1. Introduction
Current climate changes lead us to conclude that many plant species with the ability to resist or tolerate natural constraints are beginning to lose these characters. Physiological, morphological, biochemical, molecular and hormonal disturbances are attributable to various abiotic stresses [
1].
Ref. [
2] shows that salinity has a depressive effect on germination and seed production. However, this effect varies depending on the intensity of stress.
Ref. [
3] shows that the effect of NaCl is a parameter that can help determine the origin of the depressive effect of salinity on germination.
Some researchers confirm that the response to or tolerance for salt depends on the species itself, its variety, the salt concentration and the growing conditions [
4].
Ref. [
5] confirms that successful germination constitutes the fundamental condition for establishing a good culture. On the other hand, ref. [
6] shows that seed germination is a set of metabolic processes leading to the emergence of the radical. This stage of development is considered a critical step in establishing seedlings and thus determining successful agricultural production.
Okra is a plant of leguminous origin; all its parts (roots, stem, leaves, fruits, seeds) are valued on a food, medicinal, artisanal and even industrial level.
In order to ensure good okra production, the search for varieties that are tolerant of and/or resistant to salinity is becoming a necessity [
7].
The objective of our research is to study the effect of NaCl on the germination of Abelmoschus esculentus L. seeds and their development in order to specify their tolerance limits to this constraint (NaCl) during the germination phase.
2. Materials and Methods
2.1. Plant Material
2.1.1. Seed Collection
The seeds used in this experiment were collected from plants grown in a greenhouse at Mustapha Stambouli de Mascara. The seeds were interposed under prior conservation conditions until the application of the germination tests.
2.1.2. Preparing Seeds for Sowing
The seeds were disinfected with 0.8% sodium hypochlorite for three minutes. Then, the seeds were rinsed with distilled water several times to eliminate traces of chlorine and dried on sterile filter paper.
The germination tests were carried out with 11 repetitions of 10 seeds per sterile Petri dish, each filled with moistened Wattman filter paper. The Petri dishes were placed in the dark at the optimal germination temperature (25 °C).
2.1.3. Application of Treatments
In each Petri dish, 7 mL of sterile distilled water were poured for the control seeds and 7 mL for the seeds treated with the different saline solutions (100 mM and 150 mM).
We proceeded to count the germinated seeds as soon as the tip of the radical appeared through the envelope and exited 1 mm outside the seed coat and became visible to the naked eye according to the definition of [
8]. Germination followed after for a week. The
Figure 1 shows the results of germination.
2.1.4. Germination Parameters
Estimation of Germination Rate
Based on the total number of seeds used (Tn), we calculated the percentage of seeds germinating (Ni) according to the relationship:
Germination Speed
It characterizes the variation over time in germination rates from the appearance of the first tip of the radicle until germination stability. It can be expressed by:
2.1.5. Biometric Settings
Water Content of Seeds
The water content was determined by the difference between the fresh weight of the germinated seeds after one week and the dry weight obtained after drying in an oven at 80 °C for 48 h.
Radical Elongation
Radical length was assessed by measuring the length of the radical; these measurements were performed every day for a week. The
Figure 2 shows the results of Radical length.
3. Results
3.1. Effect of NaCl in the Germination Parameters of Abelmoschus esculentus L.
3.1.1. Precocity of Germination
The precocity of germination is expressed by the rate of the first germinated seeds.
Figure 3 shows the variation in the rates of the first germinated seeds under the effect of the NaCl treatment (100 mM, 150 mM).
The results obtained show that the response of the germinated seeds decreases as a function of the increase in the NaCl concentration: germination started on the 2nd day after sowing with an estimated low germination rate of (17.27%) under the effect of 100 mM as compared to the control seeds, while those under the 150 mM treatment germination started on the 3rd day with a very low rate (12.73%)
There was a reduction of 30% as compared to the controls when germination started on the 2nd day of the seedlings.
3.1.2. Kinetics of Seed Germination
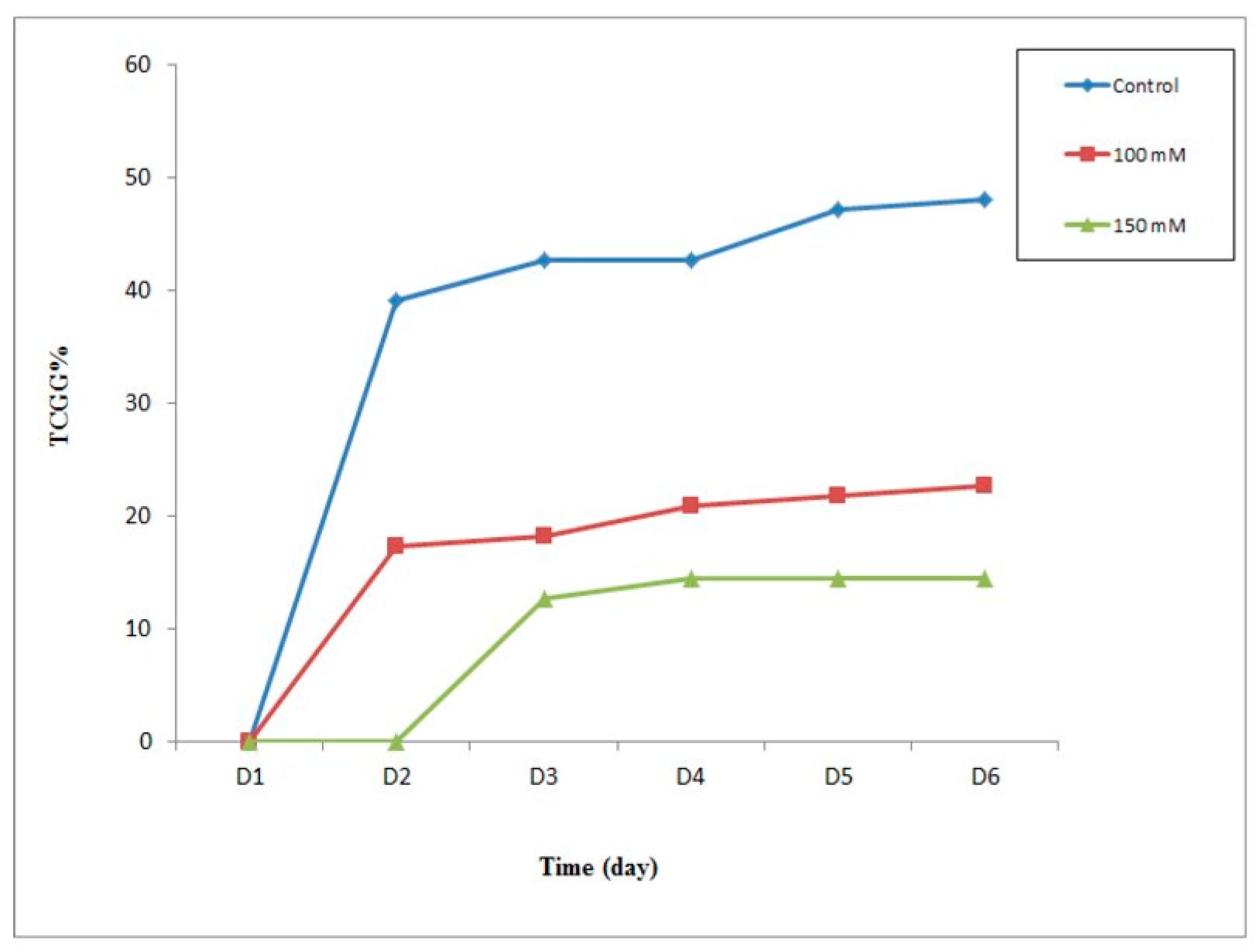
Germination kinetics most often represent the evolution of the cumulative germination percentages as a function of time.
Figure 4 shows that the variation in germination rates increased continuously in all treated batches as a function of time.
Furthermore, batches of seeds at 100 mM caused germination to progress slowly as compared to the control. In fact, the cumulative rate of germinated seeds increased from 17.27% to a maximum of 22.73% on the 6th day after sowing, a decrease of 25.45% as compared to the control.
On the other hand, the seeds treated at 150 mM presented an evolution in which the cumulative rate of the germinated seeds progressed very weakly, the reaction of which manifests itself on the 3rd day after sowing to stabilize from the 4th day at a maximum of 14.55%, an estimated reduction of 30% to 33.63% as compared to the control.
The statistical study according to the Student’s test indicates that the difference in the germination rate is highly significant for the seeds treated with 100 mM NaCl as compared to the control (≤0.05) and very highly significant for the seeds treated with 150 mM NaCl as compared to the control (≤0.05).
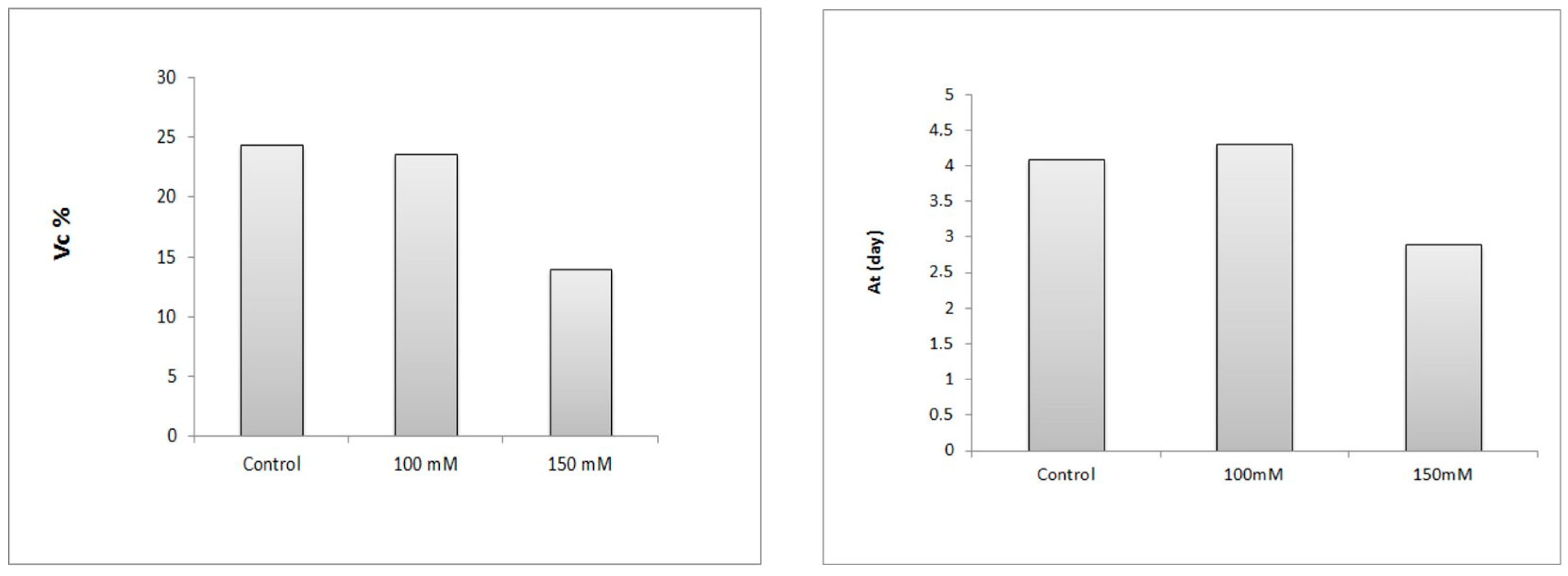
3.1.3. Germination Speed: Velocity Coefficient (Vc) and Average Time (At)
The germination speed is considered to be the time taken by the seeds to germinate, determined between the sowing and the end of germination.
To better study the factors acting on seed germination, we adopted two simple formulas: the velocity coefficient (Vc) and the average germination time (At) proposed by [
9].
The results in
Figure 5 illustrate that the treatment with 100 mM NaCl caused a decrease in the germination speed (23.58%) and a slight prolongation over time as compared to the control. On the other hand, the treatment with 150 mM induced a remarkable decrease in the germination speed (13.96% against 24.39%) and the average time as compared to the control (2.9 days against 4.1 days).
The statistical analysis according to the Student’s test shows that the treatment of 100 mM had no significant effect on the germination speed and the average time as compared to the control (≤0.05). On the other hand, the treatment with 150 mM NaCl had a highly significant effect on the germination speed as compared to the control (≤0.05).
3.1.4. Water Content
The humidity level must remain high in order for the seed to begin its germination phase successfully.
Figure 6 shows that the water content in the seeds decreased remarkably under the treatment of 100 mM and 150 mM NaCl.
In fact, the humidity of the seeds treated at 100 mM reached half that of the controls, while the humidity levels of the batches of seeds treated at 150 mM were very low as compared to the control.
The statistical study according to the Student’s test indicates that the treatment with 100 mM and 150 Mm of NaCl had a very highly significant effect on the moisture content of the seeds of Abelmoschus esculentus as compared to the control (≤0.05).
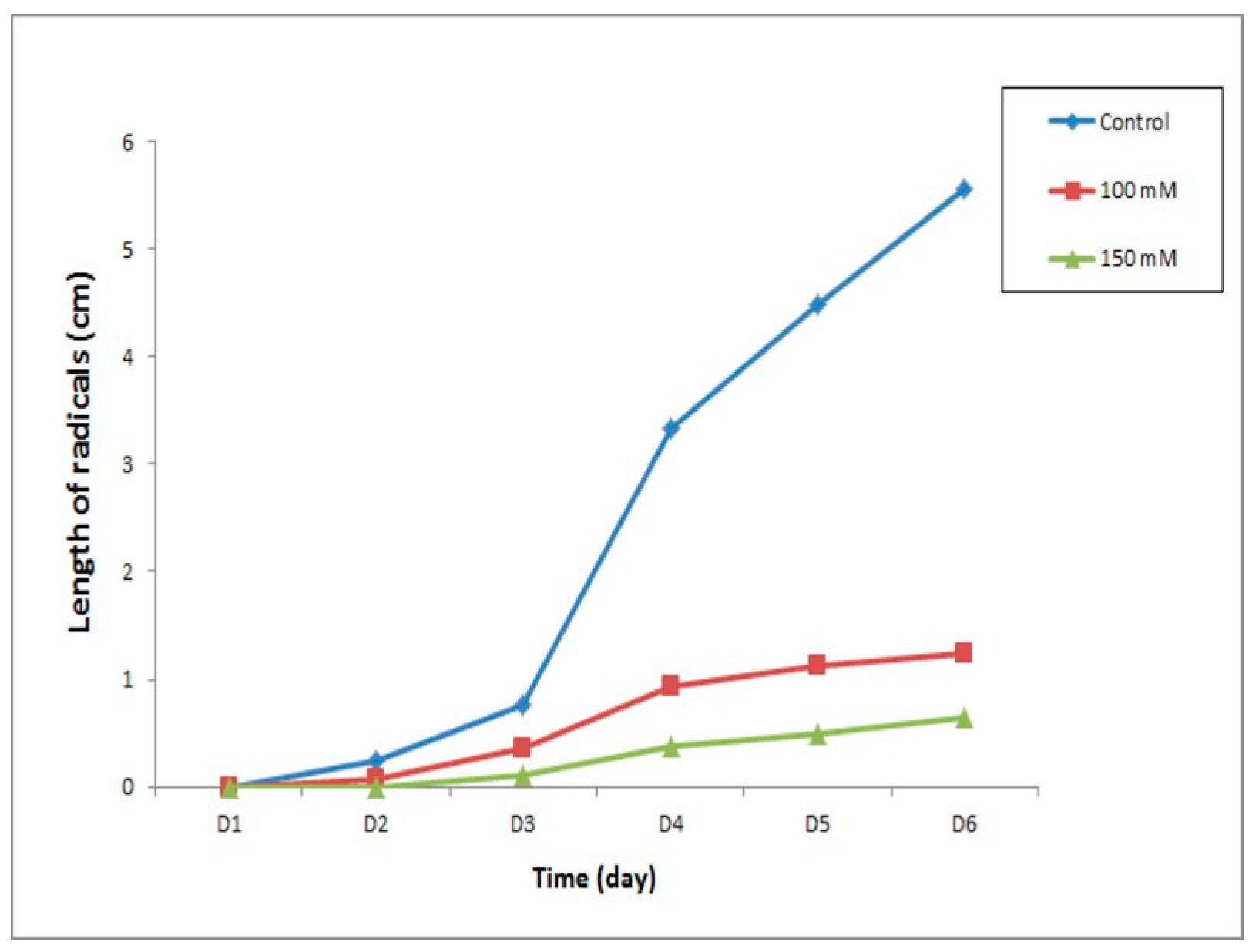
3.1.5. Radicle Length
Figure 7 shows that the radical length of the control seeds increased rapidly as a function of time until a maximum of 5.56 cm was reached; on the other hand, the growth in the radical length of the seeds treated with NaCl, whatever the concentration (100 mM, 150 mM), increased slowly as compared to the control.
In fact, the radical length of the control seeds reached four times the radical length of the seeds treated at 100 mM and eight times the radical length of the seeds treated at 150 mM on the 6th day of sowing.
The statistical analysis according to the Student t test reveals that the treatment with 150 mM NaCl had a highly significant effect on the length of the roots at the germination stage as compared to the control (≤0.05), unlike the treatment with 100 mM n, which had no significant effect on root length at the seed germination phase as compared to the control (≤0.05).
4. Discussion
Seed germination is a set of metabolic processes leading to the emergence of the radical. This stage of development is considered a critical stage in establishing seedlings and thus determining successful agricultural production [
6].
Knowledge of salinity tolerance at the time of germination reveals the ability of the species to grow on very saline soils [
10].
Ref. [
11] shows that the germination rate could be considered as an early criterion for the selection of plant species tolerant to saline stress.
Some researchers show that increasing the salt concentration reduces the final germination percentage [
12].
Several studies show that in halophytic and glycophytic plants, salinity delays the initiation of germination and reduces the germinative capacity of seeds [
13,
14,
15,
16].
Similar remarks are recorded in our conditions, whose treatment with 100 mM and 150 mM NaCl causes a remarkable decrease in the germination rate of Abelmoschus esculentus L. seeds.
Recent work reveals that higher concentrations of sodium chloride have an effect on time and speed [
12,
17,
18].
Ref. [
11] shows that the germination speeds of the three legumes tested (soybeans, beans, cowpeas) are strongly affected, and they decrease with the increase in the NaCl concentration.
Our results reveal that changing the NaCl concentrations induces a decrease in germination speed and average time as compared to controls.
Ref. [
19] reveals that the saline treatment of okra seeds during germination influences the growth and development of the seedlings and slows down the elongation of the root axis.
Similar observations are recorded in our conditions, in which the radical length of control seeds reaches four times the radical length of seeds treated at 100 mM and eight times the radical length of seeds treated at 150 mM.
In addition, our results confirm that there is a negative correlation between the fresh and dry weight of okra seeds and the evolution of the NaCl concentration.
5. Conclusions
Monitoring the germination of Abelmoschus esculentus L. seeds indicates that this species seems to express sensitivity to the action of salinity. However, the degree of sensitivity depends on the intensity of the stress.
According to the results obtained, it is possible to retain the essential points:
- -
Germination starts on the 2nd day after sowing for batches of seeds tested at 100 mM and on the 3rd day after sowing for batches tested at 150 mM. Therefore, this NaCl treatment causes a remarkable decrease in the seed germination rate as compared to the control.
- -
A rapid decrease in the germination speed is observed in the batches of seeds stressed with NaCl as compared to those of the controls.
- -
The radical length of the control seeds reached four times the radical length of the seeds treated at 100 mM and eight times the radical length of the seeds treated at 150 mM, which explains the detrimental effect of NaCl on the growth of radicals of stressed seeds. In addition, there is a negative correlation between the fresh and dry weight of okra seeds and the evolution of the NaCl concentration.
Author Contributions
Conceptualization, methodology, investigation, resources, data curation, writing—original draft preparation, and visualization, K.D.; Software, validation, writing—review and editing, supervision, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy reason.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and droughttolerance in plants. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Nedjimi, B.; Bekai, Z.; Guit, B.; Toumi Met Daoud, Y. Germination et croissance d’Atriplex halimus subsp. schweinfurthii en présence de CaCl2. Alger. J. Arid. Environ. 2013, 3, 15–23. [Google Scholar]
- Nichols, P.G.H.; Malik, A.I.; Stockdale, M.; Colmer, T.D. Salt tolerance and avoidance mechanisms at germination of annual pasture legumes: Importance for adaptation to saline environments. Plant Soil 2009, 315, 241–255. [Google Scholar] [CrossRef]
- Mrani Alaoui, M.; El Jourmi, L.; Ouarzane, A.; Lazar, S.; El Antri, S.; Zahouily, M.; Hmyene, A. Effet du stress salin sur la germination et la croissance de six variétés marocaines de blé. J. Mater. Environ. Sci. 2013, 4, 997–1004. [Google Scholar]
- Benidire, L.; Daoui, K.; Fatemi, Z.A.; Achouak, W.; Bouarab, L.; Oufdou, K. Effet du stress salin sur la germination et le développement des plantules de Vicia faba L. J. Mater. Environ. Sci. 2015, 6, 840–851. [Google Scholar]
- Ibn Maaouia-Houimli, S.; Denden, M.; Bouthaina, D.M.; Ben Mansour-Gueddes, S. Caractéristiques de la croissance et de la production en fruits chez trois variétés de piment (Capsicum annuum L.) sous stress salin. Tropicultura 2011, 29, 75–81. [Google Scholar]
- Nana, R.; Tamini, Z.; Sawadogo, M. Effets d’un stress hydrique intervenu pendant le stade végétatif et la phase de floraison chez le gombo. Int. J. Biol. Chem. Sci. 2009, 3, 1161–1170. [Google Scholar] [CrossRef][Green Version]
- Côme, D. Les Obstacles à la Germination (Monographie et Physiologie Végétale); Masson et Cie: Paris, France, 1970; p. 162. [Google Scholar]
- Kotowski, F. Les relations de température à lagermination des semences de légumes. Actes L’american Soc. Hortic. Sci. 1926, 23, 176–184. [Google Scholar]
- Jaouadi, W.; Hamrouni, L.; Souayeh, N.; Khouja, M.L. Etude de la germination des graines d’Acaciatortilis subsp. Raddiana sous différentes contraintes abiotiques. Biotechnol. Agron. Soc. Environ. 2010, 14, 643–652. [Google Scholar]
- Camara, B.; Sanogo, S.; Cherif, M.; Kone, D. Effet du stress salin sur la germination des graines de trois légumineuses (Phaseolusvulgaris, Glycinemax et Vignaunguiculata). J. Appl. Biosci. 2018, 124, 12424–12432. [Google Scholar]
- El-Goumi, Y.; Fakiri, M.; Lamsaouri, O.; Benchekroun, M. Salt stress effect on seed germination and some physiological traits in three Maroccan barley (Hordeumvulgare L.) cultivas. J. Mater. Environ. Sci. 2014, 5, 625–632. [Google Scholar]
- Abbes, S.T.; Pervez, M.A.; Ayyub, C.M.; Ahmed, R. Assessement of morphologicale, antioxidand, biochemical and ionic reponses of salt tolerant and sensitive okra (Abelmoschus esculentus L.) under saline regime. Pak J. Life Soc. Sci. 2013, 11, 147–153. [Google Scholar]
- Bouda, S.; Haddioui, A. Effet du stress salin sur la germinationde quelques espèces du genre Atriplex sp. Nat. Technol. 2011, 5, 72–79. [Google Scholar]
- Ly, M.O.; Kumar, D.; Diouf, M.; Nautiyal, S.; Diop, T. Effet de la salinité sur la croissance et la production de biomasse de deux provenances de Jatrophacurcas L. cultivés en serre. Int. J. Biol. Chem. Sci. 2014, 8, 46–56. [Google Scholar] [CrossRef][Green Version]
- Salehi, M.; Arzani, A. Evaluation of triticale genotypes for salt tolerance using physiological traits. Emir. J. Food Agric 2014, 26, 277–283. [Google Scholar] [CrossRef]
- Boumia, O. Interaction Fluridone et Salinité sur la Germination des Graines du Gombo (Abelmoschus esculentus L.) Mémoire de Magisteren Physiologie Végétale. Ph.D. Thesis, University of Oran 1—Ahmed Ben Bella, Es Sénia, Algeria, 2011; pp. 1–130. [Google Scholar]
- Ouis, M. Recherche des Marqueurs Biochimique de la Tolérance à la Salinité Chez le Gombo (Abelmoschus esculentus L.). Ph.D. Thesis, University of Oran 1—Ahmed Ben Bella, Es Sénia, Algeria, 2016; pp. 1–115. [Google Scholar]
- Achour, A. Caractérisations Physiologique et Biochimique du Gombo (Abelmoschus esculentus L.) Sous Stress Salin. Ph.D. Thesis, University of Oran 1—Ahmed Ben Bella, Es Sénia, Algeria, 2016; pp. 1–120. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).