The Stomata of the Katanin Mutants, fra2, lue1 and bot1 †
Abstract
1. Introduction
2. Experiments
2.1. Plant Material
2.2. Preparation of Semithin Sections and Toluidin Blue O Staining
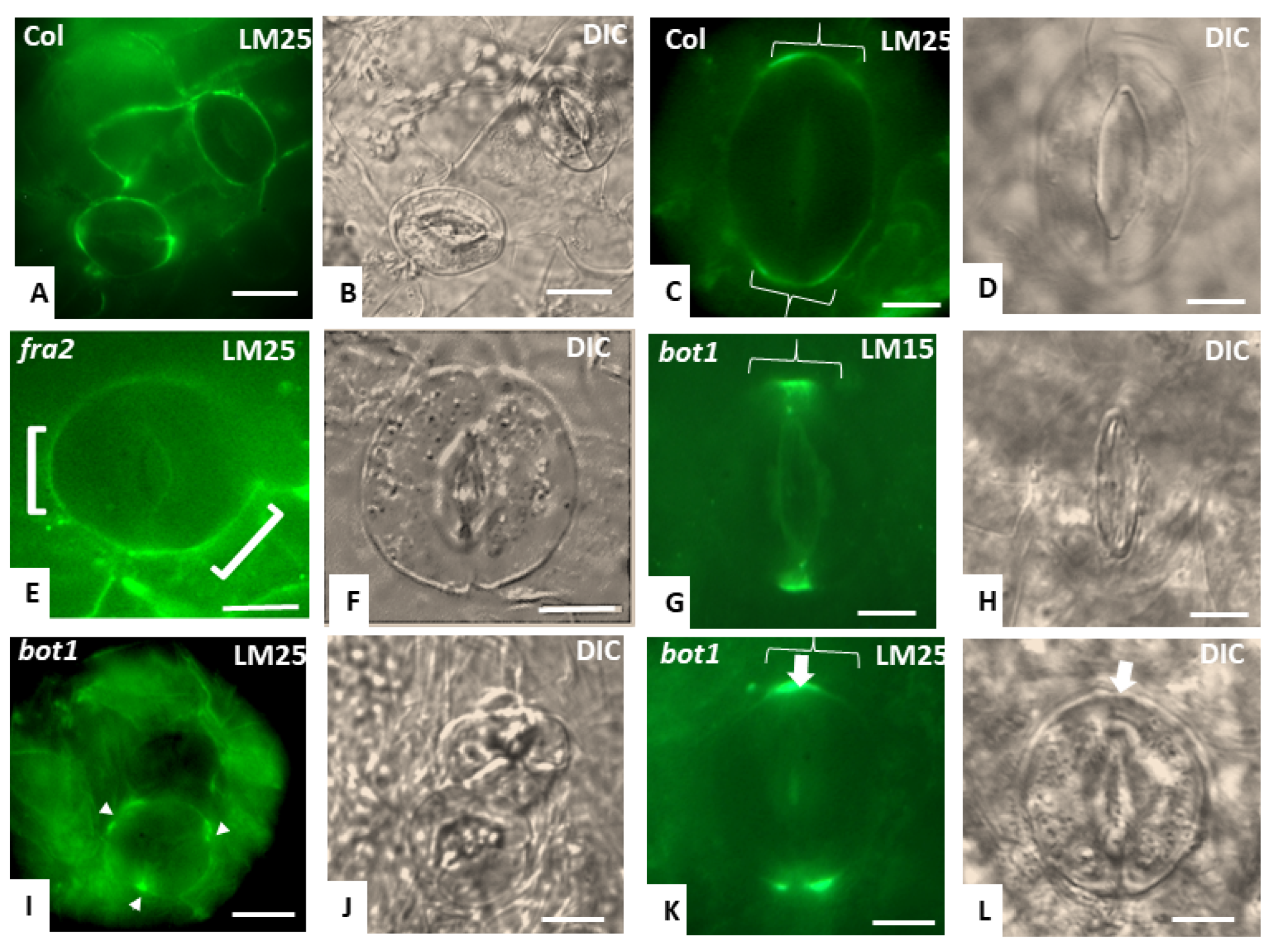
2.3. Callose Localization
2.4. Immunolocalization of Pectins, Hemicelluloses and Microtubules
2.5. Observation and Photography
3. Results
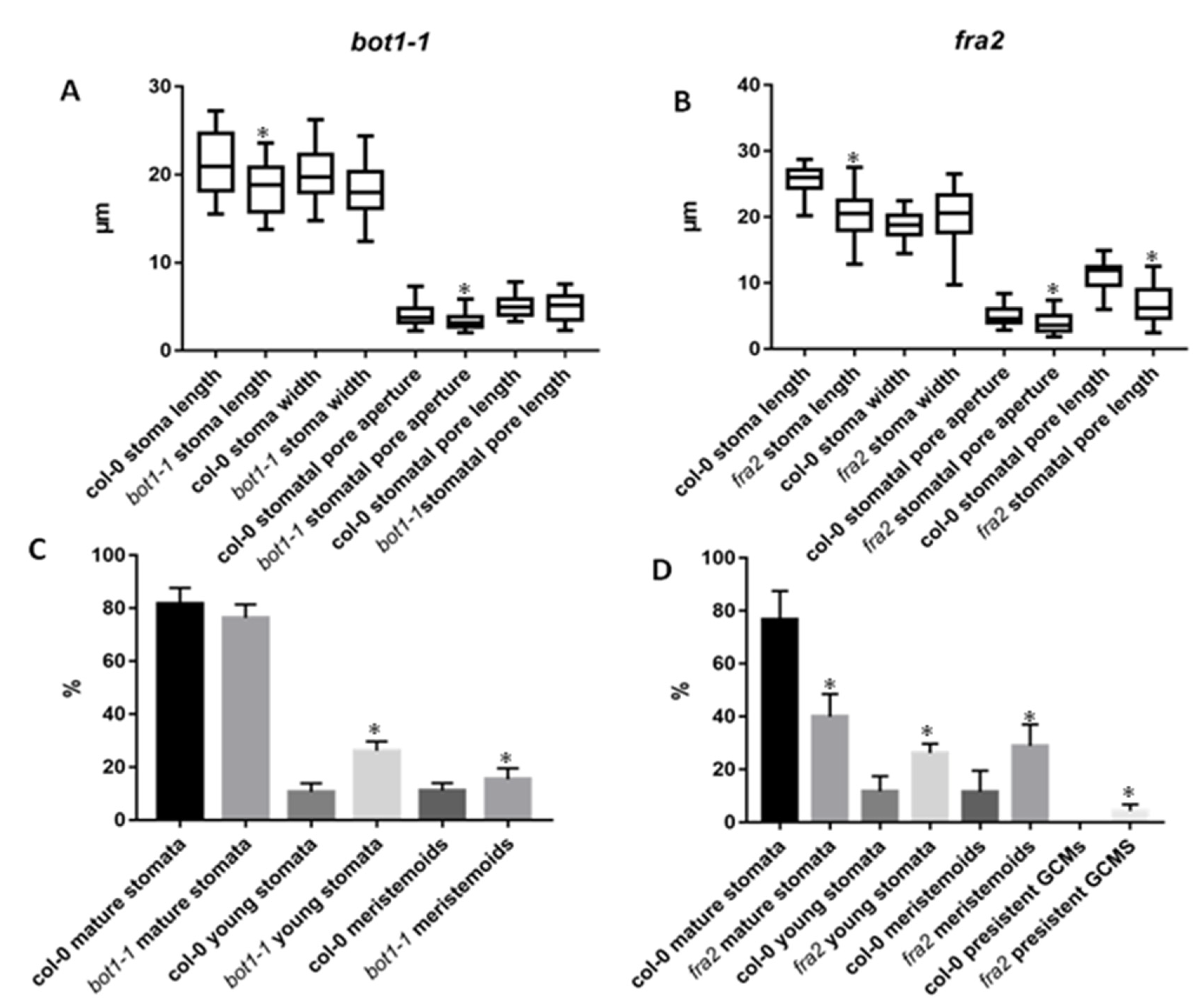
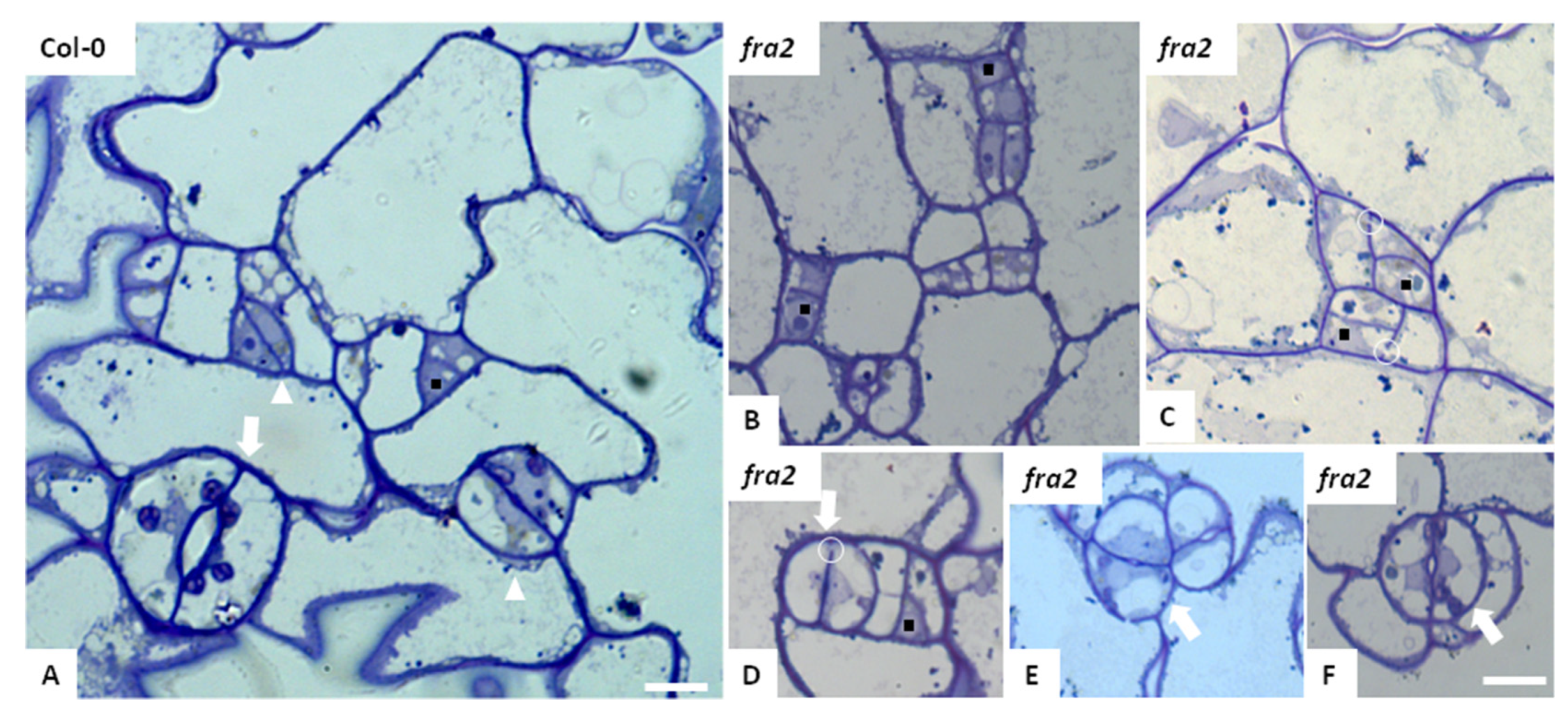
3.1. Stomatal Development and Stomatal Characteristics in Katanin Mutants
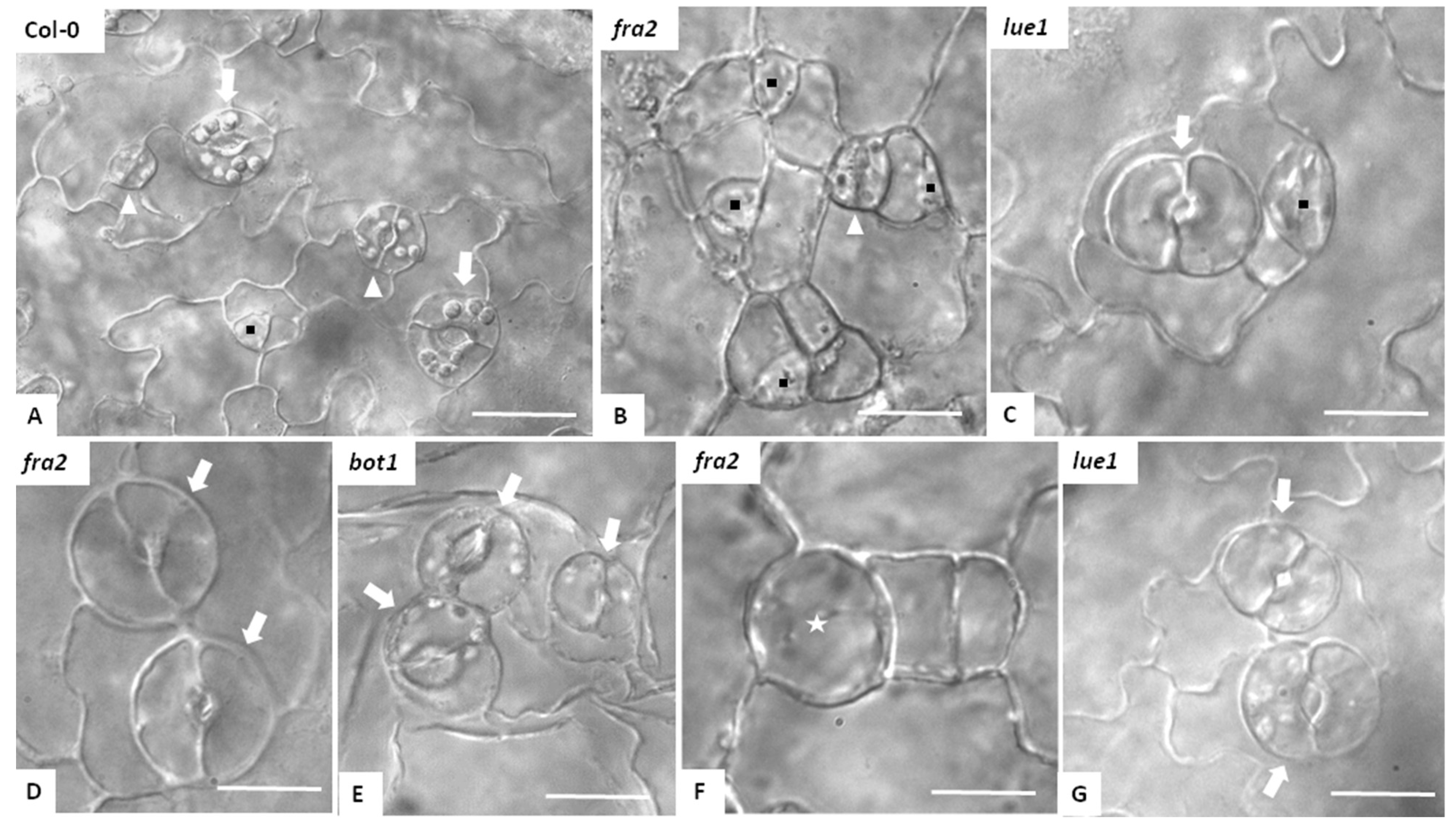
3.2. Stomatal Patterning in Katanin Mutants
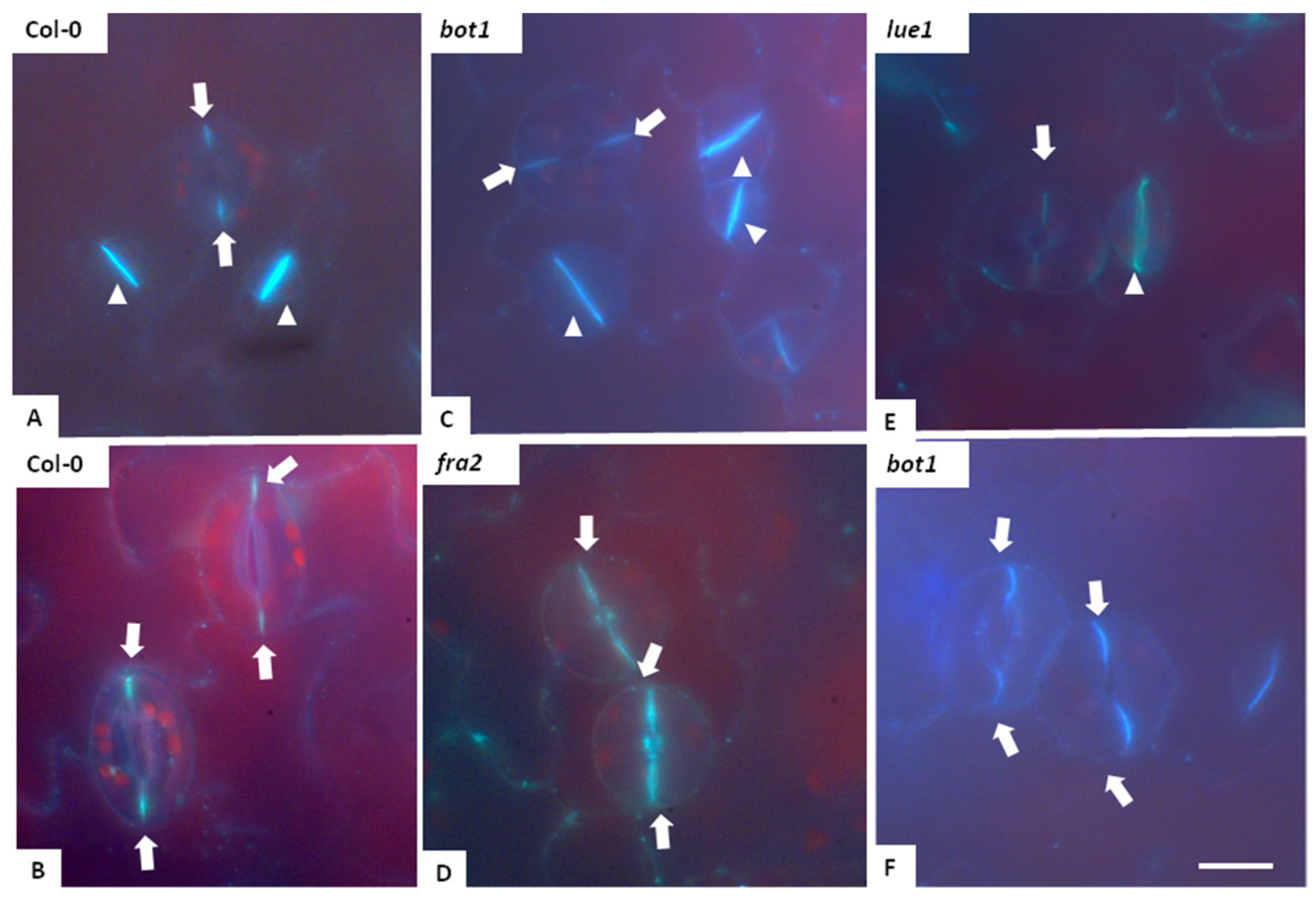
3.3. Callose Deposition in Fra2, Lue1 and Bot1 Mutants
3.4. Hemicellulose and Pectin Deposition in Stomatal Complexes of fra2 and Bot1 Mutants
3.5. Microtubules in Fra2 and Bot1 Mutants
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DIC | diffentiate interphase contrast |
| GMC | guard mother cells |
| HG | homogalacturonans |
| MMC | mereistemoid mother cells |
| PC | pavement cells |
| SLGC | stomatal-lineage ground cell |
References
- Han, S.K.; Torii, K.U. Lineage-specific stem cells, signals and asymmetries during stomatal development. Development 2016, 143, 1259–1270. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Dong, J. Stomatal Development in Arabidopsis. Arab. Book 2013, 11, e0162. [Google Scholar] [CrossRef]
- Chater, C.C.C.; Caine, R.S.; Fleming, A.J.; Gray, J.E. Origins and evolution of stomatal development. Plant Physiol. 2017, 174, 624–638. [Google Scholar] [CrossRef]
- Zoulias, N.; Harrison, E.L.; Casson, S.A.; Gray, J.E. Molecular control of stomatal development. Biochem. J. 2018, 475, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Nithianantham, S.; McNally, F.J.; Al-Bassam, J. Structural basis for disassembly of katanin heterododecamers. J. Biol. Chem. 2018, 293, 10590–10605. [Google Scholar] [CrossRef] [PubMed]
- Luptovčiak, I.; Komis, G.; Takáč, T.; Ovečka, M.; Šamaj, J. Katanin: A sword cutting microtubules for cellular, developmental, and physiological purposes. Front. Plant Sci. 2017, 8, 1982. [Google Scholar] [CrossRef] [PubMed]
- Panteris, E.; Adamakis, I.-D.S.; Voulgari, G.; Papadopoulou, G. A role for katanin in plant cell division: Microtubule organization in dividing root cells of fra2 and lue1Arabidopsis thaliana mutants. Cytoskeleton 2011, 68, 401–413. [Google Scholar] [CrossRef]
- Panteris, E.; Adamakis, I.-D.S. Aberrant microtubule organization in dividing root cells of p60-katanin mutants. Plant Signal. Behav. 2012, 7, 16–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lucas, J.R.; Nadeau, J.A.; Sack, F.D. Microtubule arrays and Arabidopsis stomatal development. J. Exp. Bot. 2006, 57, 71–79. [Google Scholar] [CrossRef]
- Moustaka, J.; Panteris, E.; Adamakis, I.-D.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ROS formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Kohari, M.; Shibuya, N.; Kaku, H. Simultaneous visualization of callose deposition and plasma membrane for live-cell imaging in plants. Plant Cell Rep. 2020, 39, 1517–1523. [Google Scholar] [CrossRef]
- Giannoutsou, E.; Sotiriou, P.; Nikolakopoulou, T.L.; Galatis, B.; Apostolakos, P. Callose and homogalacturonan epitope distribution in stomatal complexes of Zea mays and Vigna sinensis. Protoplasma 2019, 257, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.D.S.; Panteris, E.; Eleftheriou, E.P. Tungsten disrupts root growth in Arabidopsis thaliana by PIN targeting. J. Plant Physiol. 2014, 171, 1174–1187. [Google Scholar] [CrossRef]
- Komorison, M.; Ueguchi-Tanaka, M.; Aichi, I.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M.; Sazuka, T. Analysis of the rice mutant dwarf and gladius leaf 1. Aberrant katanin-mediated microtubule organization causes up-regulation of gibberellin biosynthetic genes independently of gibberellin signaling. Plant Physiol. 2005, 138, 1982–1993. [Google Scholar] [CrossRef]
- Bouquin, T.; Mattsson, O.; Næsted, H.; Foster, R.; Mundy, J. The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J. Cell Sci. 2003, 116, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Komis, G.; Luptovčiak, I.; Ovečka, M.; Samakovli, D.; Šamajová, O.; Šamaj, J. Katanin effects on dynamics of cortical microtubules and mitotic arrays in Arabidopsis thaliana revealed by advanced live-cell imaging. Front Plant Sci. 2017, 8, 866. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, D. Division plane determination during plant somatic cytokinesis. Curr. Opin. Plant Biol. 2009, 12, 745–751. [Google Scholar] [CrossRef]
- Muroyama, A.; Gong, Y.; Bergmann, D.C. Opposing, Polarity-Driven Nuclear Migrations Underpin Asymmetric Divisions to Pattern Arabidopsis Stomata. Curr. Biol. 2020, 30, 4467–4475.e4. [Google Scholar] [CrossRef] [PubMed]
- Meidani, C.; Ntalli, N.G.; Giannoutsou, E.; Adamakis, I.D.S. Cell wall modifications in giant cells induced by the plant parasitic nematode meloidogyne incognita in wild-type (Col-0) and the fra2 arabidopsis thaliana katanin mutant. Int. J. Mol. Sci. 2019, 20, 5465. [Google Scholar] [CrossRef]
- Panteris, E.; Kouskouveli, A.; Pappas, D.; Adamakis, I.-D.S. Cytokinesis in fra2 Arabidopsis thaliana p60-Katanin Mutant: Defects in cell plate/daughter wall formation. Int. J. Mol. Sci. 2021, 22, 1405. [Google Scholar] [CrossRef]
- Carter, R.; Woolfenden, H.; Baillie, A.; Amsbury, S.; Carroll, S.; Healicon, E.; Sovatzoglou, S.; Braybrook, S.; Gray, J.E.; Hobbs, J.; et al. Stomatal Opening Involves Polar, Not Radial, Stiffening Of Guard Cells. Curr. Biol. 2017, 27, 2974–2983.e2. [Google Scholar] [CrossRef] [PubMed]
- Galatis, B.; Apostolakos, P. The role of the cytoskeleton in the morphogenesis and function of stomatal complexes. New Phytol. 2004, 161, 613–639. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevopoulou, D.; Anezakis, N.; Giannoutsou, E.; Sotiriou, P.; Adamakis, I.-D.S. The Stomata of the Katanin Mutants, fra2, lue1 and bot1. Biol. Life Sci. Forum 2021, 4, 30. https://doi.org/10.3390/IECPS2020-08730
Paraskevopoulou D, Anezakis N, Giannoutsou E, Sotiriou P, Adamakis I-DS. The Stomata of the Katanin Mutants, fra2, lue1 and bot1. Biology and Life Sciences Forum. 2021; 4(1):30. https://doi.org/10.3390/IECPS2020-08730
Chicago/Turabian StyleParaskevopoulou, Dafni, Nikolaos Anezakis, Eleni Giannoutsou, Penelope Sotiriou, and Ioannis-Dimosthenis S. Adamakis. 2021. "The Stomata of the Katanin Mutants, fra2, lue1 and bot1" Biology and Life Sciences Forum 4, no. 1: 30. https://doi.org/10.3390/IECPS2020-08730
APA StyleParaskevopoulou, D., Anezakis, N., Giannoutsou, E., Sotiriou, P., & Adamakis, I.-D. S. (2021). The Stomata of the Katanin Mutants, fra2, lue1 and bot1. Biology and Life Sciences Forum, 4(1), 30. https://doi.org/10.3390/IECPS2020-08730






