Contribution of Glutathione and Ascorbate to Realization of the Protective Effect of Nitric Oxide on Wheat Plants under Drought †
Abstract
:1. Introduction
2. Experiments
2.1. Plant Materials
2.2. Glutathione Determination
2.3. Assessments of Lipid Peroxidation (MDA)
2.4. AsA Content
2.5. Determination of Glutathione Reductase Activity
3. Results and Discussion
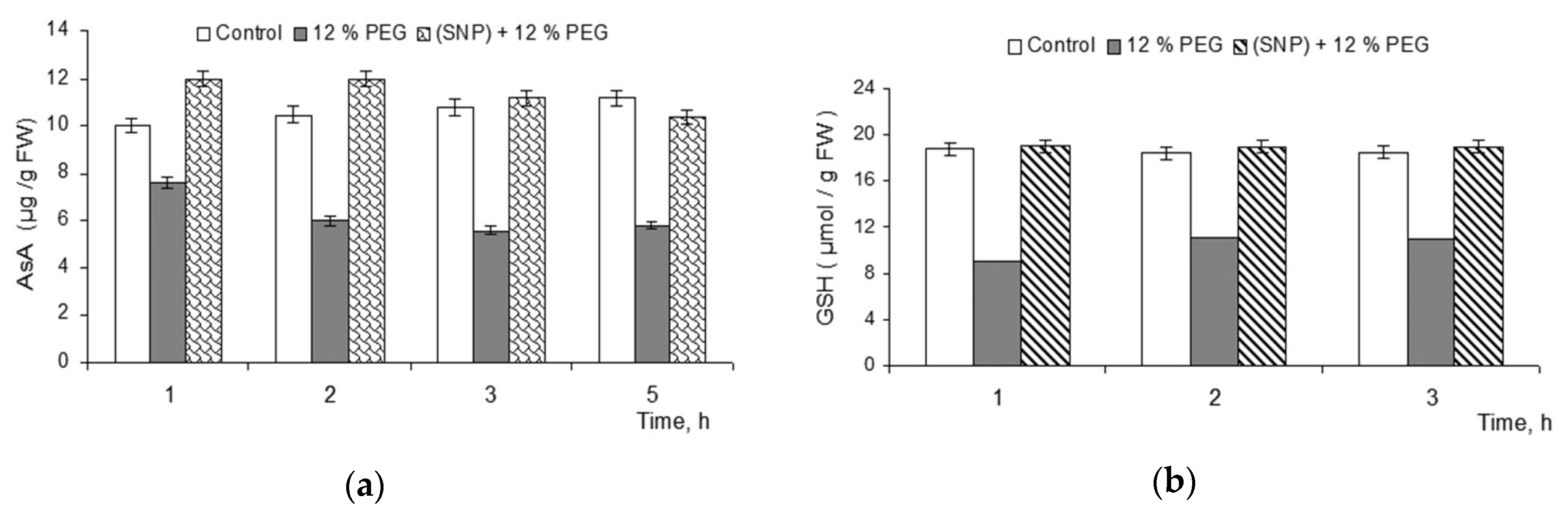
3.1. Effect of 200 μM SNP on Pool AsA and GSH in the Roots of the Wheat Plant under Drought Conditions
3.2. Effect of 200 μM SNP on Activity GR and Content MDA in the Roots of Wheat Plant under Drought Conditions
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamaeva, A.S.; Fomenkov, A.A.; Nosov, A.V.; Moshkov, I.E.; Mur, L.A.J.; Hall, M.A.; Novikova, G.V. Regulatory role of nitricoxidei n plants. Russ. J. Plant Physiol. 2015, 62, 427–440. [Google Scholar] [CrossRef]
- Nabi, R.B.C.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric oxide regulates plant responses to drought, salinity, and heavy metals tress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Ding, Y.; Gardiner, D.M.; Kazan, K. Regulators of nitric oxide signaling triggered by host perception in a plant pathogen. Proc. Natl. Acad. Sci. USA 2020, 117, 11147–11157. [Google Scholar] [CrossRef] [PubMed]
- Maslennikova, D.R.; Allagulova Ch, R.; Fedorova, K.A.; Plotnikov, A.A.; Avalbaev, A.M.; Shakirova, F.M. Cytokinins contribute to realization of nitric oxide growth-stimulating and protective effects on wheat plants. Russ. J. Plant Physiol. 2017, 64, 665–671. [Google Scholar] [CrossRef]
- Maslennikova, D.R.; Plotnikov, A.A.; Shakirova, F.M. Comparative analysis of physiological action nitricoxide and 6-benzylaminopurine on the state of the components of the glutathione complex in the roots of wheat plants. Agrochemistry 2019, 3, 37–43. [Google Scholar] [CrossRef]
- Hewitt, E.J.; Dickes, G.J. Spectrophotometric measurements on ascorbic acid and their use for the estimation of ascorbic acid and dehydroascorbic acid in plant tissues. Biochem. J. 1961, 78, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea a plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikova, D.; Shakirova, F. Contribution of Glutathione and Ascorbate to Realization of the Protective Effect of Nitric Oxide on Wheat Plants under Drought. Biol. Life Sci. Forum 2021, 4, 109. https://doi.org/10.3390/IECPS2020-08850
Maslennikova D, Shakirova F. Contribution of Glutathione and Ascorbate to Realization of the Protective Effect of Nitric Oxide on Wheat Plants under Drought. Biology and Life Sciences Forum. 2021; 4(1):109. https://doi.org/10.3390/IECPS2020-08850
Chicago/Turabian StyleMaslennikova, Dilara, and Farida Shakirova. 2021. "Contribution of Glutathione and Ascorbate to Realization of the Protective Effect of Nitric Oxide on Wheat Plants under Drought" Biology and Life Sciences Forum 4, no. 1: 109. https://doi.org/10.3390/IECPS2020-08850
APA StyleMaslennikova, D., & Shakirova, F. (2021). Contribution of Glutathione and Ascorbate to Realization of the Protective Effect of Nitric Oxide on Wheat Plants under Drought. Biology and Life Sciences Forum, 4(1), 109. https://doi.org/10.3390/IECPS2020-08850




