Phytophthora Diversity in a Sentinel Arboretum and in a Nature Reserve Area †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Areas
2.2. Sampling and Phytophthora Isolation
2.3. Morphological Characterization of Isolates
2.4. Molecular Identification of Isolates
2.5. Soil Analysis and USDA Classification
3. Results
3.1. Composition of Phytophthora Communities
3.2. Analysis of Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Scott, P.; Bader, M.K.F.; Burgess, T.; Hardy, G.; Williams, N. Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environ. Sci. Policy 2019, 101, 175–182. [Google Scholar] [CrossRef]
- Jung, T.; Scanu, B.; Brasier, C.M.; Webber, J.; Milenković, I.; Corcobado, T.; Tomšovský, M.; Pánek, M.; Bakonyi, J.; Maia, C.; et al. A survey in natural forest ecosystems of vietnam reveals high diversity of both new and described Phytophthora taxa including P. ramorum. Forests 2020, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society (APS Press): St. Paul, MN, USA, 1996. [Google Scholar]
- Hansen, E.M.; Reeser, P.W.; Sutton, W. Phytophthora Beyond Agriculture. Annu. Rev. Phytopathol. 2012, 50, 359–378. [Google Scholar] [CrossRef]
- Jung, T. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. For. Pathol. 2009, 39, 73–94. [Google Scholar] [CrossRef]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Jung, M.H.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Pers. Mol. Phylogeny Evol. Fungi 2018, 40, 182–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scibetta, S.; Schena, L.; Abdelfattah, A.; Pangallo, S.; Cacciola, S.O. Selection and experimental evaluation of universal primers to study the fungal microbiome of higher plants. Phytobiomes J. 2018, 2, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Frisullo, S.; Lima, G.; Magnano di San Lio, G.; Camele, I.; Melissano, L.; Puglisi, I.; Pane, A.; Agosteo, G.E.; Prudente, L.; Cacciola, S.O. Phytophthora cinnamomi Involved in the decline of holm oak (Quercus ilex) Stands in Southern Italy. For. Sci. 2018, 64, 290–298. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H.; Oßwald, W. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 2000, 49, 706–718. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Gumpertz, M.L. New frontiers in the study of dispersal and spatial analysis of epidemics caused by species in the Genus Phytophthora. Annu. Rev. Phytopathol. 2000, 38, 541–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biasi, A.; Martin, F.N.; Cacciola, S.O.; di San Lio, G.M.; Grünwald, N.J.; Schena, L. Genetic Analysis of Phytophthora nicotianae Populations from different hosts using microsatellite Markers. Phytopathology 2016, 106, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammella, M.A.; Cacciola, S.O.; Martin, F.; Schena, L. Genetic characterization of Phytophthora nicotianae by the analysis of polymorphic regions of the mitochondrial DNA. Fungal Biol. 2011, 115, 432–442. [Google Scholar] [CrossRef] [PubMed]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O.; La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma counteracts the challenge of Phytophthora nicotianae infections on tomato by modulating plant defense mechanisms and the expression of crinkler, necrosis-inducing phytophthora protein 1, and cellulose-binding elicitor lectin pathogenic effectors. Front. Plant Sci. 2020, 11, 1653. [Google Scholar]
- Pane, A.; Faedda, R.; Granata, G.; Puglisi, I.; Aloi, F.; La Spada, F.; Evoli, M.; Stracquadanio, C.; Cacciola, S.O. First report of root and basal stem rot caused by Phytophthora cryptogea and P. inundata on dwarf banana in Italy. Plant Dis. 2018, 102, 684. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.S.J.; Barber, P.A.; Alvarado, P.; Barnes, C.W.; Buchanan, P.K.; Heykoop, M.; Moreno, G.; et al. Fungal Planet description sheets: 558–624. Persoonia 2017, 38, 240. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, I.; De Patrizio, A.; Schena, L.; Jung, T.; Evoli, M.; Pane, A.; Van Hoa, N.; Van Tri, M.; Wright, S.; Ramstedt, M.; et al. Two previously unknown Phytophthora species associated with brown rot of Pomelo (Citrus grandis) fruits in Vietnam. PLoS ONE 2017, 12, e0172085. [Google Scholar] [CrossRef] [Green Version]
- Balcì, Y.; Halmschlager, E. Phytophthora species in oak ecosystems in Turkey and their association with declining oak trees. Plant Pathol. 2003, 52, 694–702. [Google Scholar] [CrossRef]
- Balci, Y.; Balci, S.; Mac Donald, W.L.; Gottschalk, K.W. Pathogenicity of Phytophthora species isolated from rhizosphere soil in the eastern United States. In Proceedings of the Sudden Oak Death Third Science Symposium, Monterey, CA, USA, 15–18 December 2002; Frankel, S.J., Kliejunas, J.T., Palmieri, K.M., Eds.; Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2008; pp. 225–226. [Google Scholar]
- Cooke, D.E.L.; Schena, L.; Cacciola, S.O. Tools to detect, identify and monitor Phytophthora species in natural ecosystems. J. Plant Pathol. 2007, 89, 13–28. [Google Scholar]
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.J.; White, D.; Paap, T.; Dunstan, W.A.; Burgess, T.I. Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: Evolutionary and ecological implications. Persoonia 2011, 26, 13–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sierra, A.; López-García, C.; León, M.; García-Jiménez, J.; Abad-Campos, P.; Jung, T. Previously unrecorded low-temperature Phytophthora species associated with Quercus decline in a Mediterranean forest in eastern Spain. For. Pathol. 2013, 43, 331–339. [Google Scholar] [CrossRef]
- Rea, A.J.; Burgess, T.I.; Hardy, G.E.S.J.; Stukely, M.J.C.; Jung, T. Two novel and potentially endemic species of Phytophthora associated with episodic dieback of Kwongan vegetation in the south-west of Western Australia. Plant Pathol. 2011, 60, 1055–1068. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Schena, L.; Agosteo, G.E.; Magnano di San Lio, G.; Cacciola, S.O. Phytophthora oleae sp. nov. causing fruit rot of olive in southern Italy. Plant Pathol. 2018, 67, 1362–1373. [Google Scholar] [CrossRef]
- Riolo, M.; Aloi, F.; La Spada, F.; Sciandrello, S.; Moricca, S.; Santilli, E.; Pane, A.; Cacciola, S.O. Diversity of Phytophthora communities across different types of mediterranean vegetation in a nature reserve area. Forests 2020, 11, 853. [Google Scholar] [CrossRef]
- Santilli, E.; Riolo, M.; La Spada, F.; Pane, A.; Cacciola, S.O. First report of root rot caused by Phytophthora bilorbang on Olea europaea in Italy. Plants 2020, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Scanu, B.; Linaldeddu, B.T.; Deidda, A.; Jung, T. Diversity of Phytophthora species from declining mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 2015, 10, e0143234. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef] [Green Version]
- Prigigallo, M.I.; Mosca, S.; Cacciola, S.O.; Cooke, D.E.L.; Schena, L. Molecular analysis of Phytophthora diversity in nursery-grown ornamental and fruit plants. Plant Pathol. 2015, 64, 1308–1319. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linaldeddu, B.T.; Scanu, B.; Maddau, L.; Franceschini, A. Diplodia corticola and Phytophthora cinnamomi: The main pathogens involved in holm oak decline on Caprera Island (Italy). For. Pathol. 2014, 44, 191–200. [Google Scholar] [CrossRef]
- Simamora, A.V.; Paap, T.; Howard, K.; Stukely, M.J.C.; Hardy, G.E.S.J.; Burgess, T.I. Phytophthora contamination in a nursery and its potential dispersal into the natural environment. Plant Dis. 2018, 102, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, L.; Tjosvold, S.; Chambers, D.; Garbelotto, M. Control of Phytophthora species in plant stock for habitat restoration through best management practices. Plant Pathol. 2019, 68, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Sims, L.L.; Chee, C.; Bourret, T.; Hunter, S.; Garbelotto, M. Genetic and phenotypic variation of Phytophthora crassamura isolates from California nurseries and restoration sites. Fungal Biol. 2019, 123, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Pane, A.; Cacciola, S.O.; Scibetta, S.; Bentivenga, G.; Di San Lio, G.M. Four Phytophthora species causing foot and root rot of apricot in Italy. Plant Dis. 2009, 93, 844. [Google Scholar] [CrossRef]
- Rizza, C.; Faedda, R.; Pane, A.; Cacciola, S.O. First report of root and basal stem rot caused by Phytophthora nicotianae on tree aeonium (Aeonium arboreum) in Italy. Plant Dis. 2011, 95, 362. [Google Scholar] [CrossRef] [PubMed]
- Saville, A.C.; La Spada, F.; Faedda, R.; Migheli, Q.; Scanu, B.; Ermacora, P.; Gilardi, G.; Lenzi, N.; Testa, A.; Bechir Allagui, M.; et al. Population structure of Phytophthora infestans collected on potato and tomato in Italy. Plant Pathol. 2021, 70, 898–911. [Google Scholar] [CrossRef]
- Cacciola, S.O.; Gullino, M.L. Emerging and re-emerging fungus and oomycete soil-borne plant diseases in Italy. Phytopathol. Mediterr. 2019, 58, 451–472. [Google Scholar]
- Cacciola, S.O.; Agosteo, G.E.; Magnano di San Lio, G. Collar and root rot of olive trees caused by Phytophthora megasperma in Sicily. Plant Dis. 2001, 86, 96. [Google Scholar] [CrossRef]
- Cacciola, S.O.; Pane, A.; Raudino, F.; Davino, S. First report of root and crown rot of sage caused by Phytophthora cryptogea in Italy. Plant Dis. 2002, 86, 1176. [Google Scholar] [CrossRef]
- Cacciola, S.O.; Chimento, A.; Pane, A.; Cooke, D.E.L.; Magnano di San Lio, G. Root and foot rot of lantana caused by Phytophthora cryptogea. Plant Dis. 2005, 89, 909. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, S.O.; Pane, A.; Cooke, D.E.L.; Raudino, F.; Magnano di San Lio, G. First report of brown rot and wilt of fennel caused by Phytophthora megasperma in Italy. Plant Dis. 2006, 90, 110. [Google Scholar] [CrossRef]
- Davino, S.; Cacciola, S.O.; Pennisi, A.M.; Li Destri Nicosia, M.G. Phytophthora palmivora a new pathogen of lavender in Italy. Plant Dis. 2002, 86, 561. [Google Scholar] [CrossRef] [PubMed]
- Faedda, R.; Cacciola, S.O.; Pane, A.; Szigethy, A.; Bakonyi, J.; Man in’t Veld, W.A.; Martini, P.; Schena, L.; di San Lio, G.M. Phytophthora × pelgrandis causes root and collar rot of Lavandula stoechas in Italy. Plant Dis. 2013, 97, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pane, A.; Agosteo, G.E.; Cacciola, S.O. Phytophthora species causing crown and root rot of tomato in southern Italy. EPPO Bull. 2000, 30, 251–255. [Google Scholar] [CrossRef]
- Jung, T.; La Spada, F.; Pane, A.; Aloi, F.; Evoli, M.; Jung, M.H.; Scanu, B.; Faedda, R.; Rizza, C.; Puglisi, I.; et al. Diversity and distribution of Phytophthora species in protected natural areas in Sicily. Forests 2019, 10, 259. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.; Blaschke, H.; Neumann, P. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. Eur. J. Plant Pathol. 1996, 26, 253–272. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Pérez-Sierra, A.; Jung, T.; Solla, A. First report of Phytophthora gonapodyides involved in the decline of Quercus ilex in xeric conditions in Spain. New Dis. Rep. 2010, 22, 33. [Google Scholar] [CrossRef] [Green Version]
- Cooke, D.E.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol 2000, 30, 17–32. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- FinchTV v.1.4.0. Available online: https://digitalworldbiology.com/FinchTV (accessed on 18 May 2020).
- BLAST Searches. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 18 May 2020).
- Phytophthora Database. Available online: http://www.phytophthoradb.org/ (accessed on 18 May 2020).
- Ditzler, C.; Scheffe, K.; Monger, H.C. Soil Science Division Staff Soil Survey Manual. In Agriculture Handbook; US Department of Agriculture: Washington, DC, USA, 2017. [Google Scholar]
- Jung, T.; Jung, M.H.; Scanu, B.; Seress, D.; Kovács, G.M.; Maia, C.; Pérez-Sierra, A.; Chang, T.T.; Chandelier, A.; Heungens, K.; et al. Six new Phytophthora species from ITS Clade 7a including two sexually functional heterothallic hybrid species detected in natural ecosystems in Taiwan. Persoonia 2017, 38, 100–135. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.; Jung, M.H.; Cacciola, S.O.; Cech, T.; Bakonyi, J.; Seress, D.; Mosca, S.; Schena, L.; Seddaiu, S.; Pane, A.; et al. Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus 2017, 8, 219–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcobado, T.; Solla, A.; Madeira, M.A.; Moreno, G. Combined effects of soil properties and Phytophthora cinnamomi infections on Quercus ilex decline. Plant Soil 2013, 373, 403–413. [Google Scholar] [CrossRef]
- Burgess, T.I.; White, D.; McDougall, K.M.; Garnas, J.; Dunstan, W.A.; Català, S.; Carnegie, A.J.; Worboys, S.; Cahill, D.; Vettraino, A.M.; et al. Distribution and diversity of Phytophthora across Australia. Pac. Conserv. Biol. 2017, 23, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Bose, T.; Wingfield, M.J.; Roux, J.; Vivas, M.; Burgess, T.I. Community composition and distribution of Phytophthora species across adjacent native and non-native forests of South Africa. Fungal Ecol. 2018, 36, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Barber, P.A.; Paap, T.; Burgess, T.I.; Dunstan, W.; Hardy, G.E.S.J. A diverse range of Phytophthora species are associated with dying urban trees. Urban For. Urban Green. 2013, 12, 569–575. [Google Scholar] [CrossRef]
- Migliorini, D.; Khdiar, M.Y.; Padrón, C.R.; Vivas, M.; Barber, P.A.; Hardy, G.E.S.J.; Burgess, T.I. Extending the host range of Phytophthora multivora, a pathogen of woody plants in horticulture, nurseries, urban environments and natural ecosystems. Urban For. Urban Green. 2019, 46, 126460. [Google Scholar] [CrossRef]
- Paap, T.; Burgess, T.I.; Wingfield, M.J. Urban trees: Bridge-heads for forest pest invasions and sentinels for early detection. Biol. Invasions 2017, 19, 3515–3526. [Google Scholar] [CrossRef] [Green Version]
- Summerell, B.A.; Liew, E.C.Y. Phytophthora root rot: Its impact in botanic gardens and on threatened species conservation. Sibbaldia Int. J. Bot. Gard. Hortic. 2020, 18, 89–104. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Roques, A.; Yart, A.; Fan, J.T.; Sun, J.H.; Vannini, A. Sentinel trees as a tool to forecast invasions of alien plant pathogens. PLoS ONE 2015, 10, e0120571. [Google Scholar] [CrossRef] [PubMed]
- Vettraino, A.M.; Li, H.M.; Eschen, R.; Morales-Rodriguez, C.; Vannini, A. The sentinel tree nursery as an early warning system for pathway risk assessment: Fungal pathogens associated with Chinese woody plants commonly shipped to Europe. PLoS ONE 2017, 12, e0188800. [Google Scholar] [CrossRef] [Green Version]
- Prigigallo, M.I.; Abdelfattah, A.; Cacciola, S.O.; Faedda, R.; Sanzani, S.M.; Cooke, D.E.L.; Schena, L. Metabarcoding analysis of Phytophthora diversity using genus-specific primers and 454 pyrosequencing. Phytopathology 2016, 106, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Morales-Rodríguez, C.; Bastianelli, G.; Aleandri, M.; Tuğba Doğmuş-Lehtijärvi, H.; Oskay, F.; Vannini, A. Revealing novel interactions between oak and Tubakia species: Evidence of the efficacy of the sentinel arboreta strategy. Biol. Invasions 2021, 1–17. [Google Scholar] [CrossRef]
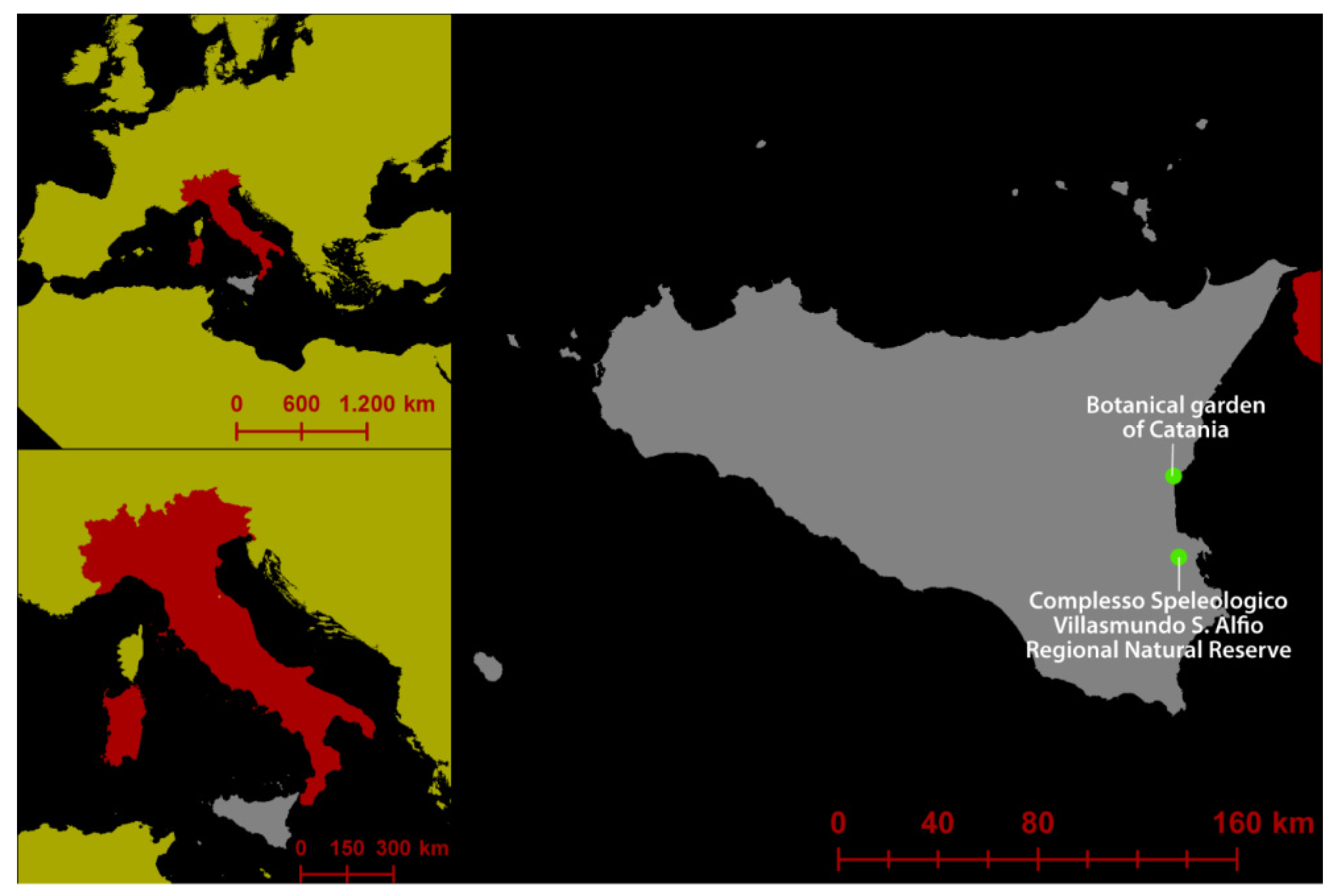
| Sampling Site | Rhizosphere Soil Sample ID | Location—Municipality and Geographic Coordinates (DATUM WGS84) | Plant Species |
|---|---|---|---|
| Complesso Speleologico Villasmundo S. Alfio Regional Nature Reserve | NR_1903_S1 | Melilli—37°13′17.54″ N; 15°6′19.52″ E | Salix pedicellata |
| NR_1903_S2 | Melilli—37°13′17.66″ N; 15°6′19.28″ E | S. pedicellata | |
| NR_1903_S3 | Melilli—37°13′17.753″ N; 15°6′18.93″ E | Platanus orientalis | |
| NR_1903_S4 | Melilli—37°13′17.86″ N; 15°6′18.81″ E | P. orientalis | |
| NR_1903_S5 | Melilli—37°13′17.25″ N; 15°6′15.30″ E | Euphorbia dendroides | |
| NR_1903_S6 | Melilli—37°13′17.48″ N; 15°6′15.31″ E | Cynara cardunculus | |
| NR_1903_S7 | Melilli—37°13′17.60″ N; 15°6′15.30″ E | Asphodelus sp. | |
| NR_1903_S8 | Melilli—37°13′11.75”N; 15° 6′1.20″ E | Quercus ilex | |
| NR_1903_S9 | Melilli—37°13′11.00″ N; 15°5′59.69″ E | Q. ilex | |
| NR_1903_S10 | Melilli—37°13′10.93″ N; 15°5′59.95″ E | Q. ilex | |
| NR_1903_S11 | Melilli—37°13′11.788″ N; 15°6′0.547″ E | Q. pubescens sensu latu | |
| NR_1903_S12 | Melilli—37°13′17.52″ N; 15°6′7.94″ E | Sarcopoterium spinosum | |
| NR_1903_S13 | Melilli—37°13′17.50″ N; 15°6′8.57″ E | S. spinosum | |
| NR_1903_S14 | Melilli—37°13′17.28″ N; 15°6′4.77″ E | Pistacia lentiscus | |
| NR_1903_S15 | Melilli—37°13′17.50″ N; 15°6′5.13″ E | P. lentiscus + Pyrus sp., mixed sample | |
| NR_1903_S16 | Melilli—37°13′16.94″ N; 15°6′7.66″ E | P. lentiscus | |
| NR_1903_S17 | Melilli—37°13′16.93″ N; 15°6′6.24″ E | P. lentiscus | |
| Botanical garden of Catania | BG_1903_S1 | Catania—37°30′57.29″ N; 15°5′2.27″ E | Araucaria cokii |
| BG_1903_S2 | Catania—37°30′55.92″ N; 15°5′1.95″ E | Phytolacca dioica | |
| BG_1903_S3 | Catania—37°30′55.08″ N; 15°4′59.75″ E | Grevillea robusta | |
| BG_1903_S4 | Catania—37°30′57.56″ N; 15°5′1.47″ E | Pistacia atlantica | |
| BG_1903_S5 | Catania—37°30′57.47″ N; 15°5′0.81″ E | Sterculia diversifolia | |
| BG_1903_S6 | Catania—37°30′57.69″ N; 15°5′1.80″ E | Eucalyptus citridora | |
| BG_1903_S7 | Catania—37°30′53.46″ N; 15°5′2.38″ E | Zelkowa sicula | |
| BG_1903_S8 | Catania—37°30′53.35″ N; 15°5′1.89″ E | Q. suber | |
| BG_1903_S9 | Catania—37°30′53.19″ N; 15°5′2.42″ E | Olea europea | |
| BG_1903_S10 | Catania—37°30′53.34″ N; 15°5′2.40″ E | Pistacia lentiscus | |
| BG_1903_S11 | Catania—37°30′57.92″ N; 15°5′0.74″ E | Coffea arabica | |
| BG_1903_S12 | Catania—37°30′57.95″ N; 15°5′0.86″ E | Mangifera indica |
| Sampling Site | Rhizosphere Soil Sample ID. | Host | Baited Phytophthora spp. a | Soil Properties | ||||
|---|---|---|---|---|---|---|---|---|
| pH | Electrical Conductivity at 25 °C (μS/cm) | Soil Texture | Nitrates (mg/kg) | Organic Matter (%) | ||||
| Complesso Speleologico Villasmundo S. Alfio Regional Nature Reserve | NR_1903_S1 | Salix pedicellata | PSC | 7.6 ± 0.1 | 1497.0 ± 49 | Sandy clay loam | 11.0 ± 1 | 6.5 ± 0.3 |
| NR_1903_S2 | S. pedicellata | CRY | 7.7 ± 0.1 | 938.0 ± 43 | Sandy clay loam | 1.6 ± 0.2 | 2.8 ± 0.1 | |
| NR_1903_S3 | Platanus orientalis | - | 7.0 ± 0.1 | 913.0 ± 43 | Sandy clay | 7.1 ± 0.7 | 4.9 ± 0.2 | |
| NR_1903_S4 | P. orientalis | BIL | 7.1 ± 0.1 | 1023.0 ± 44 | Sandy clay loam | 6.9 ± 0.7 | 5.4 ± 0.3 | |
| NR_1903_S5 | Euphorbia dendroides | - | 7.3 ± 0.1 | 976.0 ± 44 | Sandy clay | 5.9 ± 0.6 | 7.1 ± 0.4 | |
| NR_1903_S6 | Cynara cardunculus | - | 7.5 ± 0.1 | 822.0 ± 41 | Sandy clay | 7.3 ± 0.7 | 5.5 ± 0.3 | |
| NR_1903_S7 | Asphodelus sp. | - | 7.5 ± 0.1 | 1122.0 ± 45 | Sandy clay | 7.2 ± 0.7 | 5.4 ± 0.3 | |
| NR_1903_S8 | Quercus ilex | GON | 7.3 ± 0.1 | 1463.0 ± 48 | Clay loam | 13.0 ± 1 | 13.1 ± 0.7 | |
| NR_1903_S9 | Q. ilex | PLU | 7.4 ± 0.1 | 1617.0 ± 53 | Loamy sand | 17.0 ± 2 | 21.0 ± 1 | |
| NR_1903_S10 | Q. ilex | - | 7.6 ± 0.1 | 1397.0 ± 46 | Sandy loam | 11.0 ± 1 | 16.3 ± 0.8 | |
| NR_1903_S11 | Q. pubescens sensu latu | GON | 7.2 ± 0.1 | 1174.0 ± 45 | Clay loam | 11.0 ± 1 | 11.4 ± 0.6 | |
| NR_1903_S12 | Sarcopoterium spinosum | - | 7.2 ± 0.1 | 922.0 ± 42 | Sandy clay | 6.1 ± 0.5 | 5.1 ± 0.2 | |
| NR_1903_S13 | S. spinosum | - | 7.3 ± 0.1 | 1102.0 ± 49 | Sandy clay | 7.1 ± 0.7 | 4.2 ± 0.1 | |
| NR_1903_S14 | Pistacia lentiscus | - | 7.4 ± 0.1 | 831.0 ± 41 | Sandy clay | 6.7 ± 0.7 | 8.2 ± 0.4 | |
| NR_1903_S15 | P. lentiscus + Pyrus sp., mixed sample | - | 7.2 ± 0.1 | 856.0 ± 43 | sandy clay loam | 5.3 ± 0.7 | 7.2 ± 0.2 | |
| NR_1903_S16 | P. lentiscus | - | 7.3 ± 0.1 | 796.0 ± 41 | Sandy clay | 1.7 ± 0.2 | 7.7 ± 0.4 | |
| NR_1903_S17 | P. lentiscus | - | 7.3 ± 0.1 | 1056.0 ± 44 | Sandy clay | 3.6 ± 0.4 | 8.7 ± 0.4 | |
| Botanical garden of Catania (Arboretum) | BG_1903_S1 | Eucalyptus citridora | MUL, NIC | 7.99 ± 0.1 | 877.5 ± 48 | Loamy sand | 145.3 ± 0.4 | 1.07 ± 0.2 |
| BG_1903_S2 | Araucaria cooki | MUL, NIC | 8.19 ± 0.1 | 3437.5 ± 46 | Sandy loam | 1210.9 ± 0.6 | 1.29 ± 0.1 | |
| BG_1903_S3 | Gravillea robusta | - | 8.14 ± 0.1 | 852.5 ± 40 | Loamy sand | 145.3 ± 0.6 | 0.86 ± 0.1 | |
| BG_1903_S4 | Phytolacca dioica | - | 8.26 ± 0.1 | 997.5 ± 43 | Sandy clay loam | 188.2 ± 0.7 | 1.01 ± 0.1 | |
| BG_1903_S5 | Pistacia atlantica | MUL | 7.43 ± 0.1 | 3945.0 ± 45 | Loamy sand | 1076.6 ± 0.1 | 1.49 ± 0.1 | |
| BG_1903_S6 | Quercus suber | - | 8.14 ± 0.1 | 765.0 ± 45 | Loamy sand | 45.3 ± 0.2 | 1.48 ± 0.2 | |
| BG_1903_S7 | Zelkova sicula | MUL | 8.55 ± 0.1 | 970.0 ± 44 | Sandy clay loam | 103.9 ± 0.8 | 0.73 ± 0.1 | |
| BG_1903_S8 | Sterculia diversifolia | MUL, NIC | 8.10 ± 0.1 | 1675.0 ± 48 | Clay loam | 1366.6 ± 1.0 | 0.11 ± 0.05 | |
| BG_1903_S9 | Mangifera indica | MUL, NIC | 8.60 ± 0.1 | 765.0 ± 41 | Clay loam | 176.7 ± 0.4 | 1.0 ± 0.1 | |
| BG_1903_S10 | Olea europaea | - | 7.10 ± 0.1 | 1540.0 ± 43 | Sandy clay loam | 55.3 ± 0.4 | 0.66 ± 0.1 | |
| BG_1903_S11 | Pistacia lentiscus | PAR | 8.64 ± 0.1 | 867.5 ± 46 | Sandy loam | 148.2 ± 0.5 | 0.99 ± 0.1 | |
| BG_1903_S12 | Coffea arabica | - | 8.40 ± 0.1 | 677.5 ± 50 | Loamy sand | 41.0 ± 0.3 | 0.82 ± 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riolo, M.; La Spada, F.; Aloi, F.; Giusso del Galdo, G.; Santilli, E.; Pane, A.; Cacciola, S.O. Phytophthora Diversity in a Sentinel Arboretum and in a Nature Reserve Area. Biol. Life Sci. Forum 2021, 4, 51. https://doi.org/10.3390/IECPS2020-08748
Riolo M, La Spada F, Aloi F, Giusso del Galdo G, Santilli E, Pane A, Cacciola SO. Phytophthora Diversity in a Sentinel Arboretum and in a Nature Reserve Area. Biology and Life Sciences Forum. 2021; 4(1):51. https://doi.org/10.3390/IECPS2020-08748
Chicago/Turabian StyleRiolo, Mario, Federico La Spada, Francesco Aloi, Giampietro Giusso del Galdo, Elena Santilli, Antonella Pane, and Santa Olga Cacciola. 2021. "Phytophthora Diversity in a Sentinel Arboretum and in a Nature Reserve Area" Biology and Life Sciences Forum 4, no. 1: 51. https://doi.org/10.3390/IECPS2020-08748
APA StyleRiolo, M., La Spada, F., Aloi, F., Giusso del Galdo, G., Santilli, E., Pane, A., & Cacciola, S. O. (2021). Phytophthora Diversity in a Sentinel Arboretum and in a Nature Reserve Area. Biology and Life Sciences Forum, 4(1), 51. https://doi.org/10.3390/IECPS2020-08748







