S.A.P.; Marrão, R.; Pinheiro, L.A.; López-Saez, J.A.; Mexia, A.; Bento, A.; Pereira, J.A. Syrphids feed on multiple patches in heterogeneous agricultural landscapes during the autumn season, a period of food scarcity. Agric. Ecosyst. Environ. 2016, 233, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.; Santos, S.A.P.; Mexia, A.; Bento, A.; Pereira, J.A. Ground cover management affects parasitism of Prays oleae (Bernard). Biol. Control 2016, 96, 72–77. [Google Scholar] [CrossRef]
- Villa, M.; Marrão, R.; Mexia, A.; Bento, A.; Pereira, J.A. Are wild flowers and insect honeydews potential food resources for adults of the olive moth, Prays oleae? J. Pest Sci. 2016, 90, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.; Somavilla, I.; Santos, S.A.P.; López-Sáez, J.A.; Pereira, J.A. Pollen feeding habits of Chrysoperla carnea s.l. adults in the olive grove agroecosystem. Agric. Ecosyst. Environ. 2019, 283, 106573. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.; Santos, S.A.P.; Sousa, J.P.; Ferreira, A.; Martins da Silva, P.; Patanita, I.; Ortega, M.; Pascual, S.; Pereira, J.A. Landscape composition and configuration affect the abundance of the olive moth (Prays oleae, Bernard) in olive grove. Agric. Ecosyst. Environ. 2020, 294, 106854. [Google Scholar] [CrossRef]
- Paredes, D.; Cayuela, L.; Campos, M. Synergistic effects of ground cover and adjacent vegetation on natural enemies of olive insect pests. Agric. Ecosyst. Environ. 2013, 173, 72–80. [Google Scholar] [CrossRef]
- Gómez, J.A.; Campos, M.; Guzmán, G.; Castillo-Llanque, F.; Vanwalleghem, T.; Lora, A.; Giráldez, J.V. Soil erosion control, plant diversity, and arthropod communities under heterogeneous cover crops in an olive orchard. Environ. Sci. Pollut. Res. 2018, 25, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, H.A.; Morente, M.; Campos, M.; Ruano, F. La madurez de las cubiertas vegetales aumenta la presencia de enemigos naturales y la resiliencia de la red trófica de la copa del olivo. Ecosistemas 2019, 28, 92–106. [Google Scholar] [CrossRef] [Green Version]
- Ortega, M.; Sánchez-Ramos, I.; González-Núñez, M.; Pascual, S. Time course study of Bactrocera oleae (Diptera: Tephritidae) pupae predation in soil: The effect of landscape structure and soil condition. Agric. Forest Entomol. 2018, 20, 201–207. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Community Sampling: The Relevé Method. Aims and Methods of Vegetation Ecology; John Wiley and Sons: Hoboken, NJ, USA, 1974; pp. 45–66. [Google Scholar]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 November 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 November 2020).
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-78. 2020. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 1 November 2020).
- Nelder, J.A. A reformulation of linear models. J. R. Stat. Soc. Ser. A Ger. 1977, 140, 48–77. [Google Scholar] [CrossRef]
- Álvarez, H.A.; Morente, M.; Shigeo, F.; Rodríguez, E.; Campos, M.; Ruano, F. Semi-natural habitat complexity affects abundance and movement of natural enemies in organic olive orchards. Agric. Ecosyst. Environ. 2019, 285, 106618. [Google Scholar] [CrossRef]
- Villa, M.; Rodrigues, I.; Baptista, P.; Fereres, A.; Pereira, J.A. Populations and host/non-host plants of spittlebugs nymphs in olive orchards from northeastern Portugal. Insects 2020, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Plazio, E.; Altamura, G.; Tauru, D.; Gilioli, G.; et al. Spittlebugs of Mediterranean olive groves: Host-plant exploitation throughout the year. Insects 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Spring | Summer | Autumn | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Biodiversity Indexes | Olive | Herb | Scrub | Olive | Herb | Scrub | Olive | Herb | Scrub |
| Richness | 109 | 97 | 105 | 40 | 53 | 38 | 14 | 35 | 6 |
| Pielou | 0.749 | 0.644 | 0.664 | 0.593 | 0.642 | 0.718 | 0.605 | 0.624 | 0.150 |
| Shannon | 3.514 | 2.946 | 3.089 | 2.188 | 2.548 | 2.612 | 1.596 | 2.217 | 0.269 |
| Simpson | 0.957 | 0.917 | 0.915 | 0.790 | 0.879 | 0.879 | 0.666 | 0.830 | 0.109 |
| df | SS | R2 | Pseudo-F | p-Value | ||
|---|---|---|---|---|---|---|
| Spring | Location | 2 | 12.525 | 0.044 | 20.479 | 0.001 |
| Land use | 2 | 34.441 | 0.122 | 56.313 | 0.001 | |
| Location: Land use | 4 | 21.601 | 0.076 | 17.660 | 0.001 | |
| Residual | 702 | 214.672 | 0.758 | |||
| Total | 710 | 283.239 | 1.000 | |||
| Summer | Location | 2 | 13.188 | 0.072 | 22.556 | 0.001 |
| Land use | 2 | 16.827 | 0.092 | 28.781 | 0.001 | |
| Location: Land use | 4 | 20.925 | 0.114 | 17.895 | 0.001 | |
| Residual | 452 | 132.134 | 0.722 | |||
| Total | 460 | 183.074 | 1.000 | |||
| Autumn | Location | 2 | 9.045 | 0.136 | 17.299 | 0.001 |
| Land use | 2 | 8.594 | 0.129 | 16.435 | 0.001 | |
| Location: Land use | 4 | 10.058 | 0.151 | 9.618 | 0.001 | |
| Residual | 149 | 38.955 | 0.584 | |||
| Total | 157 | 66.651 | 1.000 |
| Season | Site | Olive Orchard | Herbaceous Plot | Scrubland Plot |
|---|---|---|---|---|
| Spring | Cedães | Ornithopus compressus L. | Chamaemelum mixtum (L.) All. | Lavandula pedunculata (Mill.) Cav. |
| Guribanes | Crepis capillaris (L.) Wallr. | Trifolium michelianum Savi | L. pedunculata | |
| Paradela | Bunias erucago L. | Chrysanthemum segetum L. | L. pedunculata | |
| Summer | Cedães | Chondrilla juncea L. | Chondrilla juncea L. | Helichrysum stoechas (L.) Moench |
| Guribanes | Chondrilla juncea L. | Hypochaeris radicata L. | Centaurea aristata subsp. langeana (Arènes) Dostál | |
| Paradela | Andryala integrifolia L. | Tolpis barbata (L.) Gaertn. | Daphne gnidium L. | |
| Autumn | Cedães | Chondrilla juncea L. | Chondrilla juncea L. | Daphne gnidium L |
| Guribanes | Chondrilla juncea L. | Hypochaeris radicata L. | Arbutus unedo L. | |
| Paradela | Chenopodium album L. | Chondrilla juncea L. | Daphne gnidium L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, M.; Santos, S.A.P.; Aguiar, C.; Pereira, J.A. Plants Biodiversity in Olive Orchards and Surrounding Landscapes from a Conservation Biological Control Approach. Biol. Life Sci. Forum 2021, 4, 66. https://doi.org/10.3390/IECPS2020-08604
Villa M, Santos SAP, Aguiar C, Pereira JA. Plants Biodiversity in Olive Orchards and Surrounding Landscapes from a Conservation Biological Control Approach. Biology and Life Sciences Forum. 2021; 4(1):66. https://doi.org/10.3390/IECPS2020-08604
Chicago/Turabian StyleVilla, María, Sónia A. P. Santos, Carlos Aguiar, and José Alberto Pereira. 2021. "Plants Biodiversity in Olive Orchards and Surrounding Landscapes from a Conservation Biological Control Approach" Biology and Life Sciences Forum 4, no. 1: 66. https://doi.org/10.3390/IECPS2020-08604
APA StyleVilla, M., Santos, S. A. P., Aguiar, C., & Pereira, J. A. (2021). Plants Biodiversity in Olive Orchards and Surrounding Landscapes from a Conservation Biological Control Approach. Biology and Life Sciences Forum, 4(1), 66. https://doi.org/10.3390/IECPS2020-08604








