Lactide Synthesis Using ZnO Aqueous Nanoparticles as Catalysts †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
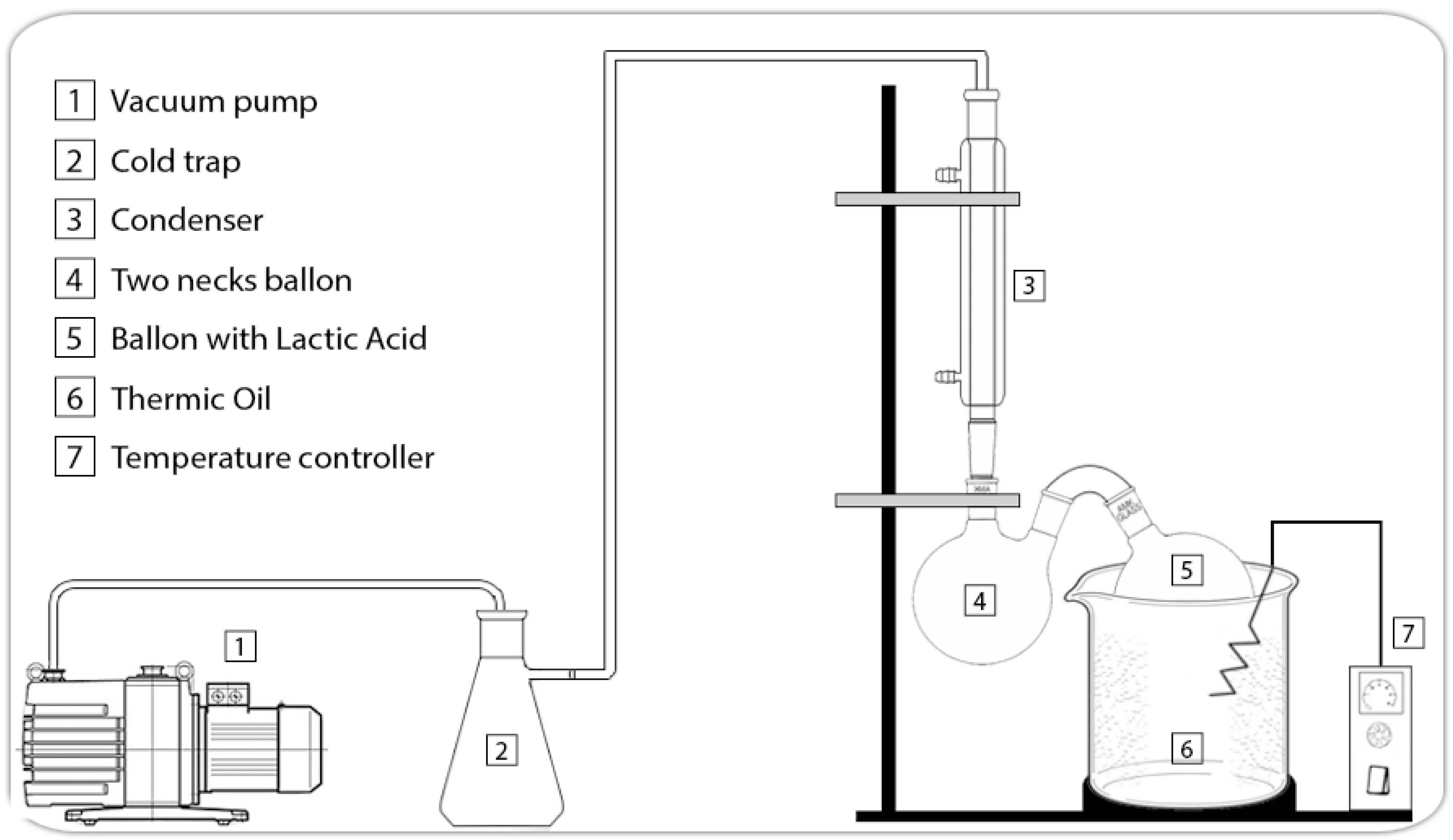
2.2. Lactide Synthesis
2.3. Lactide Purification
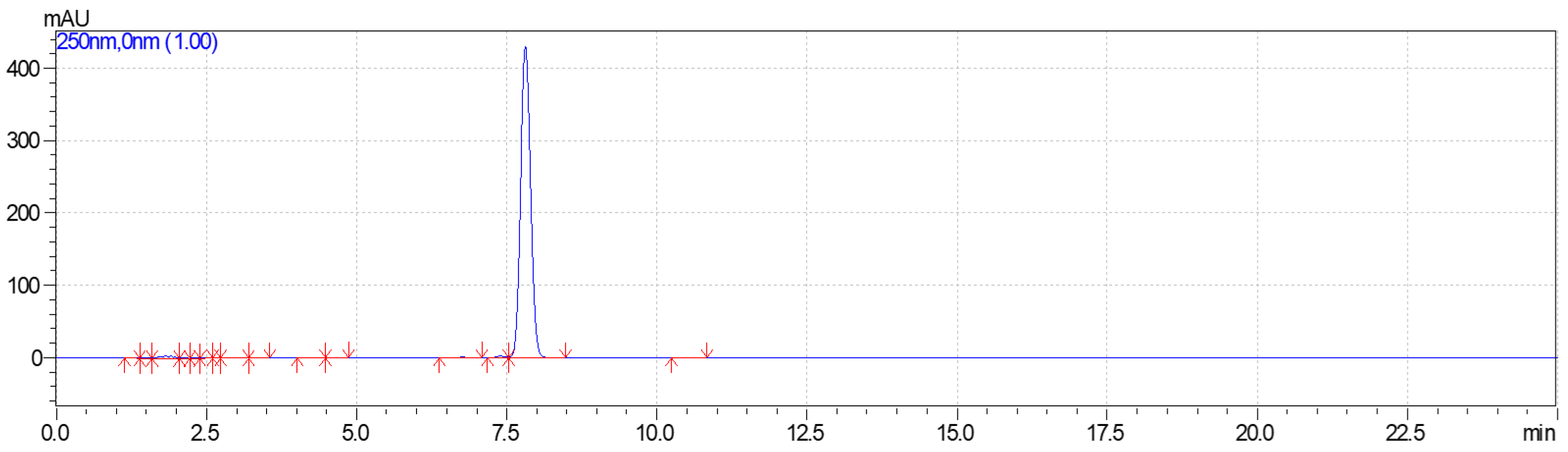
2.4. Lactide Quantification
2.5. Lactide Characterization
3. Results
3.1. Lactide Purification
3.2. Lactide Quantification
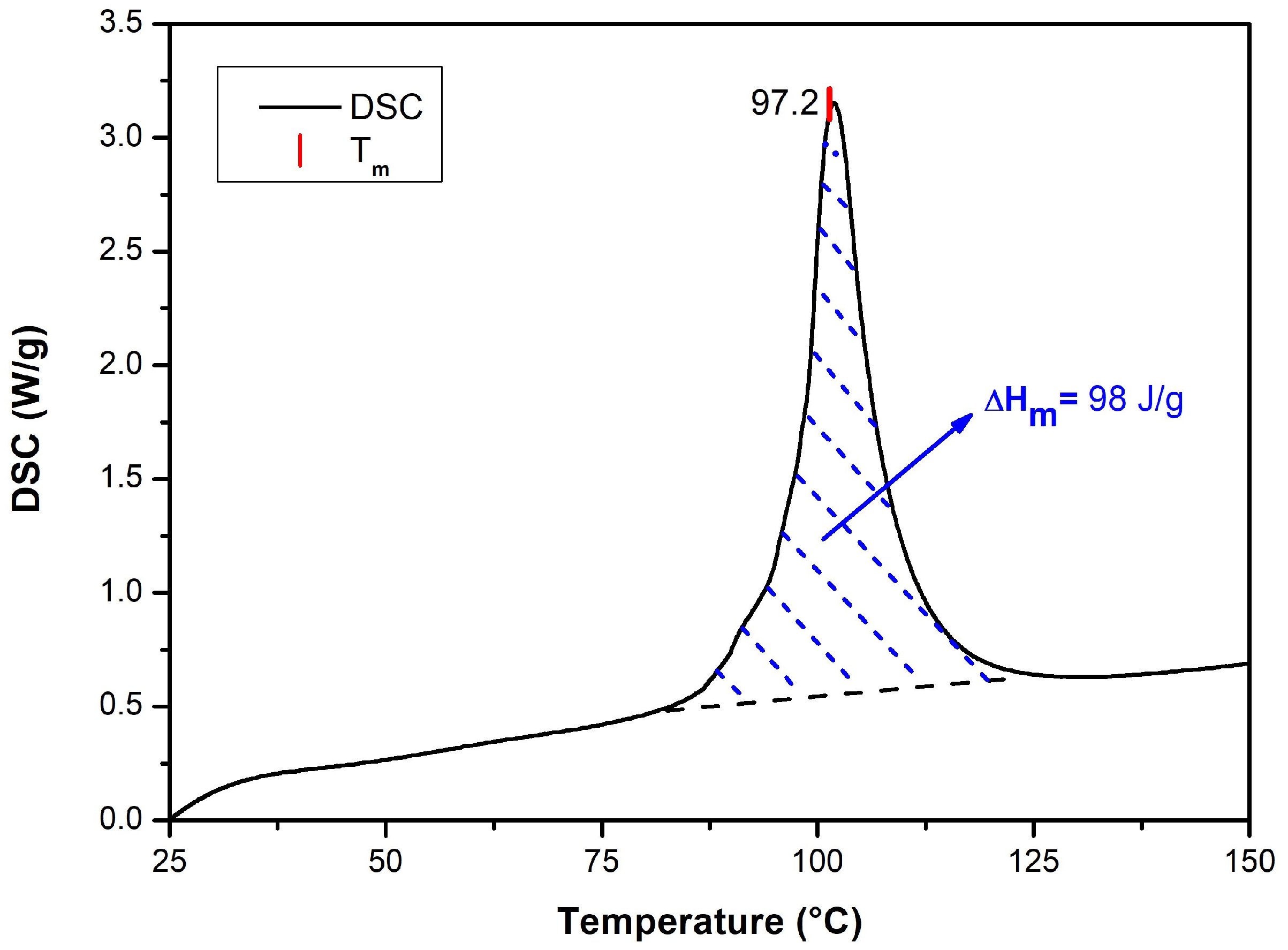
3.3. Lactide Characterization
4. Discussion
4.1. Conversion and Production Yields of Lactide
4.2. Characterization of Lactide
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butbunchu, N.; Pathom-Aree, W. Actinobacteria as Promising Candidate for Polylactic Acid Type Bioplastic Degradation. Front. Microbiol. 2019, 10, 2834. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.; Kamal, M.; Heuzey, M. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- NatureWorks. Ingeo. 2022. Available online: https://www.natureworksllc.com/ (accessed on 19 October 2022).
- Nuyken, O.; Pask, S. Ring-opening polymerization-An introductory review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- De Albuquerque, T.; Marques Júnior, J.; de Queiroz, L.; Ricardo, A.; Rocha, M. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S. Synthesis of poly(lactic acid): A review. J. Macromol. Sci. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.; Cheuk, K.; Lin, C. Newly developed techniques on polycondensation, ring-opening polymerization and polymer modification: Focus on poly(lactic acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef]
- Vink, E.; Rábago, K.; Glassner, D.; Gruber, P. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Xu, X.; Liu, L. A study on highly concentrated lactic acid and the synthesis of lactide from its solution. J. Chem. Res. 2021, 10, 856–864. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.A.; Fei, B.; Chen, L.; Kwan, T.H.; Lin, C.S. Efficient ZnO aqueous nanoparticle catalysed lactide synthesis for poly(lactic acid) fibre production from food waste. J. Clean. Prod. 2017, 165, 157–167. [Google Scholar] [CrossRef]
- Tefara, S.F.; Jiru, E.B. Lactide synthesis via thermal catalytic depolymerization of poly lactic acid oligomer using ZnO nanoparticle dispersion. J. Polym. Res. 2023, 30, 146. [Google Scholar] [CrossRef]
- Dong, K.; Kim, D.; Doo, S. Synthesis of lactide from oligomeric PLA: Effects of temperature, pressure, and catalyst. Macromol. Res. 2006, 14, 510–516. [Google Scholar]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Sanglard, P.; Adamo, V.; Bourgeois, J.; Chappuis, T.; Vanoli, E. Universities of applied sciences. Chimia 2012, 6, 951–954. [Google Scholar] [CrossRef]
- Feng, L.; Gao, Z.; Bian, X.; Chen, Z.; Chen, X.; Chen, W. A quantitative HPLC method for determining lactide content using hydrolytic kinetics. Polym. Test. 2009, 28, 592–598. [Google Scholar] [CrossRef]
- Cunha, B.; Bahú, J.; Xavier, L.; Crivellin, S.; de Souza, S.; Lodi, L.; Jardini, A.; Maciel Filho, R.; Schiavon, M.; Cárdenas Concha, V.; et al. Lactide: Production Routes, Properties, and Applications. Bioengineering 2022, 9, 164. [Google Scholar] [CrossRef]
- Marchesan, A.; Motta, I.; Filho, R.; Maciel, M. Simulation of Multi-stage Lactic Acid Salting-out Extraction using Ethanol and Ammonium Sulfate. Comput. Aided Chem. Eng. 2020, 48, 1645–1650. [Google Scholar]
- Zhang, Y.; Qi, Y.; Yin, Y.; Sun, P.; Li, A.; Zhang, Q.; Jiang, W. Efficient Synthesis of Lactide with Low Racemization Catalyzed by Sodium Bicarbonate and Zinc Lactate. ACS Sustain. Chem. Eng. 2020, 8, 2865–2873. [Google Scholar] [CrossRef]
- Huang, W.; Qi, Y.; Cheng, N.; Zong, X.; Zhang, T.; Jiang, W.; Li, H.; Zhang, Q. Green synthesis of enantiomerically pure l-lactide and d-lactide using biogenic creatinine catalyst. Polym. Degrad. Stab. 2014, 101, 18–23. [Google Scholar] [CrossRef]
- Upare, P.; Hwang, Y.; Chang, J.; Hwang, D. Synthesis of lactide from alkyl lactate via a prepolymer route. Ind. Eng. Chem. Res. 2012, 51, 4837–4842. [Google Scholar] [CrossRef]
- Glotova, V.; Zamanova, M.; Yarkova, A.; Krutas, D.; Izhenbina, T.; Novikov, V. Influence of Storage Conditions on the Stability of Lactide. Procedia Chem. 2014, 10, 252–257. [Google Scholar] [CrossRef][Green Version]
- Peñaranda, J.; Agudelo, C.; Zuluaga, F.; Valencia, C. Synthesis of poly (lactic acid) and production of scaffolds by electrospinning. MOJ Proteomics Bioinform. 2016, 4, 334–338. [Google Scholar]
- Nyiavuevang, B.; Sodkampang, S.; Dokmaikun, S.; Thumanu, K.; Boontawan, A.; Junpirom, S. Effect of temperature and time for the production of polylactic acid without initiator catalyst from lactide synthesized from ZnO powder catalyst. J. Phys. Conf. Ser. 2022, 2175, 012042. [Google Scholar] [CrossRef]



| Run Number | ||||
|---|---|---|---|---|
| 1 | 68.71 | 77.91 | 54.51 | |
| 2 | 67.75 | 76.70 | 47.63 | 36.59 |
| 3 | 71.04 | 79.55 | 57.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, S.; Dullak, A.; Ferreira, F.P.; Oddone, M.; Riveros, D. Lactide Synthesis Using ZnO Aqueous Nanoparticles as Catalysts. Biol. Life Sci. Forum 2023, 28, 13. https://doi.org/10.3390/blsf2023028013
Duarte S, Dullak A, Ferreira FP, Oddone M, Riveros D. Lactide Synthesis Using ZnO Aqueous Nanoparticles as Catalysts. Biology and Life Sciences Forum. 2023; 28(1):13. https://doi.org/10.3390/blsf2023028013
Chicago/Turabian StyleDuarte, Shirley, Axel Dullak, Francisco P. Ferreira, Marcelo Oddone, and Darío Riveros. 2023. "Lactide Synthesis Using ZnO Aqueous Nanoparticles as Catalysts" Biology and Life Sciences Forum 28, no. 1: 13. https://doi.org/10.3390/blsf2023028013
APA StyleDuarte, S., Dullak, A., Ferreira, F. P., Oddone, M., & Riveros, D. (2023). Lactide Synthesis Using ZnO Aqueous Nanoparticles as Catalysts. Biology and Life Sciences Forum, 28(1), 13. https://doi.org/10.3390/blsf2023028013






