Essential Oil Composition and Glandular Trichome Structure of the Weather Prophet Dimorphoteca pluvialis †
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
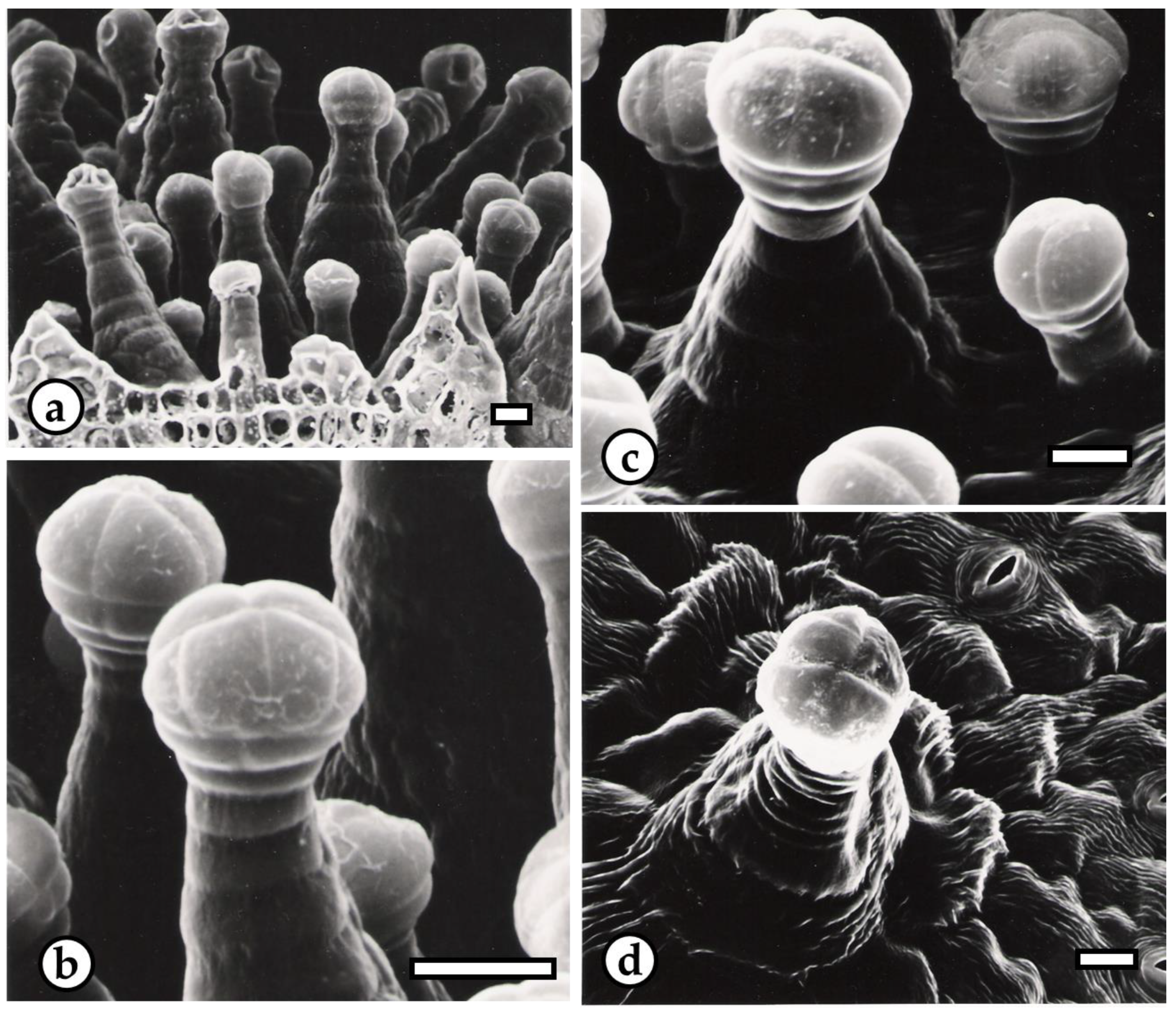
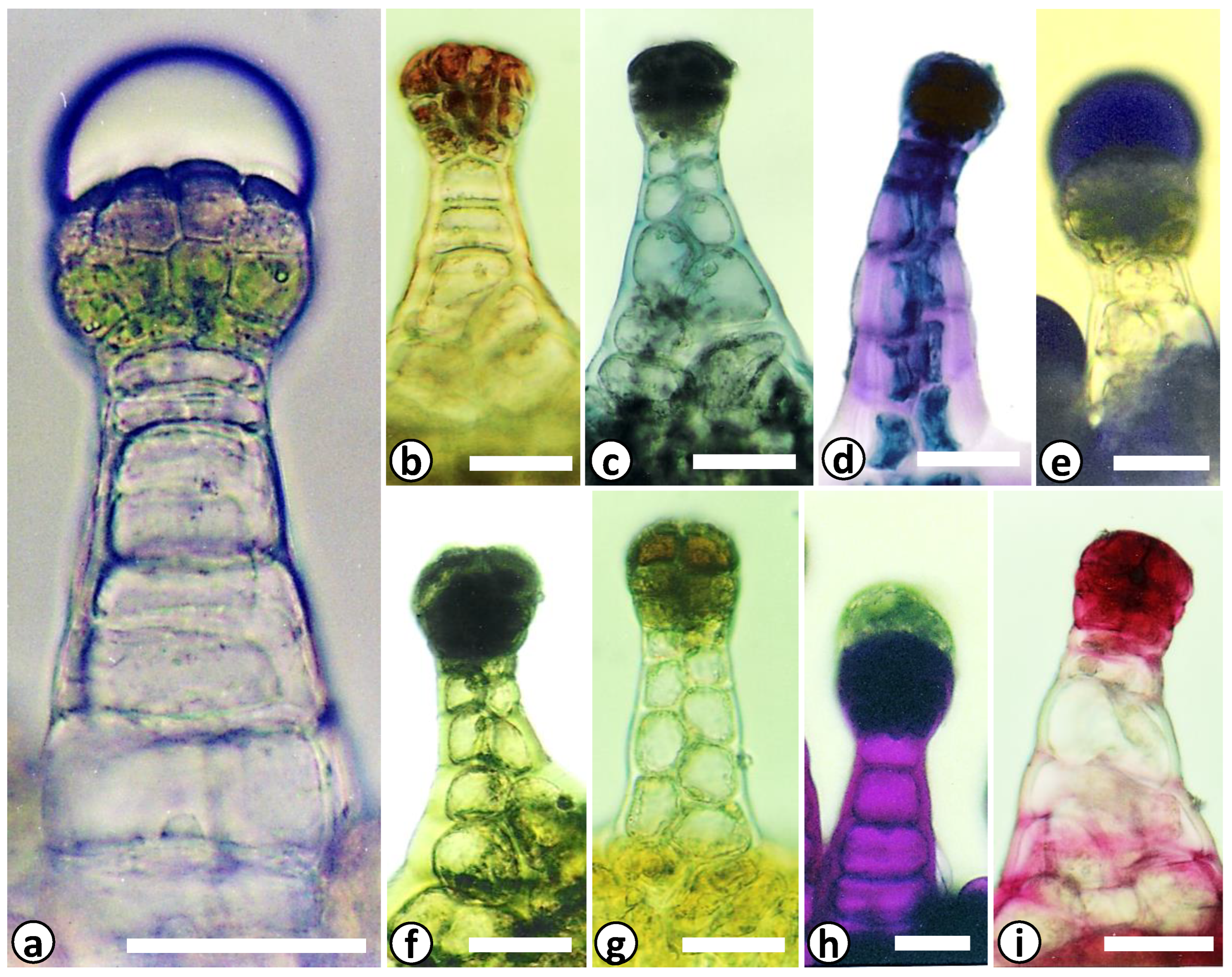
2.2. Structural and Chemical Characterization of Glandular Trichomes
2.3. Essential Oil Extraction and Analysis
2.4. Data Treatment and Statistical Analysis
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WFO—The World Flora Online Dimorphotheca Pluvialis Moench. Available online: http://www.worldfloraonline.org/taxon/wfo-0000127997 (accessed on 29 March 2023).
- Hof, L.; Nieboer, I.G.; Dolstra, O. Response to mass selection and estimation of heritability for oil content in Dimorphotheca pluvialis. Euphytica 1999, 106, 111–116. [Google Scholar] [CrossRef]
- Marvin, H.J.P.; Mastebroek, H.D.; Becu, D.M.S.; Janssens, R.J.J. Investigation into the Prospects of Five Novel Oilseed Crops within Europe. Outlook Agric. 2000, 29, 47–53. [Google Scholar] [CrossRef]
- Ascensao, L.; Marques, N.; Pais, M.S. Peltate glandular trichomes of Leonotis leonurus leaves: Ultrastructure and histochemical characterization of secretions. Int. J. Plant Sci. 1997, 158, 249–258. [Google Scholar] [CrossRef]
- Council of Europe; Commission European Pharmacopoeia European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia; European Directorate for the Quality of Medicines, Ed.; Council of Europe, European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2010; p. 241. [Google Scholar]
- Laterre, R.; Pottier, M.; Remacle, C.; Boutry, M. Photosynthetic Trichomes Contain a Specific Rubisco with a Modified pH-Dependent Activity. Plant Physiol. 2017, 173, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Corallo, A.; Gastaldo, P.; Romussi, G.; Ciarallo, G.; Fontana, N.; De Tommasi, N.; Profumo, P. Glandular hairs and secreted material in Salvia blepharophylla Brandegee ex Epling grown in Italy. Ann. Bot. 1999, 83, 441–452. [Google Scholar] [CrossRef]
- Wakai, J.; Kusama, S.; Nakajima, K.; Kawai, S.; Okumura, Y.; Shiojiri, K. Effects of trans-2-hexenal and cis-3-hexenal on post-harvest strawberry. Sci. Rep. 2019, 9, 10112. [Google Scholar] [CrossRef] [PubMed]
- Nádasi, E.; Varjas, T.; Pajor, L.; Ember, I. Carcinogenic potential of trans-2-hexenal is based on epigenetic effect. In Vivo 2005, 19, 559–562. [Google Scholar] [PubMed]
| Components | RI | Shoots | Flowers | ||||
|---|---|---|---|---|---|---|---|
| Time (min) | 15 | 30 | 60 | 15 | 30 | 60 | |
| Octene | 799 | 3.9 ± 0.1 a | 1.7 ± 0.1 b | 1.0 ± 0.4 b | 1.6 ± 0.2 a | 1.7 ± 0.4 a | 1.5 ± 0.4 a |
| Hexanal | 800 | 0.4 ± 0.1 b | 1.7 ± 0.2 a | 1.7 ± 0.1 a | 1.3 ± 0.1 a | 0.9 ± 0.1 a | 1.2 ± 0.2 a |
| Octane | 800 | 0.7 ± 0.1 b | 1.3 ± 0.0 a | 1.7 ± 0.1 a | 1.2 ± 0.2 a | 1.7 ± 0.6 a | 1.1 ± 0.2 a |
| 2-trans-Hexenal | 866 | 23.0 ± 0.1 a | 25.3 ± 3.3 a | 28.9 ± 0.8 a | 56.3 ± 4.1 a | 34.2 ± 3.5 b | 22.3 ± 3.7 b |
| cis-3-Hexen-1-ol | 868 | 24.0 ± 1.6 a | 16.4 ± 1.0 b | 26.7 ± 0.3 a | 3.5 ± 1.7 b | 16.8 ± 3.5 a | 18.6 ± 2.4 a |
| Hexanol | 882 | 21.6 ± 0.2 a | 18.8 ± 1.1 b | 16.2 ± 1.6 b | 18.4 ± 0.9 a | 11.4 ± 2.0 a | 18.4 ± 2.9 a |
| α-Thujene | 924 | 0.2 ± 0.1 a | 0.0 ± 0.0 b 1 | 0.0 ± 0.0 b | 0.3 ± 0.1 a | 1.0 ± 0.5 a | 1.5 ± 0.7 a |
| α-Pinene | 930 | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.1 ± 0.1 a | 0.5 ± 0.1 a | 1.1 ± 0.5 a |
| Camphene | 938 | 0.4 ± 0.0 a | 0.2 ± 0.0 b | 0.2 ± 0.0 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 1.7 ± 1.2 a |
| Sabinene | 958 | 2.3 ± 0.0 a | 2.1 ± 0.3 a | 2.4 ± 0.4 a | 3.9 ± 0.3 a | 1.5 ± 0.4 b | 4.1 ± 0.2 a |
| β-Pinene | 963 | 2.3 ± 0.0 a | 0.9 ± 0.4 b | 1.0 ± 0.4 b | 1.6 ± 0.3 a | 1.3 ± 0.3 a | 1.4 ± 0.3 a |
| Dehydro-1.8-cineole | 973 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.1 a |
| 2-Pentyl furan | 973 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.7 ± 0.3 a |
| n-Octanal | 973 | 0.0 ± 0.0 a | 0.1 ± 0.1 a | 1.1 ± 0.6 a | 0.0 ± 0.0 a | 0.2 ± 0.1 a | 0.3 ± 0.1 a |
| β-Myrcene | 975 | 0.0 ± 0.0 a | 0.2 ± 0.1 a | 0.1 ± 0.1 a | 0.1 ± 0.0 a | 0.6 ± 0.2 a | 0.5 ± 0.2 a |
| α-Phellandrene | 995 | 0.1 ± 0.1 b | 0.5 ± 0.1 a | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 0.4 ± 0.2 a | 0.5 ± 0.1 a |
| Benzene acetaldehyde | 1002 | 0.1 ± 0.0 a | 0.0 ± 0.0 a | 0.1 ± 0.1 a | 0.1 ± 0.0 a | 0.1 ± 0.1 a | 0.1 ± 0.1 a |
| α-Terpinene | 1002 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| p-Cymene | 1003 | 0.1 ± 0.0 a | 0.1 ± 0.1 a | 0.1 ± 0.1 a | 0.0 ± 0.0 b | 0.2 ± 0.1 a | 0.3 ± 0.1 a |
| β-Phellandrene | 1005 | 0.0 ± 0.0c | 0.2 ± 0.0 a | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.2 ± 0.1 a | 0.5 ± 0.1 a |
| Limonene | 1009 | 0.1 ± 0.1 b | 0.4 ± 0.0 a | 0.5 ± 0.0 a | 0.0 ± 0.0 b | 0.5 ± 0.2 a | 0.5 ± 0.1 a |
| γ-Terpinene | 1035 | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.3 ± 0.0 a |
| Terpinolene | 1064 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 0.2 ± 0.1 a | 0.1 ± 0.1 a | 0.3 ± 0.1 a | 0.2 ± 0.1 a |
| n-Nonanal | 1073 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b | 0.3 ± 0.1 a | 0.2 ± 0.1 a |
| Terpinen-4-ol | 1148 | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.1 ± 0.1 a | 0.3 ± 0.1 a | 0.3 ± 0.1 a | 0.5 ± 0.1 a |
| α-Terpineol | 1159 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| n-Decanal | 1180 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.4 ± 0.1 a | 0.4 ± 0.1 a |
| β-Damascenone | 1356 | 0.0 ± 0.0 a | 0.3 ± 0.2 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.1 ± 0.0 a |
| n-Dodecanal | 1397 | 0.0 ± 0.0 a | 0.2 ± 0.1 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| β-Caryophyllene | 1414 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.4 ± 0.2 a | 0.8 ± 0.2 a | 1.0 ± 0.3 a | 0.6 ± 0.2 a |
| α-Humulene | 1447 | 0.6 ± 0.1 a | 0.5 ± 0.3 a | 0.0 ± 0.0 a | 0.4 ± 0.3 a | 0.2 ± 0.1 a | 0.1 ± 0.0 a |
| Bicyclogermacrene | 1487 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.2 ± 0.1 a | 1.9 ± 0.9 a | 0.2 ± 0.0 a |
| Elemol | 1530 | 1.3 ± 0.5 a | 1.3 ± 0.3 a | 0.9 ± 0.2 a | 0.1 ± 0.1 b | 1.7 ± 0.5 a | 0.8 ± 0.1 a |
| Spathulenol | 1551 | 0.2 ± 0.1 a | 0.3 ± 0.2 a | 0.0 ± 0.0 a | 0.3 ± 0.2 b | 0.9 ± 0.2 a | 0.4 ± 0.1 b |
| β-Caryophyllene oxide | 1561 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.1 ± 0.0 a |
| γ-Eudesmol | 1609 | 0.7 ± 0.2 a | 0.8 ± 0.5 a | 0.3 ± 0.1 a | 0.1 ± 0.1 b | 1.0 ± 0.2 a | 0.5 ± 0.1 a |
| β-Eudesmol | 1620 | 0.7 ± 0.1 a | 1.3 ± 0.3 a | 0.5 ± 0.0 b | 0.0 ± 0.0 b | 1.4 ± 0.2 a | 0.5 ± 0.1 a |
| α-Eudesmol | 1634 | 1.3 ± 0.4 a | 2.3 ± 0.7 a | 1.1 ± 0.2 a | 0.0 ± 0.0 b | 2.0 ± 0.7 a | 0.8 ± 0.2 b |
| % Identification | 84.1 ± 1.1 a | 77.3 ± 3.5 a | 85.6 ± 2.9 a | 91.5 ± 1.5 a | 87.2 ± 0.6 a b | 82.0 ± 3.8 b | |
| Grouped compounds | |||||||
| Monoterpene hydrocarbons | 5.7 ± 0.2a | 4.8 ± 0.1 b | 5.0 ± 0.1 b | 6.4 ± 0.9 b | 7.0 ± 1.3 b | 12.5 ± 1.9 a | |
| Oxygen-containing monoterpenes | 0.2 ± 0.0a | 0.2 ± 0.1 a | 0.1 ± 0.0 a | 0.3 ± 0.1 a | 0.4 ± 0.1 a | 0.6 ± 0.1 a | |
| Sesquiterpene hydrocarbons | 0.6 ± 0.1a | 0.5 ± 0.3 a | 0.4 ± 0.2 a | 1.5 ± 0.4 a b | 3.1 ± 0.7 a | 0.9 ± 0.1 b | |
| Oxygen-containing sesquiterpenes | 4.2 ± 1.1a | 6.0 ± 1.9 a | 2.7 ± 0.1 a | 0.5 ± 0.1 b | 8.9 ± 1.3 a | 3.1 ± 0.5 b | |
| C13 Norisoprenoid | 0 ± 0.0 a | 0.3 ± 0.2 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.1 ± 0.0 a | |
| Others | 73.5 ± 2.0 b | 65.5 ± 1.4 b | 77.5 ± 3.3 a | 82.5 ± 2.4 a | 67.8 ± 2.1 b | 64.9 ± 5.5 b | |
| Yield (%, V/fresh weight) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S. Essential Oil Composition and Glandular Trichome Structure of the Weather Prophet Dimorphoteca pluvialis. Biol. Life Sci. Forum 2023, 27, 4. https://doi.org/10.3390/IECAG2023-15145
Faria JMS. Essential Oil Composition and Glandular Trichome Structure of the Weather Prophet Dimorphoteca pluvialis. Biology and Life Sciences Forum. 2023; 27(1):4. https://doi.org/10.3390/IECAG2023-15145
Chicago/Turabian StyleFaria, Jorge M. S. 2023. "Essential Oil Composition and Glandular Trichome Structure of the Weather Prophet Dimorphoteca pluvialis" Biology and Life Sciences Forum 27, no. 1: 4. https://doi.org/10.3390/IECAG2023-15145
APA StyleFaria, J. M. S. (2023). Essential Oil Composition and Glandular Trichome Structure of the Weather Prophet Dimorphoteca pluvialis. Biology and Life Sciences Forum, 27(1), 4. https://doi.org/10.3390/IECAG2023-15145






