Profiling the Variability of Eucalyptus Essential Oils with Activity against the Phylum Nematoda †
Abstract
1. Introduction
2. Chemical Variability of Eucalyptus Essential Oils
3. Chemical Composition of Active EOs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Marbot, R.; Quert, R.; García, H. Study of essential oils of Eucalyptus resinifera Smith, E. tereticornis Smith and Corymbia maculata (Hook.) K. D. Hill & L. A. S. Johnson, grown in Cuba. Flavour Fragr. J. 2002, 17, 1–4. [Google Scholar] [CrossRef]
- Keszei, A.; Brubaker, C.L.; Foley, W.J. A molecular perspective on terpene variation in Australian Myrtaceae. Aust. J. Bot. 2008, 56, 197–213. [Google Scholar] [CrossRef]
- Keszei, A.; Brubaker, C.L.; Carter, R.; Köllner, T.; Degenhardt, J.; Foley, W.J. Functional and evolutionary relationships between terpene synthases from Australian Myrtaceae. Phytochemistry 2010, 71, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.L.; Keszei, A.; Foley, W.J. Intensive sampling identifies previously unknown chemotypes, population divergence and biosynthetic connections among terpenoids in Eucalyptus tricarpa. Phytochemistry 2013, 94, 148–158. [Google Scholar] [CrossRef]
- Padovan, A.; Keszei, A.; Külheim, C.; Foley, W.J. The evolution of foliar terpene diversity in Myrtaceae. Phytochem. Rev. 2014, 13, 695–716. [Google Scholar] [CrossRef]
- Bustos-Segura, C.; Dillon, S.; Keszei, A.; Foley, W.J.; Külheim, C. Intraspecific diversity of terpenes of Eucalyptus camaldulensis (Myrtaceae) at a continental scale. Aust. J. Bot. 2017, 65, 257. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Lima, A.S.; Mendes, M.D.; Leiria, R.; Geraldes, D.A.; Figueiredo, A.C.; Trindade, H.; Pedro, L.G.; Barroso, J.G.; Sanches, J. Eucalyptus from Mata Experimental do Escaroupim (Portugal): Evaluation of the essential oil composition from sixteen species. Acta Hortic. 2011, 925, 61–66. [Google Scholar] [CrossRef]
- Figueiredo, A.C. Biological properties of essential oils and volatiles: Sources of variability. Nat. Volatiles Essent. Oils 2017, 4, 1–13. [Google Scholar]
- Rodrigues, A.M.; Mendes, M.D.; Lima, A.S.; Barbosa, P.M.; Ascensão, L.; Barroso, J.G.; Pedro, L.G.; Mota, M.M.; Figueiredo, A.C. Pinus halepensis, Pinus pinaster, Pinus pinea and Pinus sylvestris essential oils chemotypes and monoterpene hydrocarbon enantiomers, before and after inoculation with the pinewood nematode Bursaphelenchus xylophilus. Chem. Biodivers. 2017, 14, e1600153. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Basyoni, M.M.A.; Rizk, E.M.A. Nematodes ultrastructure: Complex systems and processes. J. Parasit. Dis. 2016, 40, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.K.; Traboulsi, A.F.; El-Haj, S. Effect of essential oils and plant extracts on hatching, migration and mortality of Meloidogyne incognita. Phytopathol. Mediterr. 2006, 45, 238–246. [Google Scholar] [CrossRef]
- Macedo, I.T.F.; Bevilaqua, C.M.L.; de Oliveira, L.M.B.; CamurÇa-Vasconcelos, A.L.F.; Vieira, L.D.S.; Oliveira, F.R.; Queiroz-Junior, E.M.; Portela, B.G.; Barros, R.S.; Chagas, A.C.S. Atividade ovicida e larvicida in vitro do óleo essencial de Eucalyptus globulus sobre Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2009, 18, 62–66. [Google Scholar] [CrossRef]
- Macedo, I.T.F.; Bevilaqua, C.M.L.; de Oliveira, L.M.B.; Camurça-Vasconcelos, A.L.F.; Vieira, L.D.S.; Oliveira, F.R.; Queiroz-Junior, E.M.; Tomé, A.D.R.; Nascimento, N.R.F. Anthelmintic effect of Eucalyptus staigeriana essential oil against goat gastrointestinal nematodes. Vet. Parasitol. 2010, 173, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Macedo, I.T.F.; Bevilaqua, C.M.L.; de Oliveira, L.M.B.; Camurça-Vasconcelos, A.L.F.; Vieira, L.D.S.; Amóra, S.D.S.A. Evaluation of Eucalyptus citriodora essential oil on goat gastrointestinal nematodes. Rev. Bras. Parasitol. Vet. 2011, 20, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Phytochemistry and nematicidal activity of the essential oils from 8 greek lamiaceae aromatic plants and 13 terpene components. J. Agric. Food Chem. 2010, 58, 7856–7863. [Google Scholar] [CrossRef]
- Mesquita, M.D.A.; Júnior, J.B.E.S.; Panassol, A.M.; de Oliveira, E.F.; Vasconcelos, A.L.C.F.; de Paula, H.C.B.; Bevilaqua, C.M.L. Anthelmintic activity of Eucalyptus staigeriana encapsulated oil on sheep gastrointestinal nematodes. Parasitol. Res. 2013, 112, 3161–3165. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, W.L.C.; Macedo, I.T.F.; dos Santos, J.M.L.; de Oliveira, E.F.; Camurça-Vasconcelos, A.L.F.; de Paula, H.C.B.; Bevilaqua, C.M.L. Activity of chitosan-encapsulated Eucalyptus staigeriana essential oil on Haemonchus contortus. Exp. Parasitol. 2013, 135, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Ribeiro, W.L.C.; Camurça-Vasconcelos, A.L.F.; Macedo, I.T.F.; Santos, J.M.L.; Paula, H.C.B.; Araújo Filho, J.V.; Magalhães, R.D.; Bevilaqua, C.M.L. Efficacy of free and nanoencapsulated Eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Vet. Parasitol. 2014, 204, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Laquale, S.; Candido, V.; Avato, P.; Argentieri, M.P.; D’Addabbo, T. Essential oils as soil biofumigants for the control of the root-knot nematode Meloidogyne incognita on tomato. Ann. Appl. Biol. 2015, 167, 217–224. [Google Scholar] [CrossRef]
- Ribeiro, W.L.C.; Camurça-Vasconcelos, A.L.F.; Macedo, I.T.F.; dos Santos, J.M.L.; de Araújo-Filho, J.V.; Ribeiro, J.D.C.; Pereira, V.D.A.; Viana, D.D.A.; de Paula, H.C.B.; Bevilaqua, C.M.L. In vitro effects of Eucalyptus staigeriana nanoemulsion on Haemonchus contortus and toxicity in rodents. Vet. Parasitol. 2015, 212, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, W.L.C.; Camurça-Vasconcelos, A.L.F.; dos Santos, J.M.L.; Macedo, I.T.F.; Ribeiro, J.D.C.; de Oliveira, E.F.; de Paula, H.C.B.; Bevilaqua, C.M.L. The use of Eucalyptus staigeriana nanoemulsion for control of sheep haemonchosis. Pesqui. Vet. Bras. 2017, 37, 221–226. [Google Scholar] [CrossRef][Green Version]
- De Araújo-Filho, J.V.; Ribeiro, W.L.C.; André, W.P.P.; Cavalcante, G.S.; Guerra, M.D.C.M.; Muniz, C.R.; Macedo, I.T.F.; Rondon, F.C.M.; Bevilaqua, C.M.L.; de Oliveira, L.M.B. Effects of Eucalyptus citriodora essential oil and its major component, citronellal, on Haemonchus contortus isolates susceptible and resistant to synthetic anthelmintics. Ind. Crops Prod. 2018, 124, 294–299. [Google Scholar] [CrossRef]
- De Araújo-Filho, J.V.; Ribeiro, W.L.C.; André, W.P.P.; Cavalcante, G.S.; Rios, T.T.; Schwinden, G.M.; da Rocha, L.O.; Macedo, I.T.F.; de Morais, S.M.; Bevilaqua, C.M.L.; et al. Anthelmintic activity of Eucalyptus citriodora essential oil and its major component, citronellal, on sheep gastrointestinal nematodes. Rev. Bras. Parasitol. Vet. 2019, 28, 644–651. [Google Scholar] [CrossRef]
- Kundu, A.; Dutta, A.; Mandal, A.; Negi, L.; Malik, M.; Puramchatwad, R.; Antil, J.; Singh, A.; Rao, U.; Saha, S.; et al. A Comprehensive in vitro and in silico Analysis of Nematicidal Action of Essential Oils. Front. Plant Sci. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lanzerstorfer, P.; Sandner, G.; Pitsch, J.; Mascher, B.; Aumiller, T.; Weghuber, J. Acute, reproductive, and developmental toxicity of essential oils assessed with alternative in vitro and in vivo systems. Arch. Toxicol. 2021, 95, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Maleita, C.M.N.; Abrantes, I.; Bennett, R.; Mota, M.; Figueiredo, A.C.D.S. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. 2016, 89, 207–217. [Google Scholar] [CrossRef]

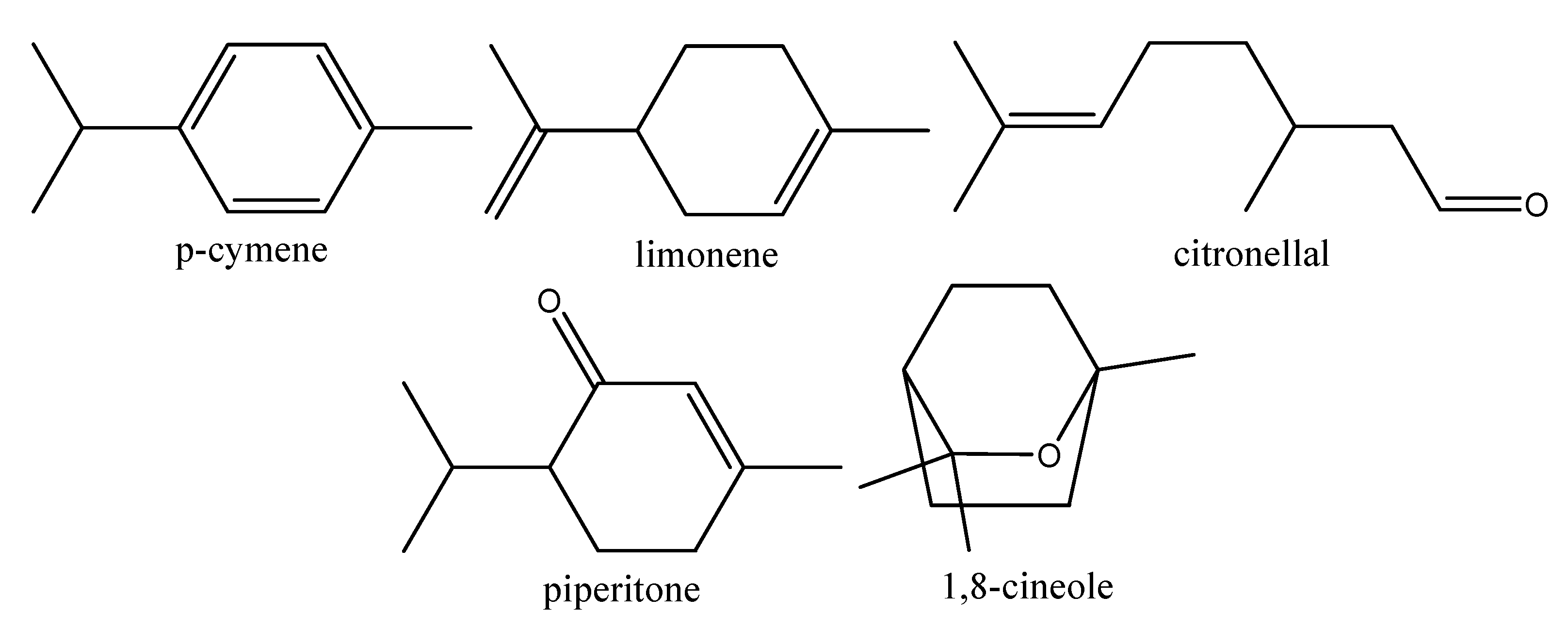
| EO Components (%) | Plant Parasitic Nematodes | Animal Parasitic Nematodes | Free Living Nematodes |
|---|---|---|---|
| p-Cymene | 3.1–25.0 | 2.6 | 3.8 |
| 1,8-Cineole | 1.3–91.5 | 1.7–83.9 | 82.6 |
| Limonene | - | 7.0–72.9 | 7.7 |
| Citronellal | 35.8–83.8 | 5.5–71.8 | - |
| Piperitone | 40.2 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.M.; Faria, J.M.S. Profiling the Variability of Eucalyptus Essential Oils with Activity against the Phylum Nematoda. Biol. Life Sci. Forum 2021, 2, 26. https://doi.org/10.3390/BDEE2021-09425
Rodrigues AM, Faria JMS. Profiling the Variability of Eucalyptus Essential Oils with Activity against the Phylum Nematoda. Biology and Life Sciences Forum. 2021; 2(1):26. https://doi.org/10.3390/BDEE2021-09425
Chicago/Turabian StyleRodrigues, Ana Margarida, and Jorge M. S. Faria. 2021. "Profiling the Variability of Eucalyptus Essential Oils with Activity against the Phylum Nematoda" Biology and Life Sciences Forum 2, no. 1: 26. https://doi.org/10.3390/BDEE2021-09425
APA StyleRodrigues, A. M., & Faria, J. M. S. (2021). Profiling the Variability of Eucalyptus Essential Oils with Activity against the Phylum Nematoda. Biology and Life Sciences Forum, 2(1), 26. https://doi.org/10.3390/BDEE2021-09425







