1. Introduction
The introduction of vegetable crops with a high content of biologically active compounds into agriculture remains a relevant task. Sweet potato (
Ipomoea batatas) is the seventh most important food crop grown in many countries. Its root tubers are rich in sugars, which determine its taste, as well as in proteins, B vitamins, ascorbic acid, carotenoids, and macro- and microelements [
1]. Sweet potato is vegetatively propagated, and a consistent supply of virus-free planting material is critical for sustainable production. Cryotherapy is a promising procedure for eliminating pathogens from infected plant tissue. Cryopreservation is widely used for the long-term preservation of plant genetic resources. The standard procedure usually involves cutting out the shoot tips, immersing them into liquid nitrogen and, after thawing and post-cultivation, regenerating into plants. Recently, cryotherapy has been demonstrated to eradicate seven unrelated groups of viruses and two types of bacteria-like pathogens from several species of economic importance, i.e.,
Prunus,
Musa spp.,
Vitis vinífera,
Fragaria ananassa,
Solanum tuberosum,
Rubus idaeus,
Ipomea batatas,
Dioscorea opposite, and
Allium sativum [
2,
3]. For cryotherapy of plants, cooling conditions must allow survival of the cells only in apical dome and in the youngest (1st–2nd) leaves’ primordia. The basal part of the apical dome and more advanced leaves’ primordia are to be killed by liquid nitrogen (LN) as far as these cells are generally infected by plant pathogens, in particular viruses [
3]. Shoot tips used for cryotherapy can thus be relatively large; they can show relatively high regenerative capacity [
2]. Based on previously published data, cryotherapy can be used as a proper and easily implemented approach for eradicating various pathogens from plant tissue, and can replace traditional methodologies [
2]. Cryotherapy has a number of advantages compared to traditional approaches, i.e., simplicity and time efficiency, ability to treat simultaneously a large number of samples, low cost, and high frequency of plants free from viruses after recovery [
2].
The greatest challenge for the wider application of cryotherapy is that different genotypes of the same species may differently respond to cryotherapy [
3,
4,
5]. Several protocols with different cryotherapy methods have been reported and the results differ between laboratories, with varying levels of survival and regeneration [
2]. Since the cryotherapy is impossible without the effective techniques of cryopreservation, in this work, we compared efficacy of different cryopreservation technique for sweet potatoes’ meristems (Admiral cultivar) preservation.
2. Experiments
Sterile explants were obtained from the shoots of sprouted Admiral cultivar sweet potato tubers. Parts of the stems were sterilized in 30% sodium hypochlorite solution for 25 min and washed 5 times with sterile distilled water. Then they were transferred to vials with agar nutrient medium Murashige and Skoog (MS) [
6], supplemented with 3% sucrose, 6% agar vitamins, 0.1 mg/L benzylaminopurine, 0.5 mg/L naphthylacetic acid, and 2.0 mg/L gibberellic acid. To obtain a sufficient number of samples, the in vitro shoots were divided into parts, each containing a stem with a leaf and a lateral bud. The cuttings were planted in vials with solid nutrient medium MS, supplemented with 3% sucrose, 6% agar, and 0.01 mg/L indoleacetic acid. The in vitro material was cultured at 20 ± 2 °C, with 16 h of light and 8 h of darkness and 2 kilolux light intensity. The explants were propagated by micro-grafting every 45 days.
For cryopreservation the apical and axillar meristems up to 1–2 mm were isolated from three-week in vitro cultured plants. The isolated samples were transferred into a liquid MS medium, supplemented with 12% sucrose and exposed at dark for 24 h. Prior to freezing the meristems were divided into the experimental groups. The first group contained meristems dehydrated for 120 min with sterile airflow (AD), frozen by plunging into LN on a needle tip, and re-warmed in MS medium, enriched with 12% sucrose. Other groups consisted of meristems incubated for 60 min at 22 °C with the following cryoprotectant solutions: (i) PVS 2 (30% glycerol + 15% ethylene glycol + 15% dimethyl sulfoxide and 0.4 M sucrose [
7]), (ii) 88% PVS 3 (44% glycerol + 44% sucrose [
8]), (iii) modified PVS 1 (22% glycerol + 13% 1,2-propylene glycol + 13% ethylene glycol + 6% dimethyl sulfoxide and 0.4 M sucrose [
9]), and (iv) PVS N (1 M sucrose + 15% glycerol + 14% ethylene glycol [
10]). The meristems of all groups after PVSs treatment were cooled in 1.8 mL cryovials («Corning», USA) or 50 µL hermetically sealed aluminum pans for differential scanning calorimetry (DSC) and were directly immersed into LN for 1 h. The specimens were re-warmed in water bath at 40 °C. The cryoprotectants were removed by two subsequent transfers of the meristems in fresh medium MS, enriched with 0.3 M sucrose. To examine the influence of the pretreatment steps on the explants the part of meristems were treated excluding low-temperature exposure. The meristems that were not dehydrated or cooling were selected as a control group. The post-thaw explants of all the groups were transferred into the agar MS medium, supplemented with 3% sucrose, 3 mg/L gibberellic acid, and 0.01 mg/L indoleacetic acid. The survival was determined by the number of meristems which had a green color for 30 days. In all experiments, 15–25 meristems were used per experimental condition and the experiments were replicated 3–4 times. The results were statistically analyzed using Software Past 3. The results are presented as arithmetic mean and standard deviation. For establishing statistical significance we used non-parametric Mann-Whitney criterion. The difference was considered statistically significant at
p < 0.05.
The phase and glass transitions of PVSs in the temperature range from −196 °C to complete media melting were investigated using low-temperature DSC [
11]. Glass transition temperature (Tg) was determined as a midpoint between the onset and endset of the inflectional tangent. Crystallization (Tc) and melting (Tm) temperatures were determined as an extrapolated onset-temperature (the designed point of intersection of the extrapolated baseline and the inflectional tangent at the beginning of the melting or crystallization peak). In this study, the samples were frozen by immersion into LN, with an average cooling rate of 200 degrees/min. The thermograms were recorded during warming with the rate of 0.5 degrees/min. The weight of all the investigated samples was 1 g.
3. Subsection
For successful cryopreservation, it is essential to avoid the lethal intracellular freezing that occurs during rapid cooling in LN. Thus, in any cryogenic procedure, the specimen must be sufficiently dehydrated to avoid freezing and to allow vitrification upon rapid cooling in LN. Currently, the most popular cryogenic procedures are «vitrification» and «encapsulation/dehydration» [
12]. The difference between the two methods is the method of dehydration. In «vitrification», cells are dehydrated with a highly concentrated cryoprotective solution. In «encapsulation/dehydration», air-drying is used; then, dehydrated samples can be vitrified by rapid cooling (immersion into LN).
For successful dehydration, additional pretreatment of the meristems is often carried out by preliminary cultivation on a nutrient medium. Sucrose preculture was determined to be necessary for the adaptation of sweet potato shoot tips to cryoprotection with PVS and highly significantly affected survival of sweet potato meristems after cryopreservation [
13]. In our studies, the meristems were preliminarily cultured in a nutrient medium MS with the addition of 12% sucrose and kept under dark conditions. After such an exposure, all the meristems formed plantlets during further cultivation with an MS medium.
An important indicator of successful cryopreservation is a high level of meristems preservation after pre-treatment before immersion into LN. It was shown that the survival rate meristems did not exceed 55% after treatment with 88% PVS 3. The preservation rates after PVS 2 and PVS N exposure were 83 and 85% respectively. The highest number of preserved specimens was noted after dehydration with airflow (89–95) and modified PVS 1 (88–94%). Since a decrease in the viability of the meristems was also observed in the control meristems (88%), most likely it was connected with their damage during isolation (
Figure 1).
The decrease in the number of viable meristems after exposure in 88% PVS 3 may be associated with the toxic effects of high concentrations of cryoprotectants or with osmotic reactions that lead to damage of specimens.
The meristems dehydrated with sterile airflow provided a preservation level about 85% after warming, which was not significantly different from non-cooling ones.
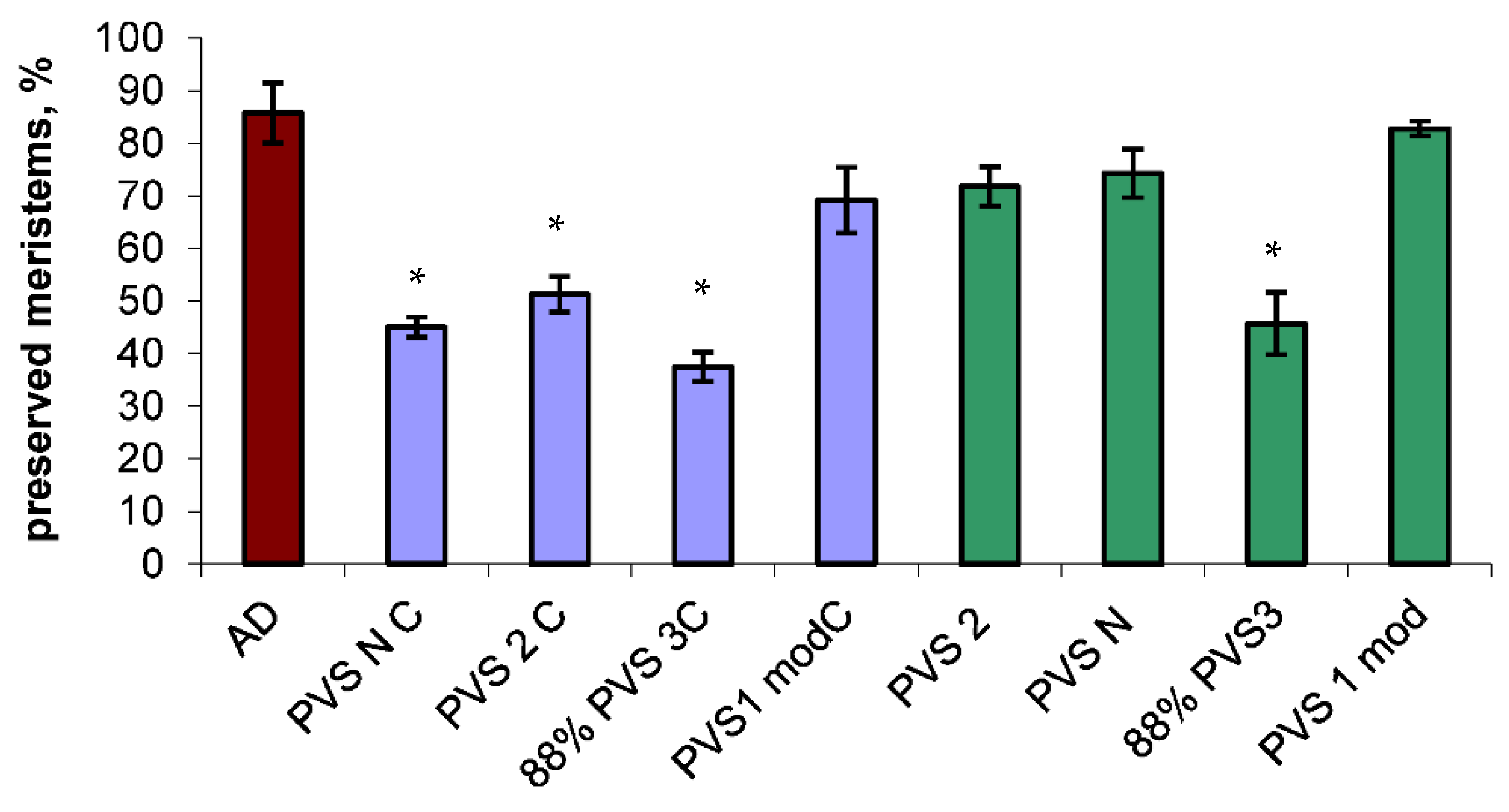
After cryopreservation in cryovials, the meristems survival rates of 35–75% were observed (
Figure 2). The highest post-thaw preservation was found in pretreatment with modified PVS 1 (60–75%). The lowest survival rate was observed in case with 88% PVS 3 (35–40%). Meristems treated with PVS 2 and PVS N demonstrated a survival index of 45–55%.
After cryopreservation in hermetically sealed aluminum pans for DSC the meristems preservation rates of 40–82% were observed (
Figure 2). The highest post-thaw preservation was found for pretreatment with modified PVS 1 (81–84%). The lowest survival rates were observed in the case with 88% PVS 3 (40–51%). The meristems treated with PVS 2 and PVS N showed survival indices of 68–75 and 70–78% respectively.
4. Discussion
According to our results, the use of aluminum pans for DSC significantly determined higher survival rate of cryopreserved sweet potato meristems, compared to those of the vitrification using cryovials. We believe it may depend on different cooling and warming rates in these containers. The cooling rate at the step from 22 down to −70 °C made 49 ± 12 deg/s for 1.8 mL cryovials and 125 ± 14 deg/s for the 50 µL aluminum pans for DSC. The rate of warming from −196 to 22 °C was 42 ± 15 for the cryovials and 348 ± 26 deg/s for the metal container.
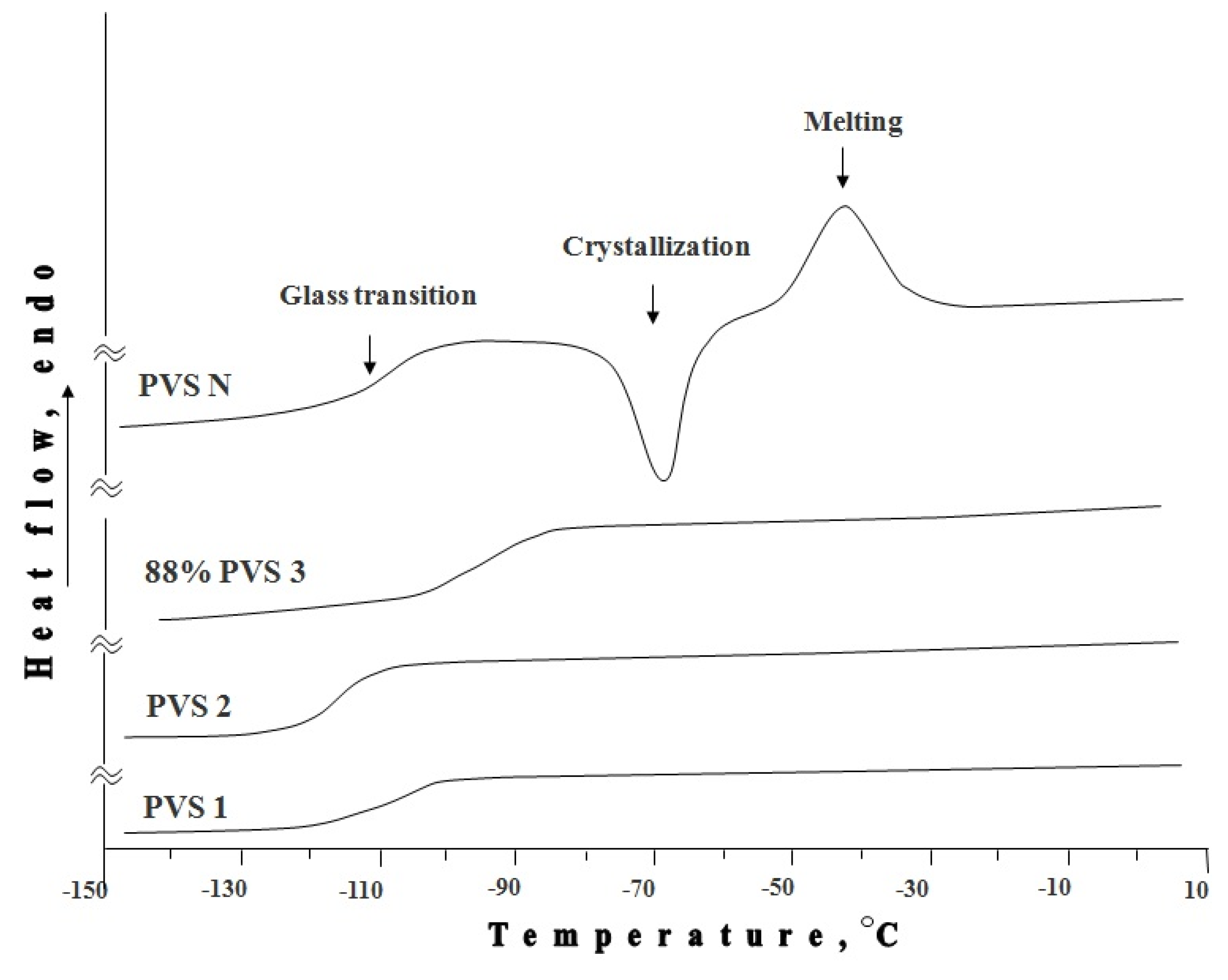
The high meristems preservation rate observed by us is most likely associated with the stability of the vitrified state of modified PVS 1. This was shown by differential scanning calorimetry, recorded during warming with the rate of 0.5 deg/min (
Figure 3).
DSC thermograms of modified PVS 1, PVS 2, and 88% PVS 3 revealed only one heat capacity jump at temperature Tg (−109; −115.3; −93.9 respectively), associated with a reverse glass transition process (transition from a solid amorphous to of supercooled liquid state). No exo- or endothermic peaks were recorded, indicating no crystallization, both at cooling and warming stage. This fact testifies that at the cooling stage the modified PVS 1, PVS 2, and 88% PVS 3 are completely transformed into a glassy state with highly stable amorphous phase, which does not crystallize even under slow warming above the glass transition temperature. In the thermogram of PVS N, in addition to the glass transition, an exothermic crystallization from an amorphous state (devitrification) peak and an endothermic peak of melting were recorded (
Figure 3). It should be noted that the area under crystallization and melting curves does not differ significantly.
This indicates that the crystallization occurs only at the warming stage. In spite of the fact that in the PVS N there were recorded the devitrification and endothermic peaks of melting at warming stage, we obtained the survival rate of meristems at the level of 42–79%. This is due to the fact that the heating rates we applied in our studies were quite high. Since PVS 3 had a negative effect on meristems at the treatment stage, its stable amorphous phase did not affect the preservation rate of the meristems after cryopreservation.
Cryopreservation of sweet potato meristems for long-term storage of germplasm and cryotherapy is carried out by vitrification [
14], encapsulation vitrification [
15], encapsulation dehydration [
16], and droplet vitrification [
13,
14,
17].
The date obtained [
14] showed that despite the high level of survival, the regeneration may be absent. Vollmer and co-workers reported successful cryopreservation of apical meristems with a regeneration rate from 1.7 to 66% for 24 tested sweet potato cultivars [
5]. The date obtained [
18] for tested cultivars showed regeneration rates of cryopreserved meristems ranging from 9.5 to 83.9%.
In view of the above, we are going to develop cryopreservation protocols with high level of regeneration for sweet potato varieties grown in Ukraine, which will be used to obtain virus-free plants (cryotherapy). Since the highest survival rates of sweet potato meristems occur after dehydration with airflow and PVS 1 and 2, we will use these protocols in further work. It is also necessary to select post-cultivation conditions, such as phytohormone composition, ammonium concentration in the nutrient medium and lighting conditions.
5. Conclusions
The survival rates of the sweet potatoes’ meristems after PVS 3, PVS 2, and PVS N treatment were 51.75, 85, and 83% correspondingly. The highest percentage of preserved specimens was detected after dehydration with airflow or modified PVS 1–92%.
The meristems dehydrated with sterile airflow provided 85% survival rate after warming. Meristems treated with 88% PVS 3, PVS 2, and PVS N demonstrated a survival index of 37.4 and 51.25 and 45% after cryopreservation in cryovials. The highest post-thaw survival was found after dehydration with modified PVS 1–69%. The results demonstrated that the post-thaw survival after cryopreservation in aluminum pans for DSC did not change compared with non-cooling but dehydrated with PVSs meristems (82.75% for modified PVS 1, 74.25–PVS N, 45.7–88% PVS 3, and 71.75–PVS 2).
The use of aluminum pans for DSC significantly determined higher survival rate of cryopreserved sweet potato meristems, compared to those of the vitrification using cryovials. It may depend on different cooling and warming rates in these containers.
At the cooling stage the modified PVS 1, PVS 2, and 88% PVS 3 are completely transformed into a glassy state with highly stable amorphous phase, which does not crystallize even under slow warming above the glass transition temperature. In the thermogram of PVS N, in addition to the glass transition, an exothermic crystallization from an amorphous state (devitrification) peak and an endothermic peak of melting were recorded. The area under crystallization and melting curves did not differ significantly.
This indicates that the crystallization occurs only at the warming stage. In spite of the fact that in the PVS N the devitrification and endothermic peaks of melting at warming stage were recorded, we obtained the survival rate of meristems at the level of 45 and 74.25%. This is due to the fact that the heating rates we applied in our studies were quite high. Since PVS 3 had a negative effect on meristems at the treatment stage, its stable amorphous phase did not affect the preservation rate of the meristems after cryopreservation.
The meristems cryopreservation method based on dehydrated with the sterile air flow is of a special interest, since no cryoprotectant use is needed.
Author Contributions
N.S., O.B. and T.I. conceived and designed the experiments; N.S., N.B., A.M., G.K. and O.B. performed the experiments; T.I., O.B. and N.S. performed statistical data processing and analysis of results; N.B., A.M. and T.I. contributed reagents, materials, and analysis tools; N.S. and O.B. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee) of Institute for Problems of Cryobiology and Cryomedicine of the National Academy of Sciences of Ukraine (protocol №12, on 24 December 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data obtained has not been previously presented anywhere.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DSC | differential scanning calorimetry |
| LN | liquid nitrogen |
| MS | nutrient medium Murashige&Skoog |
| PVS | plant vitrification solution |
| Tg | glass transition temperature |
| Tc | crystallization temperature |
| Tm | melting temperature |
References
- Kourouma, V.; Mu, T.H.; Zhang, M.; Sun, H.N. Comparative study on chemical composition, polyphenols, flavonoids, carotenoids and antioxidant activities of various cultivars of sweet potato. Int. J. Food Sci. Technol. 2020, 55, 369–378. [Google Scholar] [CrossRef]
- Wang, Q.; Valkonen, J.P. Elimination of two viruses which interact synergistically from sweetpotato by shoot tip culture and cryotherapy. J. Virol. Methods 2008, 154, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, J.C.; Costa, M.D.; Gardin, J.P.P.; Kretzschmar, A.A.; Pathirana, R. Cryotherapy: A new technique to obtain grapevine plants free of viruses. Rev. Bras. Frutic. 2016, 38, 1–13. [Google Scholar] [CrossRef]
- Feng, C.; Yin, Z.; Ma, Y.; Zhang, Z.; Chen, L.; Wang, B.; Li, B.; Huang, Y.; Wang, Q. Cryopreservation of sweet potato (Ipomoea batatas) and its pathogen eradication by cryotherapy. Biotechnol. Adv. 2011, 29, 84–93. [Google Scholar] [CrossRef]
- Wang, B.; Wang, R.R.; Cui, Z.H.; Bi, W.L.; Li, J.W.; Li, B.Q.; Ozudogru, E.A.; Volk, G.M.; Wang, Q.C. Potential applications of cryogenic technologies to plant genetic improvement and pathogen eradication. Biotechnol. Adv. 2014, 32, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Uragami, A.; Sakai, A.; Nagai, M.; Takahashi, T. Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep. 1989, 8, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Ivchenko, T.V.; Vitsenya, T.I.; Shevchenko, N.A.; Bashtan, N.O.; Kornienko, S.I. Hypothermic and low-temperature storage of garlic (Allium sativum L.) for in vitro collections. Probl. Cryobiol. Cryomed. 2017, 27, 110–120. [Google Scholar] [CrossRef]
- Teixeira, A.S.; Faltus, M.; Zámečník, J.; González-Benito, M.E.; Molina-García, A.D. Glass transition and heat capacity behaviors of plant vitrification solutions. Thermochim. Acta 2014, 593, 43–49. [Google Scholar] [CrossRef]
- Matsumoto, T. Cryopreservation of plant genetic resources: Conventional and new methods. Rev. Agric. Sci. 2017, 5, 13–20. [Google Scholar] [CrossRef]
- Park, S.U.; Kim, H.H. Cryopreservation of sweet potato shoot tips using a droplet-vitrification procedure. CryoLetters 2015, 36, 344–352. [Google Scholar] [PubMed]
- Pennycooke, J.C.; Towill, L.E. Medium alterations improve regrowth of sweet potato (Ipomoea batatas [L.] Lam.) shoot tips cryopreserved by vitrification and encapsulation-dehydration. CryoLetters 2001, 22, 381–389. [Google Scholar] [PubMed]
- Hirai, D.; Sakai, A. Simplified cryopreservation of sweet potato [Ipomoea batatas (L.) Lam.] by optimizing conditions for osmoprotection. Plant Cell Rep. 2003, 21, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lee, G.; Lee, Y.; Gwag, J.; Son, E.; Park, H. Cryopreservation of in vitro grown shoot tips of sweet potato (Ipomoea batatas L.) by the encapsulation-vitrification method. Korean J. Plant Res. 2016, 29, 635–641. [Google Scholar] [CrossRef][Green Version]
- Vollmer, R.; Panta, A.; Tay, D.; Roca, W.; Ellis, D. Effect of sucrose preculture and PVS2 exposure on the cryopreservation of sweet potato shoot tips [Ipomoea batatas (L.) lam.] using the PVS2 droplet vitrification. Acta Hortic. 2014, 1039, 265–272. [Google Scholar] [CrossRef]
- Wilms, H.; Sleziak, N.F.; Van der Auweraer, M.; Brands, M.; Verleije, M.; Hardeman, D.; Andre, E.; Panis, B. Development of a fast and user-friendly cryopreservation protocol for sweet potato genetic resources. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).