Abstract
Horticultural significance in Hoya hybrids stems from their distinctive foliage and flowers. Morphological characterization of hybrids aids in understanding genetic diversity and in forming the basis for breeding new varieties that meet market demands and enhance sustainable horticultural practices through a diversity of attributes. In this study, two first-generation offspring, GTX-021 (H. deleoniorum × H. peninsularis), GTX-067 (H. deleoniorum × H. subquintuplinervis), and their respective parents underwent phenetic examination. This encompassed the assessment of 13 vegetative traits, including aspects of leaf shape, size, and indumentum, as well as 23 reproductive traits, which included features related to inflorescence, corolla, and corona. The traits were analyzed using the UPGMA clustering method, employing the Jaccard similarity coefficient for qualitative traits and the Euclidean distances for quantitative traits. Polymorphism appeared in 14 out of 24 qualitative traits, with significant variations in all quantitative metrics except corona height (p < 0.05). Cluster analysis revealed that GTX-021 exhibited an intermediate overall morphology, comprising both qualitative and quantitative traits, falling between its parents. Notable traits include shared corolla pubescence with H. peninsularis and a distinct corona column similar to H. deleoniorum. Furthermore, GTX-067 resembled its pollen father, H. subquintuplinervis, exhibiting less twinning, horizontal stem growth, and reflexed corolla lobes. Morphometrically, it clustered close to the seed parent, with corona measurements distinguishing it from the pollen parent. This characterization emphasizes the hybrids’ distinctiveness, suggesting their potential as ornamental plants. Additionally, their contribution to enhanced genetic diversity is crucial for developing future varieties, benefiting the horticultural industry with more robust and diverse plant options.
1. Introduction
Hoya R. Br., also referred to as wax plants, is a tropical and subtropical flowering plant genus in the Apocynaceae family. In their natural habitats, hoyas have evolved alongside specific pollinators, such as bees, wasps, and moths, which are crucial to their ability to reproduce successfully [1]. By offering incentives like nectar and alluring scents, hoya flowers are made to entice these pollinators [2,3]. Their flower structure consists of five fused petals (corolla) and a distinctive corona, a crown-shaped feature that varies in color and shape amongst hoya species. One fascinating pollination mechanism found in certain hoya species is the transfer of pollinaria facilitated by medium-sized moths. As they walk around and search for nectar, a portion of their legs become clipped to the pollinaria between the staminal corona due to the restricted footholds provided by the inflorescence. This efficient mechanism ensures that the pollinaria are transferred to the moths’ legs as they move, facilitating pollination [4,5]. The insect may go to another hoya flower after being freed, which would help pollination efforts even more. In cultivated settings, hoya enthusiasts and breeders frequently utilize hand-pollination in cultivated environments to produce hybrids and add novel features [6,7]. By carefully moving pollen between different hoya species, breeders may produce hybrids with unique flower hues, patterns, and attractive foliage, making them highly sought after among hoya collectors.
In the Philippines, the size, shape, texture, and amount of pubescence of the leaf of the hoya species varies. The same findings may be observed in floral features that are distinguished by various corolla types, corona attributes, and morphometric aspects. One of the popularly known species in the horticultural market today is the unique species first discovered in Siargao, Island, Surigao Del Norte, Mindanao‒Hoya deleoniorum. The amount of hairiness on the leaves and outer surface of the corolla, the presence of a noticeable column, and the size and form of the pollinarium serve as distinguishing characteristics of this species to a close kin, H. cutis-porcelana [8,9]. Other tropical and subtropical countries in Asia and the Western Pacific also serve as habitats for other distinct hoya species. H. subquintuplinervis (syn. H. pachyclada); naturally distributed in Cambodia, Laos, Vietnam, and Thailand; has unusually wide, succulent leaves with five-ply nerves [10,11]. The Peninsular Malaysian species H. peninsularis, sometimes known as Teddy Bear in the trade, is noteworthy for having a highly hairy corolla and pronounced leaf venations [12].
Plant breeders and horticulturists frequently utilize interspecific hybridization to increase the gene pool of a specific group of plants [13]. In hoyas, a number of hybrids with good leaf and flower traits had previously been created and were widely available [14]. Even though some species have successfully hybridized, the bulk of the species in the genus with noteworthy traits have not yet had their interspecific compatibility assessed. The goal of the present study was to determine the interspecific reproductive compatibility of a Philippine hoya species (H. deleoniorum) with two species from Southeast Asia (H. peninsularis, H. subquintuplinervis). The morphological characterization of the hybrids and how closely they resemble their parental species are expected to provide information regarding their potential for hybridization and possible utility in breeding programs.
2. Materials and Methods
2.1. Plant Material
In this study, Hoya deleoniorum was used as the seed parent, along with H. peninsularis and H. subquintuplinervis as the pollen parents. All the plants were cultivated at a private nursery in Barangay Rizal, Lipa City, Batangas (13.8734° N, 121.1598° E—206.7 m asl). The study site’s average annual temperature was 25.4 °C, and the annual index rainfall was 2258 mm [15].
2.2. Hybridization, Seed Germination and Cultivation
Interspecific crosses were carried out through hand pollination, with pollen transferred to the mother plant between 10:00 p.m. and 2:00 a.m. To prevent cross-pollination by other plants, all pollinated flowers were covered with lightweight plain weave cloth. In May 2021, the cross of H. deleoniorum and H. peninsularis was performed. Two of the three flowers pollinated bore fruit; the first has 10 seeds, and the second has 20 seeds. For the cross of H. deleoniorum and H. subquintuplinervis, performed in May 2022, five flowers were pollinated. Only one pollinated flower produced a follicle containing a total of 160 seeds. Apart from interspecific crosses, the sample species also showed strong self-compatibility, thriving with both self-pollen and pollen from other individuals. After 2–3 months, mature follicle seeds were harvested and germinated in polystyrene trays with sterile cocopeat substrate. The most robust seedlings, 40 from H. deleoniorum × H. peninsularis and 37 from H. deleoniorum × H. subquintuplinervis, were transplanted 180 days after emergence into a clear polyethylene terephthalate (PET) container with cocopeat, coco cubes, and pumice substrate.
2.3. Morphological Characterization
A total of 24 qualitative and 12 quantitative features were considered. The morphological characteristics of 10 living specimens from each of the parent species and the GTX-021 and GTX-067 hybrids were evaluated using phytographical descriptions [16,17,18,19,20]. The qualitative descriptors were as follows: habit (HB), stem surface (ST), stem sap color (SSC), leaf color (LC), leaf shape (LS), leaf texture (LT), leaf apex shape (AS), leaf base shape (BS), leaf venation (LV), leaf sap color (LSC), flower geotropism (GT), type of inflorescence (IT), the shape of inflorescence (IS), peduncle position (PP), peduncle indumentum (PNI), pedicel indumentum (PCI), calyx lobe shape (CLS), calyx apex shape (CAS), calyx indumentum (CAI), corolla indumentum (COI), corolla margin (CM), corona inner processes (CIP), corona outer processes (COP), and corona outer processes basal margin (COB). The quantitative descriptors, obtained using a millimeter ruler, were as follows: stem diameter (SD), leaf length (LL), leaf width (LW), pedicel length (PCL), corolla length when flattened (CLLF), corolla lobe length (CLLL), corolla lobe width (CLLW), corona height (CNH), corona diameter (CND), corona lobe length (CNLL), coronal lobe width (CNLW), and ovary height (OH).
2.4. Cluster Analysis
Qualitative traits were put into a new distribution with a mean of 0 and a standard deviation of 1, taking into account the existence or absence of each character state. The differences in quantitative traits were examined using one-way analysis of variance (ANOVA), then assessed using Tukey’s range test at 5% (p < 0.05) statistical significance. Statistics Kingdom was used to run statistical tests [21]. PAleontological STatistics (PAST) Version 4.03 [22,23] was used to compute similarity and distance coefficients and perform cluster analysis. For qualitative features, the Jaccard similarity coefficient was calculated, whereas for quantitative traits, Euclidean distances were calculated. UPGMA (Unweighted Pair Group Method with Arithmetic Mean) was the clustering approach utilized for both types of data.
3. Results and Discussion
3.1. Interspecific Hybridization
In the cross of Hoya deleoniorum and H. peninsularis, 67% of the pollinated flowers developed into follicles, and from the resulting seedling population, GTX-021 was selected. In contrast, the cross between H. deleoniorum and H. subquintuplinervis yielded a lower rate, with only 20% of pollinated flowers developing into follicles. GTX-067 stood out as the most promising candidate based on vigor and growth rate.
3.2. Morphological Variations in the Qualitative Traits
Evaluation of the qualitative morphological traits of the selected hybrids and the parent species revealed that 14 out of the 24 characters showed polymorphism; stem shape (ST), sap color (SSC, LSC), leaf texture (LT), flower geotropism (GT), type of inflorescence (IT), shape of inflorescence (IS), peduncle position (PP), corona inner processes (CIP), and corona outer processes basal margin (COB) were the non-polymorphic traits. Figure 1 shows how similar the samples are in terms of the qualitative traits examined.
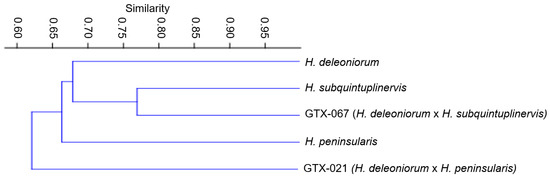
Figure 1.
Phenogram showing the qualitative feature similarity between the parent species and the hybrids using the UPGMA method based on the Jaccard similarity coefficients.
The Jaccard similarity coefficient was used in the cluster analysis for the qualitative traits, and the resulting phenogram shows that the GTX-021 did not cluster to its seed parent (H. deleoniorum) and pollen parent (H. peninsularis). Most of the qualitative traits of the hybrid exhibit values that fall between those of its progenitors. The degree of pubescence present in the corolla is one striking similarity between the hybrid and the pollen parent. The noticeable column underneath the corona is a shared character between the hybrid and the seed parent (Figure 2). Furthermore, the phenogram suggests that GTX-067 is more related to its pollen parent (H. subquintuplinervis) than to its seed parent (H. deleoniorum). The hybrid and pollen parents have a less twinning habit, stems grow horizontally, and corolla lobes are not flat.
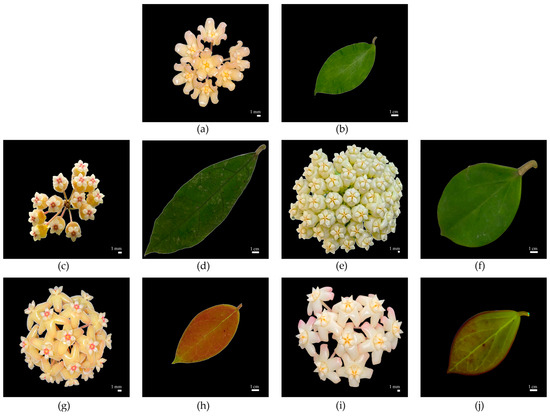
Figure 2.
Floral and leaf features of the parent species and the two hybrids: (a,b) Hoya deleoniorum (seed parent); (c,d) H. peninsularis (pollen parent); (e,f) H. subquintuplinervis (pollen parent); (g,h) GTX-021 (H. deleoniorum × H. peninsularis); and (i,j) GTX-067 (H. deleoniorum × H. subquintuplinervis).
The characters showing higher variability, and the least reliability in delineating one plant from another, were leaf shape (LS), leaf apex shape (AS), and leaf base shape (BS). Leaf features with varying forms and sizes exhibit phenotypic plasticity [24,25]. Phenotypic plasticity is one of the most essential strategies for plants to cope with stressful conditions in a variety of habitats. Aside from the documented plasticity in leaf traits, color of the leaf and the flowers were observed to be variable in the hybrids. Abiotic and biotic stressors have been identified for some reported variation in these traits [26,27,28]. This necessitates a controlled study to assess the stability of these features under varying growth settings. Changes in these features can be linked to genetic causes, including trait inheritance owing to hybridization, and may be of advantage to the expansion of plants into varied habitats [29].
3.3. Morphological Variations in the Quantitative Traits
With the exception of the corona height, differences between the hybrids and the parent species were found to be significant (p < 0.05) for all of the quantitative traits examined (Table 1).

Table 1.
Quantitative traits of the F1 hybrids and the parent species.
Figure 3 shows that GTX-021 is morphometrically similar to its pollen parent (H. peninsularis) with a Euclidean distance coefficient of 6.95. In terms of corolla measurements, however, this hybrid is more comparable to H. deleoniorum (seed parent) and has considerably larger corolla measurements than the pollen parent. The corona measurements of this hybrid, on the other hand, are much smaller than those of the pollen parent and significantly bigger than those of the seed parent. When compared to its parent species, GTX-067 is morphometrically similar to H. deleoniorum (seed parent) with a Euclidean distance coefficient of 8.04. The corona measurements can be used to distinguish between the hybrid and the pollen parent (H. subquintuplinervis); the hybrid has a substantially smaller corona.
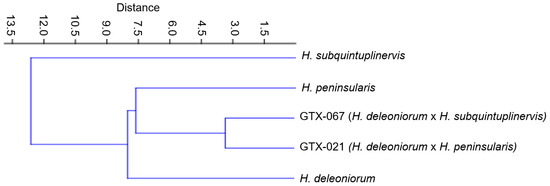
Figure 3.
Phenogram showing the quantitative feature similarity between the parent species and the hybrids using the UPGMA method based on the Jaccard similarity coefficients.
Characters with a high degree of diversity are very desirable for plant breeding [30]. Given that the quantitative features were found to be variable, especially for the corona and corolla measures, an extensive study of the additional reproductive traits of the hybrids and the parent species may serve as a guide in developing other prospective interspecific crosses.
4. Conclusions
The reproductive compatibility of H. deleoniorum with the two species sheds information on potential future crossings in which H. deleoniorum might serve as a productive seed parent. This preliminary morphological characterization underlines the distinction of the hybrids from their parents, suggesting their potential as valuable ornamental plants. To guarantee the uniformity and stability of the traits, thorough tests should be conducted to determine the limitedness of variation and certain tolerance of the hybrids in multiple growing conditions.
Author Contributions
Methodology, J.C. and N.T.; software, J.C.; writing, J.C.; resources, N.T.; visualization, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings are available within the paper.
Acknowledgments
Deep appreciation to Genoveva Tiama, in whose honor the hybrid accession code GTX was designated, for her valuable administrative aid. The continual progress of the breeding activities undertaken by the second author was made possible through the indispensable assistance provided by Maria Theresa Hartley and Rare Garden Blooms.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ollerton, J.; Liede, S. Pollination systems in the Asclepiadaceae: A survey and preliminary analysis. Biol. J. Linn. Soc. 2008, 62, 593–610. [Google Scholar] [CrossRef]
- Basir, S.; Saad, M.F.M.; Rahman, M.R.A.; Talip, N.; Baharum, S.N.; Bunawan, H. Floral Nectary and Trichome Structure of Hoya cagayanensis, Hoya lacunosa, and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae 2022, 8, 420. [Google Scholar] [CrossRef]
- Jürgens, A.; Dötterl, S.; Liede-Schumann, S.; Meve, U. Floral scent composition in early diverging taxa of Asclepiadoideae, and Secamonoideae (Apocynaceae). S. Afr. J. Bot. 2010, 76, 749–761. [Google Scholar] [CrossRef]
- Mochizuki, K.; Furukawa, S.; Kawakita, A. Pollinia transfer on moth legs in Hoya carnosa (Apocynaceae). Am. J. Bot. 2017, 104, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Landrein, S.; Zhou, Z.; Song, S. Pollinators of Hoya pottsii: Are the strongest the most effective? Flora 2021, 274, 151734. [Google Scholar] [CrossRef]
- Kato, J.; Mii, M. Production of Interspecific Hybrids in Ornamental Plants. In Plant Cell Culture Protocols. Methods in Molecular Biology; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 877, pp. 233–245. [Google Scholar] [CrossRef]
- Henny, R.; Chen, J. Cultivar Development of Ornamental Foliage Plants. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; Volume 23, pp. 245–290. [Google Scholar] [CrossRef]
- Aurigue, F.B.; Sahagun, J.R.; Suarez, W.M. Hoya cutis-porcelana (Apocynaceae): A New Species from Samar and Biliran Islands, Philippines. J. Nat. Stud. 2013, 12, 12–17. [Google Scholar]
- Aurigue, F.B.; Cabactulan, D.D.; Pimentel, R.B.; Sahagun, J.R. A remarkable new Hoya (Apocynaceae: Asclepiadoideae) from Mindanao, Philippines. Avonia 2018, 36, 146–149. [Google Scholar]
- Averyanov, L.V.; Pham, T.; Maisak, T.; Le, T.; Nguyen Van, C.; Tuan, N.; Nguyen, P.; Nguyen, K.; Nguyen, V.; Nguyen, T.; et al. Preliminary checklist of Hoya (Asclepiadaceae) in the flora of Cambodia, Laos and Vietnam. Turczaninowia 2017, 20, 103–147. [Google Scholar] [CrossRef]
- Kloppenburg, D.; Wayman, A. The World of Hoyas a Book of Pictures, 2nd ed.; Orca Publishing Company: Central Point, OR, USA, 2007. [Google Scholar]
- Rodda, M.; Zakaria, R. Hoya peninsularis (Apocynaceae, Asclepiadoideae), a new species from Peninsular Malaysia, and notes on Hoya maingayi and Gongronema wrayi. Nord. J. Bot. 2020, 38, 1–8. [Google Scholar] [CrossRef]
- Ocampo, J.; Arias, J.C.; Urrea, R. Interspecific hybridization between cultivated and wild species of genus Passiflora L. Euphytica 2016, 209, 395–408. [Google Scholar] [CrossRef]
- Noel, C. Putting a name to some old favourites. Asklepios 2014, 91, 11–14. [Google Scholar]
- Climate Data. Available online: https://en.climate-data.org (accessed on 1 May 2023).
- Beentje, H. The Kew Plant Glossary an Illustrated Dictionary of Plant Terms, 2nd ed.; Royal Botanic Gardens, Kew: London, UK, 2016; pp. 5–144. [Google Scholar]
- Kloppenburg, D. Characters for Hoya Specie[s] Determinations. Available online: http://dalekloppenburg.blogspot.com (accessed on 1 April 2023).
- Radford, A.; Dickinson, W.; Massey, J.; Bell, C. Vascular Plant Systematics; Harper and Row: New York, NY, USA, 1974; pp. 79–144. [Google Scholar]
- Simpson, M.G. Plant Systematics; Elsevier Academic Press: Burlington, VT, USA, 2006. [Google Scholar]
- Wing, S.; Ash, A.; Ellis, B.; Hickey, L.; Johnson, K.; Wilf, P. Manual of Leaf Architecture-Morphological Description and Categorization of Dicotyledonous and Net-Veined Monocotyledonous Angiosperms; Smithsonian Institution: Washington, DC, USA, 1999. [Google Scholar]
- Statistics Kingdom. Available online: https://www.statskingdom.com (accessed on 1 June 2023).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: PAleontological STatistics Software Package for Education and Data Analysis. Palaeontol. Electron 2001, 4, 1. [Google Scholar]
- Hammer, Ø. PAST PAleontological STatistics Version 4.13 Reference Manual. Available online: https://www.nhm.uio.no (accessed on 1 June 2023).
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Rahayu, S.; Jusuf, M.; Suharsono; Kusmana, C.; Abdulhadi, R. Morphological variation of Hoya multiflora Blume at different habitat type of Bodogol Research Station of Gunung Gede Pangrango National Park, Indonesia. Biodiversitas 2010, 11, 187–193. [Google Scholar] [CrossRef]
- Medina, M.N.; Amoroso, V.; Kloppenburg, R. Changes of leaf morphology of Hoya amorosoae from varying light exposure: Its implications to species description and taxonomy. J. Biodivers. Environ. Sci. 2016, 8, 232–237. [Google Scholar]
- Koski, M.H.; Galloway, L.F. Geographic Variation in Floral Color and Reflectance Correlates with Temperature and Colonization History. Front. Plant Sci. 2020, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Rusman, Q.; Poelman, E.H.; Nowrin, F.; Polder, G.; Lucas-Barbosa, D. Floral plasticity: Herbivore-species-specific-induced changes in flower traits with contrasting effects on pollinator visitation. Plant Cell Environ. 2019, 42, 1882–1896. [Google Scholar] [CrossRef] [PubMed]
- Walls, R. Hybridization and Plasticity Contribute to Divergence Among Coastal and Wetland Populations of Invasive Hybrid Japanese Knotweed s.l. (Fallopia spp.). Estuaries Coasts 2010, 22, 902–918. [Google Scholar] [CrossRef]
- Widiarsih, S.; Siar, S.V.; Lalusin, A.G.; Carandang, J.M.; Borromeo, T.H. Genetic diversity assessment in vegetative and reproductive characters of Hoya mindorensis Schlechter. Philipp. J. Crop Sci. 2012, 37, 23–29. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).