Influence of Cultivation Areas on the Seed-Borne Pathogens on Two Local Common Bean Ecotypes of “Fagioli di Sarconi” PGI (Phaseolus vulgaris L.) †
Abstract
:1. Introduction
2. Experiments
3. Results
3.1. Meteorological Parameters
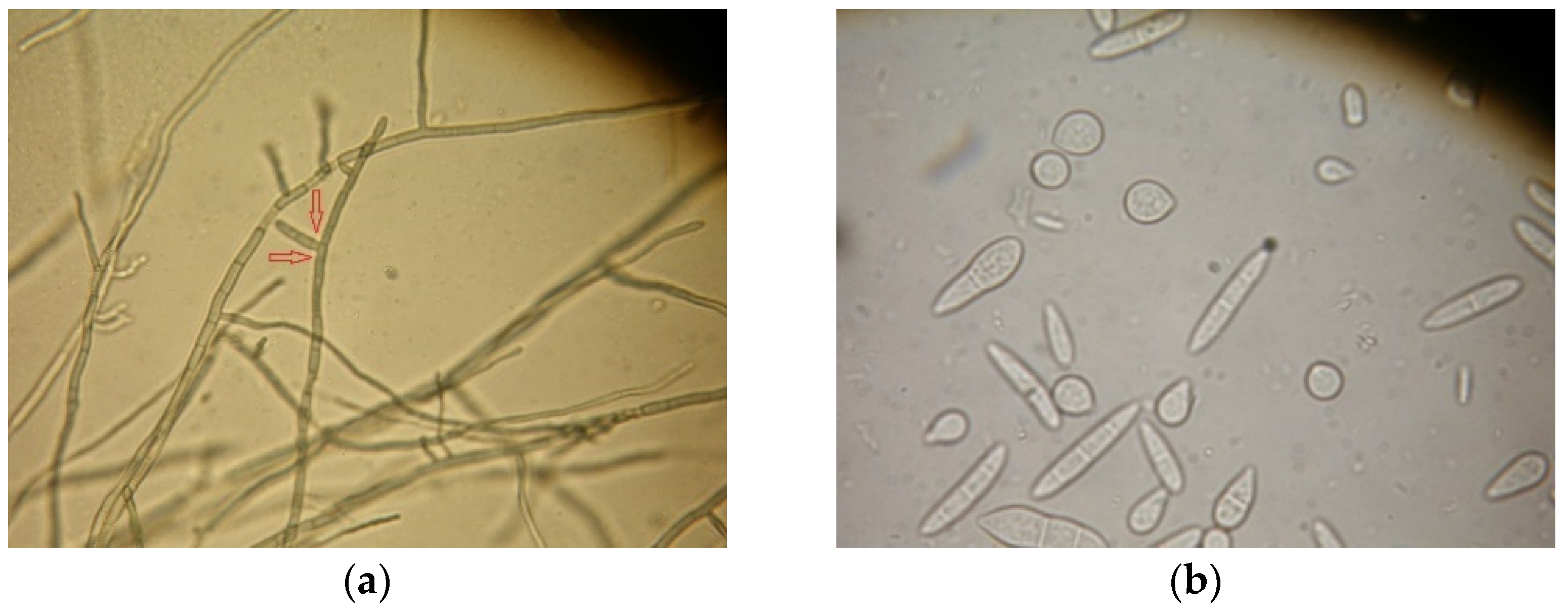
3.2. Isolation and Identification of Seed Mycoflora with Washing Test
3.3. Identification and Incidence of Rhizoctonia solani on Treated and Untreated Seeds with Blotter Test, and of Colletotrichum lindemuthianum, Fusarium oxysporum and Bacterial Disease with between Paper Test
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The Nutritional Content of Common Bean (Phaseolus vulgaris L.) Landraces in Comparison to Modern Varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Alvi, G. I Legumi da Granella: 2016 Anno Internazionale dei Legumi; Ministero delle Politiche Agricole e Forestali: Roma, Italy, 2016; pp. 1–2.
- Graham, B.R.; Ranalli, P. Common bean (Phaseolus vulgaris L.). Field Crop Res. 1997, 53, 131–146. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Laghetti, G. The common bean land-races from Basilicata (Southern Italy): An example of integrated approach applied to genetic resources management. Genet. Resour. Crop Evol. 1999, 46, 47–52. [Google Scholar] [CrossRef]
- Narayan Ghangaokar, M.; Adyodhya Kshirsagar, D. Study of seed borne fungi of different legumes. Trends Life Sci. 2013, 2, 32–35. [Google Scholar]
- Marcenaro, D.; Valkonen, J.P.T. Seedborne Pathogenic Fungi in Common Bean (Phaseolus vulgaris cv. INTA Rojo) in Nicaragua. PLoS ONE 2016, 11, e0168662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Cantore, P.; Nigro, C.; Castoro, V.; Iacobellis, N.S. Presenza e diffusione delle batteriosi in coltivazioni di fagiolo di Sarconi in Basilicata. J. Plant Pathol. 2004, 89, 43–44. [Google Scholar]
- International Rules for Tasting, Detection of Colletotrichum lindemuthianum in Phaseolus vulgaris (bean) seed. In Proceedings of the Including Changes and Editorial Corrections Adopted at the Ordinary General Meeting 2019, Hyderabad, India, 1 January 2020.
- Misra, J.K.; Merca, S.D.; Mew, T.W. A Manual of Rice Seed Healt Tasting; Mew, T.W., Misra, J.K., Eds.; IRRI, Los Banos: Laguna, CA, USA, 1994. [Google Scholar]
- Vannacci, G.; Sarrocco, S.; Porta-Puglia, A. Improved Detection and Monitoring of Seed-Borne Fungal Plant Pathogens in Europe. In Plant Pathology in the 21st Century: Global Perspectives on the Healthof Seeds and Plant Propagation Material; Gullino, M.L., Munkvold, G., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 6, pp. 67–85. [Google Scholar] [CrossRef]
- Kulshrestha, D.D.; Mathur, S.B.; Neergard, P. Identification of seed borne species of Colletotrichum. Friesia 1976, 11, 116–125. [Google Scholar]
- Miles, S.R. Handbook of tolerances and of measures of precision for seed testing. Proc. Int. Seed Test. Assoc. 1963, 28, 525–686. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and key TO Species), 3rd ed.; CRC Press: London, UK, 2010; p. 486. [Google Scholar] [CrossRef]
- Ainswoth, G.C. Introduction and keys to higher taxa. In The Fungi. An advanced Treatise IVB: A Taxonomic Review with Keys; Ainsworth, G.C., Sparrow, F.K., Sussman, A.S., Eds.; Academic Press: New York, NY, USA, 1973; pp. 1–7. [Google Scholar]
- Webster, J.; Weber, R. Introduction to Fungi, 3rd ed.; Cambridge University Press: London, UK, 2016; p. 841. [Google Scholar]
- Dragoni, I.; Cantoni, C.; Papa, A.; Vallone, L. Muffe, Alimenti e Micotossicosi; Città Studi Edizioni: Milano, Italy, 2000; p. 318. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T. Compendium os Soil Fungi; eIHW-Verlag: Braunschweig, Germany, 1993. [Google Scholar]
- Nipoti, P.; Fantino, M.G.; Filippini, G.; Gennari, S.; Di Pillo, L. Testo-Atlante dei Funghi ad Habitat Terricolo; Zanichelli: Bologna, Italy, 2006; p. 153. [Google Scholar]
- Available online: https://www.analystsoft.com/it/ (accessed on 22 April 2020).
- Available online: https://biology-assets.anu.edu.au/GenAlEx/Welcome.html (accessed on 22 April 2020).
- Kumar, R.; Gupta, A. Seed-Borne Disease of Agricultural Crops: Detection, Diagnosis e Management; Kumar, R., Gupta, A., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2020. [Google Scholar] [CrossRef]
- Vitti, A.; La Monaca, E.; Sofo, A.; Scopa, A.; Cuypers, A.; Nuzzaci, M. Beneficial effects of Trichoderma harzianum T-22 in tomato seedlings infected by Cucumber Mosaic Virus (CMV). Biocontrol 2015, 60, 135–147. [Google Scholar] [CrossRef]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 Induces Systemic Resistance in Tomato Infected by Cucumber mosaic virus. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Cett, I.; Lorito, M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, A.R.; Cerbino, D.; Brandi, M. The common bean populations from Basilicata (Southern Italy). An evaluation of their variation. Genet. Resour. Crop Evol. 2000, 47, 489–495. [Google Scholar] [CrossRef]
- Gatta, G.; Disciglio, G.; Libutti, A.; Giuliani, M.M.; Nardello, E. Caratterizzazione qualitativa del fagiolo da granella dei Monti Dauni. Italus Hortus 2010, 17, 98–100. [Google Scholar]
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.; Lucarini, M.; Carnovale, E. Solubility Digestibility relationship of legume proteins. J. Agric. Food Chem. 1997, 45, 3387–3394. [Google Scholar] [CrossRef]


| Meteorological Parameter | 2018 | 2019 | Pr (>F) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Range | Mean | Min | Max | Range | Mean | ||
| Rain (mm) | 0.00 | 38.60 | 38.60 | 2.09 | 0.00 | 56.40 | 56.40 | 1.69 | 0.530 |
| Temperature air (°C) | |||||||||
| Minimum | −4.30 | 19.80 | 24.10 | 10.03 | −0.50 | 16.50 | 17.00 | 9.58 | 0.301 |
| Maximum | 8.40 | 36.40 | 28.00 | 26.13 | 11.00 | 39.40 | 28.40 | 27.78 | 0.016 * |
| Average | 2.83 | 25.78 | 22.94 | 17.20 | 6.28 | 25.93 | 19.64 | 18.08 | 0.094 |
| Relative humidity (%) | |||||||||
| Minimum | 19.00 | 90.00 | 71.00 | 42.39 | 13.00 | 91.00 | 78.00 | 37.58 | 0.001 *** |
| Maximum | 67.80 | 100.00 | 32.20 | 96.96 | 82.00 | 99.80 | 17.80 | 96.96 | 0.998 |
| Average | 46.25 | 96.42 | 50.17 | 75.09 | 49.67 | 96.79 | 47.13 | 71.25 | 0.000 *** |
| Evapotranspiration (mm) | 0.76 | 8.13 | 7.37 | 4.44 | 0.90 | 8.08 | 7.18 | 4.87 | 0.049 * |
| Year | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ecotype | Ciuoto | Cannellino Rosso | Ciuoto | Cannellino Rosso | ||||
| Locality | Sarconi | Paterno | Sarconi | Paterno | Sarconi | Paterno | Sarconi | Paterno |
| Fungal microflora | ||||||||
| Alternaria sp. | + | − | − | − | − | + | + | − |
| Alternaria alternata | − | − | − | − | − | + | − | + |
| Aspergillus sp. | − | − | + | + | − | − | − | − |
| Aspergillus flavus | − | − | + | + | + | − | − | − |
| Aspergillus niger | + | − | − | − | − | − | + | − |
| Cladosporium cladosporioides | − | − | − | + | + | + | + | − |
| Botritys sp. | − | − | + | − | − | + | − | + |
| Colletotrichum lindemuthianum | − | + | − | − | − | + | − | − |
| Fusarium oxysporum | − | − | − | − | − | + | − | + |
| Fusarium solani | − | + | − | − | − | + | − | − |
| Mucor hiemalis | + | − | − | − | − | − | + | − |
| Penicillium sp. | − | − | + | + | − | + | + | + |
| Penicillium expansum | − | + | − | − | − | + | − | − |
| Rhizoctonia solani | − | + | − | + | + | + | + | + |
| Rhizophus nigricans | + | − | − | − | − | − | − | − |
| Trichoderma harzianum | - | − | − | + | − | − | − | − |
| Tricoderma viridae | + | − | − | − | − | − | − | − |
| Uromyces appendiculatus | − | − | − | − | − | + | − | + |
| Year | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ecotype | Ciuoto | Cannellino Rosso | Ciuoto | Cannellino Rosso | ||||
| Locality | Sarconi | Paterno | Sarconi | Paterno | Sarconi | Paterno | Sarconi | Paterno |
| BLOTTER METHOD | ||||||||
| Rhizoctonia solani | ||||||||
| treated seed | + | + | + | + | + | + | + | + |
| Disease incidence (%) 1 | 27 | 23 | 8 | 15 | 12 | 15 | 4 | 8 |
| untreated seed | + | + | + | + | + | + | + | + |
| Disease incidence (%) 1 | 19 | 35 | 24 | 27 | 11 | 23 | 12 | 24 |
| BETWEEN PAPER TEST | ||||||||
| Fusarium oxysporum | + | + | − | + | − | + | − | + |
| Disease incidence (%) 2 | 1 | 4 | 0 | 2 | 0 | 10 | 0 | 6 |
| C. lindemuthianum | + | + | + | + | + | + | + | + |
| Disease incidence (%) 2 | 4 | 40 | 8 | 20 | 4 | 65 | 4 | 30 |
| Bacterial disease | ||||||||
| P. syringae pv. phaseolicola | + | + | + | + | + | + | + | − |
| X. campestris pv. phaseoli | + | + | + | − | − | + | − | + |
| Disease incidence (%) 2 | 28 | 30 | 18 | 24 | 12 | 12 | 6 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bevilacqua, V.; Vitti, A.; Logozzo, G.; Marzario, S.; Gioia, T.; Nuzzaci, M. Influence of Cultivation Areas on the Seed-Borne Pathogens on Two Local Common Bean Ecotypes of “Fagioli di Sarconi” PGI (Phaseolus vulgaris L.). Biol. Life Sci. Forum 2021, 4, 57. https://doi.org/10.3390/IECPS2020-08593
Bevilacqua V, Vitti A, Logozzo G, Marzario S, Gioia T, Nuzzaci M. Influence of Cultivation Areas on the Seed-Borne Pathogens on Two Local Common Bean Ecotypes of “Fagioli di Sarconi” PGI (Phaseolus vulgaris L.). Biology and Life Sciences Forum. 2021; 4(1):57. https://doi.org/10.3390/IECPS2020-08593
Chicago/Turabian StyleBevilacqua, Vincenzo, Antonella Vitti, Giuseppina Logozzo, Stefania Marzario, Tania Gioia, and Maria Nuzzaci. 2021. "Influence of Cultivation Areas on the Seed-Borne Pathogens on Two Local Common Bean Ecotypes of “Fagioli di Sarconi” PGI (Phaseolus vulgaris L.)" Biology and Life Sciences Forum 4, no. 1: 57. https://doi.org/10.3390/IECPS2020-08593
APA StyleBevilacqua, V., Vitti, A., Logozzo, G., Marzario, S., Gioia, T., & Nuzzaci, M. (2021). Influence of Cultivation Areas on the Seed-Borne Pathogens on Two Local Common Bean Ecotypes of “Fagioli di Sarconi” PGI (Phaseolus vulgaris L.). Biology and Life Sciences Forum, 4(1), 57. https://doi.org/10.3390/IECPS2020-08593







