Essential Oils as Potential Biopesticides in the Control of the Genus Meloidogyne: A Review †
Abstract
:1. Introduction
2. RKN Pest Management
3. Essential Oils as RKN Biopesticides
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Barcala, M.; Cabrera, J.; Fenoll, C. Chapter One—Overview of Root-Knot Nematodes and Giant Cells. In Plant Nematode Interactions; Escobar, C., Fenoll, C., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; Volume 73, pp. 1–32. ISBN 0065-2296. [Google Scholar]
- Organization European and Mediterranean Plant Protection (EPPO) EPPO Quarentine Pests: Meloidogyne Chitwoodi. Available online: https://gd.eppo.int (accessed on 1 December 2020).
- Orion, D.; Kritzman, G.; Meyer, S.L.F.; Erbe, E.F.; Chitwood, D.J. A role of the gelatinous matrix in the resistance of root-knot nematode (Meloidogyne spp.) eggs to microorganisms. J. Nematol. 2001, 33, 203–207. [Google Scholar] [PubMed]
- Gal, T.Z.; Aussenberg, E.R.; Burdman, S.; Kapulnik, Y.; Koltai, H. Expression of a plant expansin is involved in the establishment of root knot nematode parasitism in tomato. Planta 2006, 224, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.L.; Hussey, R.S.; Mitchum, M.G.; Baum, T.J. Parasitism proteins in nematode-plant interactions. Curr. Opin. Plant Biol. 2008, 11, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.H.; Fester, T.; Taylor, C.G. Development of the Root-Knot Nematode Feeding Cell BT—Cell Biology of Plant Nematode Parasitism; Berg, R.H., Taylor, C.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 115–152. ISBN 978-3-540-85215-5. [Google Scholar]
- Kaloshian, I.; Teixeira, M. Advances in Plant−Nematode Interactions with Emphasis on the Notorious Nematode Genus Meloidogyne. Phytopathology 2019, 109, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, A.; Zhang, M.Y.; Wang, Y.Y.; Zhu, X.F.; Liu, X.Y.; Fan, H.Y.; Xuan, Y.H.; Chen, L.J.; Duan, Y.X. Review article: Meloidogyne incognita (root-knot nematode) a risk to agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1679–1690. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Lovelli, S.; Candido, V.; Avato, P. Biocide plants as a sustainable tool for the control of pests and pathogens in vegetable cropping systems. Ital. J. Agron. 2014, 9, 137–145. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Laquale, S.; Avato, P.; Argentieri, M.P.; Bellardi, M.G.; D’Addabbo, T. Nematotoxic activity of essential oils from Monarda species. J. Pest Sci. (2004). 2018, 91, 1115–1125. [Google Scholar] [CrossRef]
- Kalaiselvi, D.; Mohankumar, A.; Shanmugam, G.; Thiruppathi, G.; Nivitha, S.; Sundararaj, P. Altitude-related changes in the phytochemical profile of essential oils extracted from Artemisia nilagirica and their nematicidal activity against Meloidogyne incognita. Ind. Crops Prod. 2019, 139, 111472. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Maleita, C.M.N.; Abrantes, I.; Bennett, R.; Mota, M.; da Silva Figueiredo, A.C. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. (2004). 2016, 89, 207–217. [Google Scholar] [CrossRef]
- León-Méndez, G.; Pájaro-Castro, N.; Pájaro-Castro, E.; Torrenegra- Alarcón, M.; Herrera-Barros, A. Essential oils as a source of bioactive molecules. Rev. Colomb. Ciencias Químico-Farmacéuticas 2019, 48, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Singh, A.; Ajayakumar, P.V.; Pandey, R. Microbial interference mitigates Meloidogyne incognita mediated oxidative stress and augments bacoside content in Bacopa monnieri L. Microbiol. Res. 2017, 199, 67–78. [Google Scholar] [CrossRef] [PubMed]
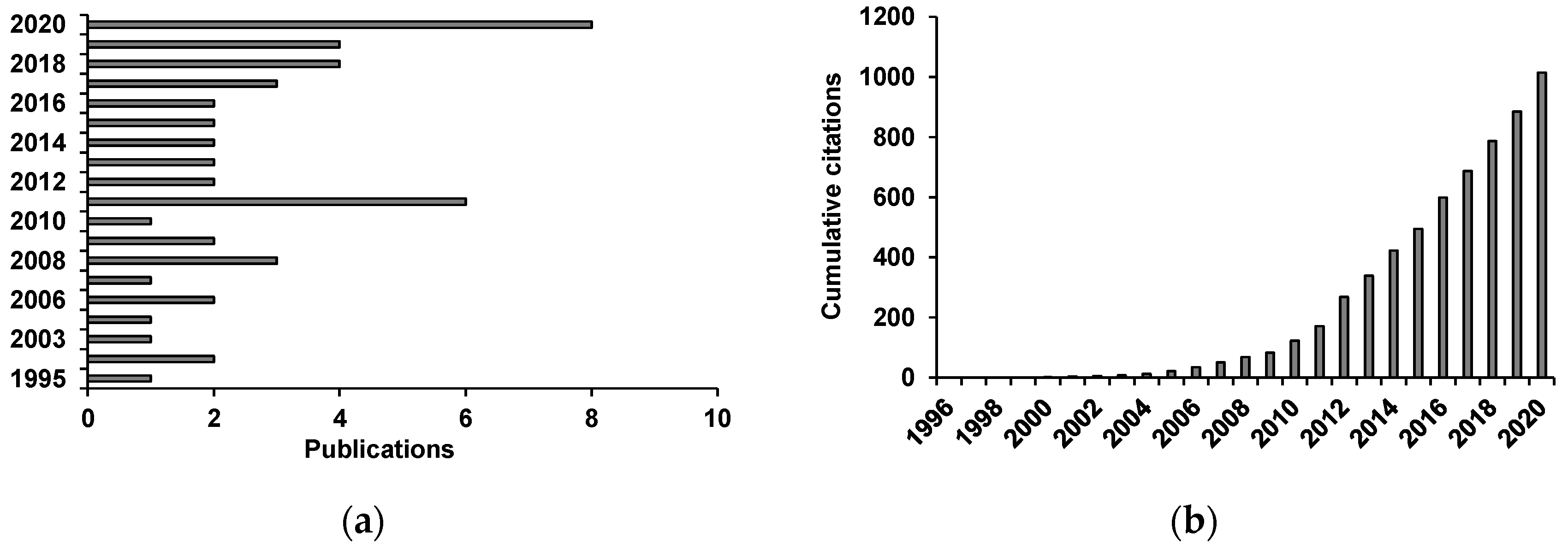
 ) of the most biologically active essential oils, organized by family and EC50.
) of the most biologically active essential oils, organized by family and EC50.
 ) of the most biologically active essential oils, organized by family and EC50.
) of the most biologically active essential oils, organized by family and EC50.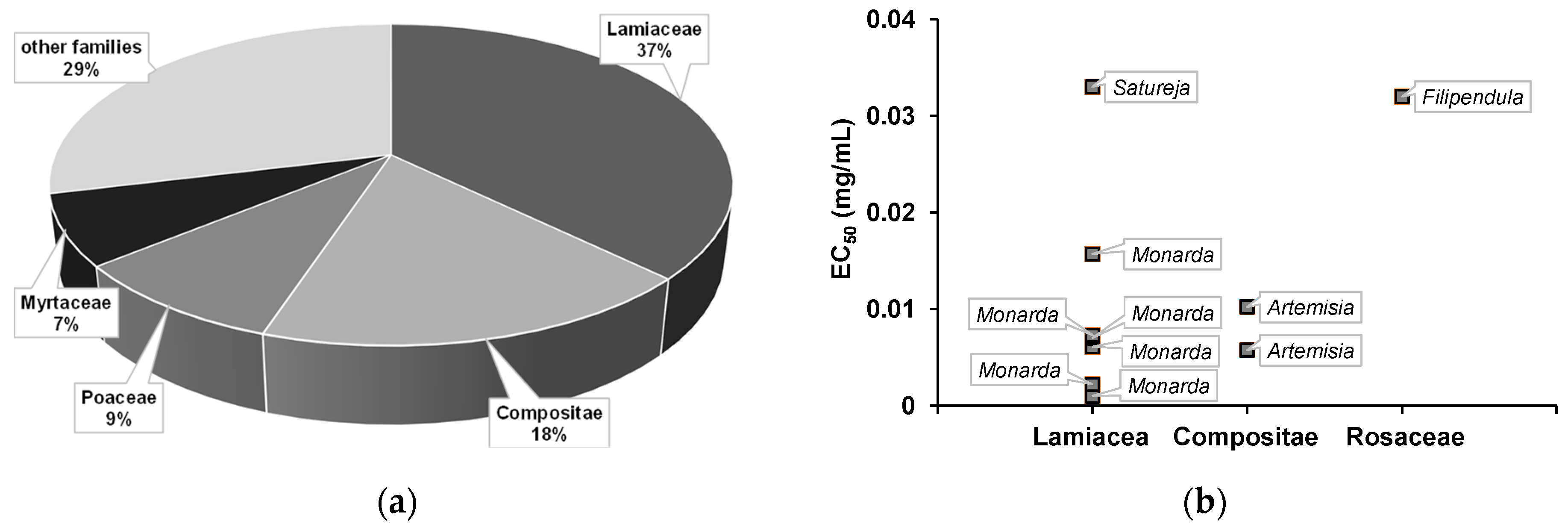
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Rodrigues, A.M. Essential Oils as Potential Biopesticides in the Control of the Genus Meloidogyne: A Review. Biol. Life Sci. Forum 2021, 3, 26. https://doi.org/10.3390/IECAG2021-09687
Faria JMS, Rodrigues AM. Essential Oils as Potential Biopesticides in the Control of the Genus Meloidogyne: A Review. Biology and Life Sciences Forum. 2021; 3(1):26. https://doi.org/10.3390/IECAG2021-09687
Chicago/Turabian StyleFaria, Jorge M. S., and Ana Margarida Rodrigues. 2021. "Essential Oils as Potential Biopesticides in the Control of the Genus Meloidogyne: A Review" Biology and Life Sciences Forum 3, no. 1: 26. https://doi.org/10.3390/IECAG2021-09687
APA StyleFaria, J. M. S., & Rodrigues, A. M. (2021). Essential Oils as Potential Biopesticides in the Control of the Genus Meloidogyne: A Review. Biology and Life Sciences Forum, 3(1), 26. https://doi.org/10.3390/IECAG2021-09687






