Higher Alcohol Preference Is Not Necessarily Linked to Higher Consumption of Palatable Food in Rats †
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets
2.3. Experimental Procedure
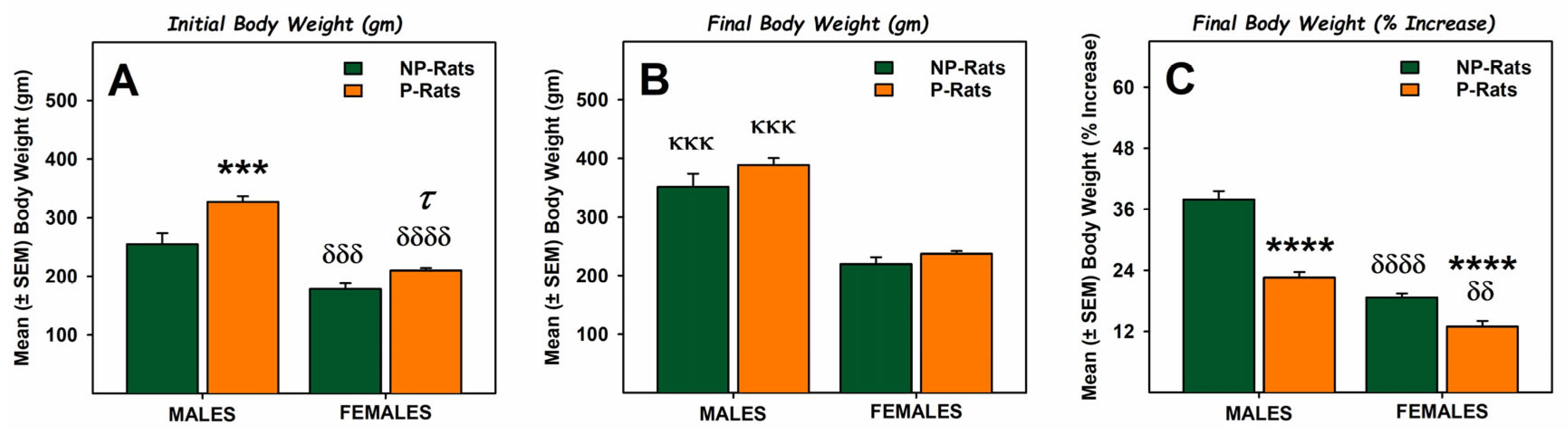
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health and Treatment of Substance Use Disorders; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Centers for Disease Control and Prevention. Alcohol and Public Health: Alcohol-Related Disease Impact. Annual Average for United States 2020–2021 Alcohol-Attributable Deaths Due to Excessive Alcohol Use, All Ages. 2024. Available online: https://nccd.cdc.gov/DPH_ARDI/Default/Report.aspx?T=AAM&P=F1F85724-AEC5-4421-BC88-3E8899866842&R=EACE3036-77C9-4893-9F93-17A5E1FEBE01&M=7F40785C-D481-440A-970F-50EFBD21B35B&F=&D= (accessed on 17 February 2025).
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef] [PubMed]
- SAMHSA. Center for Behavioral Health Statistics and Quality. 2022 National Survey on Drug Use and Health. Table 5.9A—Alcohol Use Disorder in Past Year: Among People Aged 12 or Older; by Age Group and Demographic Characteristics, Numbers in Thousands, 2022 and 2023. 2024. Available online: https://www.samhsa.gov/data/report/2023-nsduh-detailed-tables (accessed on 2 December 2024).
- Bulik, C.M.; Klump, K.L.; Thornton, L.; Kaplan, A.S.; Devlin, B.; Fichter, M.M.; Halmi, K.A.; Strober, M.; Woodside, D.B.; Crow, S.; et al. Alcohol use disorder comorbidity in eating disorders: A multicenter study. J. Clin. Psychiatry 2004, 65, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Grilo, C.M.; Sinha, R.; O’Malley, S.S. Eating Disorders and Alcohol Use Disorders. Alcohol Res. Health 2002, 26, 151–160. [Google Scholar]
- Barson, J.R.; Leibowitz, S.F. Hypothalamic neuropeptide signaling in alcohol addiction. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 321–329. [Google Scholar] [CrossRef]
- Barson, J.R.; Morganstern, I.; Leibowitz, S.F. Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiol. Behav. 2011, 104, 128–137. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 1–19. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Baler, R. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr. Top. Behav. Neurosci. 2012, 11, 1–24. [Google Scholar]
- Colditz, G.A.; Giovannucci, E.; Rimm, E.B.; Stampfer, M.J.; Rosner, B.; Speizer, F.E.; Gordis, E.; Willett, W.C. Alcohol intake in relation to diet and obesity in women and men. Am. J. Clin. Nutr. 1991, 54, 49–55. [Google Scholar] [CrossRef]
- Cummings, J.R.; Gearhardt, A.N.; Ray, L.A.; Choi, A.K.; Tomiyama, A.J. Experimental and observational studies on alcohol use and dietary intake: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e12950. [Google Scholar] [CrossRef]
- White, B.; Sirohi, S. A Complex Interplay between Nutrition and Alcohol use Disorder: Implications for Breaking the Vicious Cycle. Curr. Pharm. Des. 2024, 30, 1822–1837. [Google Scholar] [CrossRef]
- Wilkens Knudsen, A.; Jensen, J.E.B.; Nordgaard-Lassen, I.; Almdal, T.; Kondrup, J.; Becker, U. Nutritional intake and status in persons with alcohol dependency: Data from an outpatient treatment programme. Eur. J. Nutr. 2014, 53, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.; Gordis, E.; Holt, J. Dietary choices and likelihood of abstinence among alcoholic patients in an outpatient clinic. Drug Alcohol Depend. 1983, 12, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Stickel, A.; Rohdemann, M.; Landes, T.; Engel, K.; Banas, R.; Heinz, A.; Müller, C.A. Changes in Nutrition-Related Behaviors in Alcohol-Dependent Patients After Outpatient Detoxification: The Role of Chocolate. Subst. Use Misuse 2016, 51, 545–552. [Google Scholar] [CrossRef]
- Brutman, J.; Davis, J.F.; Sirohi, S. Behavioral and Neurobiological Consequences of Hedonic Feeding on Alcohol Drinking. Curr. Pharm. Des. 2020, 26, 2309–2315. [Google Scholar] [CrossRef]
- Tabakoff, B.; Hoffman, P.L. Animal models in alcohol research. Alcohol Res. Health J. Natl. Inst. Alcohol Abuse Alcohol. 2000, 24, 77–84. [Google Scholar]
- Bell, R.L.; Hauser, S.R.; Liang, T.; Sari, Y.; Maldonado-Devincci, A.; Rodd, Z.A. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 2017, 122, 201–243. [Google Scholar] [CrossRef]
- Bell, R.L.; Rodd, Z.A.; Lumeng, L.; Murphy, J.M.; McBride, W.J. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict. Biol. 2006, 11, 270–288. [Google Scholar] [CrossRef]
- McBride, W.J.; Rodd, Z.A.; Bell, R.L.; Lumeng, L.; Li, T.K. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats—Animal models of alcoholism. Alcohol 2014, 48, 209–215. [Google Scholar] [CrossRef]
- Murphy, J.M.; Stewart, R.B.; Bell, R.L.; Badia-Elder, N.E.; Carr, L.G.; McBride, W.J.; Lumeng, L.; Li, T.-K. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav. Genet. 2002, 32, 363–388. [Google Scholar] [CrossRef]
- Kesse, E.; Clavel-Chapelon, F.; Slimani, N.; van Liere, M. Do eating habits differ according to alcohol consumption? Results of a study of the French cohort of the European Prospective Investigation into Cancer and Nutrition (E3N-EPIC). Am. J. Clin. Nutr. 2001, 74, 322–327. [Google Scholar]
- Männistö, S.; Uusitalo, K.; Roos, E.; Fogelholm, M.; Pietinen, P. Alcohol beverage drinking, diet and body mass index in a cross-sectional survey. Eur. J. Clin. Nutr. 1997, 51, 326–332. [Google Scholar] [CrossRef]
- Herbeth, B.; Didelot-Barthelemy, L.; Lemoine, A.; Le Devehat, C. Dietary behavior of French men according to alcohol drinking pattern. J. Stud. Alcohol 1988, 49, 268–272. [Google Scholar] [CrossRef]
- Koehn, V.; Burnand, B.; Niquille, M.; Paccaud, F.; Magnenat, P.; Yersin, B. Prevalence of malnutrition in alcoholic and nonalcoholic medical inpatients: A comparative anthropometric study. JPEN J. Parenter. Enteral Nutr. 1993, 17, 35–40. [Google Scholar] [CrossRef]
- Glória, L.; Cravo, M.; Camilo, M.E.; Resende, M.; Cardoso, J.N.; Oliveira, A.G.; Leitão, C.N.; Mira, F.C. Nutritional deficiencies in chronic alcoholics: Relation to dietary intake and alcohol consumption. Am. J. Gastroenterol. 1997, 92, 485–489. [Google Scholar]
- Lieber, C.S. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res. Health J. Natl. Inst. Alcohol Abuse Alcohol. 2003, 27, 220–231. [Google Scholar]
- Sirohi, S.; Bakalkin, G.; Walker, B.M. Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front. Mol. Neurosci. 2012, 5, 95. [Google Scholar] [CrossRef]
- Walker, B.M.; Koob, G.F. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 2008, 33, 643–652. [Google Scholar] [CrossRef]
- Bates, M.E.; Bowden, S.C.; Barry, D. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Exp. Clin. Psychopharmacol. 2002, 10, 193–212. [Google Scholar] [CrossRef]
- Rupp, C.I.; Kemmler, G.; Kurz, M.; Hinterhuber, H.; Fleischhacker, W.W. Cognitive remediation therapy during treatment for alcohol dependence. J. Stud. Alcohol. Drugs. 2012, 73, 625–634. [Google Scholar] [CrossRef]
- Gunn, R.L.; Finn, P.R. Impulsivity partially mediates the association between reduced working memory capacity and alcohol problems. Alcohol. 2013, 47, 3–8. [Google Scholar] [CrossRef]
- Barson, J.R.; Karatayev, O.; Chang, G.Q.; Johnson, D.F.; Bocarsly, M.E.; Hoebel, B.G.; Leibowitz, S.F. Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: Counteraction by lipid-lowering drugs. Alcohol. 2009, 43, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.A.; Leibowitz, S.F.; Karatayev, O.; Hoebel, B.G. A high-fat meal or injection of lipids stimulates ethanol intake. Alcohol 2004, 34, 197–202. [Google Scholar] [CrossRef]
- Krahn, D.D.; Gosnell, B.A. Fat-preferring rats consume more alcohol than carbohydrate-preferring rats. Alcohol 1991, 8, 313–316. [Google Scholar] [CrossRef]
- Blanco-Gandía, M.C.; Ledesma, J.C.; Aracil-Fernández, A.; Navarrete, F.; Montagud-Romero, S.; Aguilar, M.A.; Manzanares, J.; Miñarro, J.; Rodríguez-Arias, M. The rewarding effects of ethanol are modulated by binge eating of a high-fat diet during adolescence. Neuropharmacology 2017, 121, 219–230. [Google Scholar] [CrossRef]
- Prasad, A.; Abadie, J.M.; Prasad, C. Can dietary macronutrient preference profile serve as a predictor of voluntary alcohol consumption? Alcohol 1993, 10, 485–489. [Google Scholar] [CrossRef]
- Takase, K.; Tsuneoka, Y.; Oda, S.; Kuroda, M.; Funato, H. High-fat diet feeding alters olfactory-, social-, and reward-related behaviors of mice independent of obesity. Obes. Silver Spring Md 2016, 24, 886–894. [Google Scholar] [CrossRef]
- Avena, N.M.; Carrillo, C.A.; Needham, L.; Leibowitz, S.F.; Hoebel, B.G. Sugar-dependent rats show enhanced intake of unsweetened ethanol. Alcohol 2004, 34, 203–209. [Google Scholar] [CrossRef]
- Williams Keith, L. Intermittent Binge-Like Sucrose Consumption Fails to Increase Ethanol Self-Administration Following Sucrose-Fade. Alcohol. Clin. Exp. Res. 2017, 41 (Suppl. S1), 37A. [Google Scholar]
- Shah, K.; Shaw, C.; Sirohi, S. Reduced alcohol drinking following patterned feeding: Role of palatability and acute contingent availability. Physiol. Behav. 2020, 224, 113020. [Google Scholar] [CrossRef]
- Crabbe, J.C.; Harris, R.A.; Koob, G.F. Preclinical studies of alcohol binge drinking. Ann. N. Y. Acad. Sci. 2011, 1216, 24–40. [Google Scholar] [CrossRef]
- Murphy, J.M.; McBride, W.J.; Lumeng, L.; Li, T.K. Contents of monoamines in forebrain regions of alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol. Biochem. Behav. 1987, 26, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Chacko, J.T.; Sindelar, D.K. Characterization of the alcohol-preferring P rat on normal chow and a high-fat diets. Appetite 2007, 49, 273. [Google Scholar] [CrossRef]
- Sirohi, S.; Van Cleef, A.; Davis, J.F. Intermittent access to a nutritionally complete high-fat diet attenuates alcohol drinking in rats. Pharmacol. Biochem. Behav. 2017, 153, 105–115. [Google Scholar] [CrossRef]
- Sirohi, S.; Van Cleef, A.; Davis, J.F. Binge-like intake of HFD attenuates alcohol intake in rats. Physiol. Behav. 2017, 178, 187–195. [Google Scholar] [CrossRef]
- Leon, Z.; Shah, K.; Bailey, L.S.; Karkhanis, A.N.; Sirohi, S. Patterned Feeding of a Hyper-Palatable Food (Oreo Cookies) Reduces Alcohol Drinking in Rats. Front. Behav. Neurosci. 2021, 15, 725856. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, S.; Sirohi, S. Higher Alcohol Preference Is Not Necessarily Linked to Higher Consumption of Palatable Food in Rats. Biol. Life Sci. Forum 2024, 38, 10. https://doi.org/10.3390/blsf2024038010
Pham S, Sirohi S. Higher Alcohol Preference Is Not Necessarily Linked to Higher Consumption of Palatable Food in Rats. Biology and Life Sciences Forum. 2024; 38(1):10. https://doi.org/10.3390/blsf2024038010
Chicago/Turabian StylePham, Sabrina, and Sunil Sirohi. 2024. "Higher Alcohol Preference Is Not Necessarily Linked to Higher Consumption of Palatable Food in Rats" Biology and Life Sciences Forum 38, no. 1: 10. https://doi.org/10.3390/blsf2024038010
APA StylePham, S., & Sirohi, S. (2024). Higher Alcohol Preference Is Not Necessarily Linked to Higher Consumption of Palatable Food in Rats. Biology and Life Sciences Forum, 38(1), 10. https://doi.org/10.3390/blsf2024038010





