Abstract
Detecting N-deficiency early in a plant’s development before visual symptoms become pronounced and irreparable damage is done is crucial to maintaining optimum grain yield and biomass production. Chlorophyll fluorescence technology (CFT) is a quick, non-invasive, non-destructive, and informative technique that is used to study the physiological status of plants at any given stage of development. The objective of the study was to determine the photosynthetic and growth responses of N-deficient maize seedlings. Two N treatments, 10 t/ha N and 50 t/ha N, were evaluated in a greenhouse in a completely randomized design with 12 replications. The results showed a significantly (p < 0.05) higher CO2 assimilation rate, maximum quantum yield of PSII photochemistry, effective quantum yield of PSII photochemistry, and chlorophyll concentration in plants that received 50 t/ha N compared to plants on 10 t/ha N at 3 and 4 weeks after fertilizer application (WAFA). In contrast, plants on 10 t/ha showed a higher level of non-photochemical stress due to up-regulation of nitric oxide production in PSII [Y(NO)] than plants on 50 t/ha. Non-photochemical quenching due to down-regulation of nitric oxide production in PSII [Y(NPQ)] was comparable (p > 0.05) in both treatments. There was no significant difference in plant height, although wider stem girth was recorded in plants on 50 t/ha. The significantly higher levels of Y(NO) in plants on 10 t/ha N suggest an alteration in nitrogen metabolism and increased production of reactive nitrogen species which may potentially cause cellular damage if not diagnosed early and managed adequately.
1. Introduction
Maize (Zea mays) is one of the world’s most important cereal crops [1], serving as a staple food source, animal feed, and a critical component of various industrial products [2]. The ability to optimize its growth and yield potential is of paramount importance in ensuring food security and economic stability on a global scale. Due to its many varieties, high financial returns, and nutritional benefits, maize is produced in a variety of climates [3,4]. Fast-growing maize cultivars, for instance, may produce even in regions with limited rainfall [5]. Due to climate fluctuation, the majority of the SSA region is undergoing climate stress, which has a detrimental effect on maize output [6]. Agronomic consultation services are inaccessible [7], there are insufficient soil nutrient supplies [8], and there is rapid soil degradation as a result of the growing human population, culminating in yield decline [9]. Low maize output and a subsequent increase in poverty have been caused by variations in the rainfall pattern and low soil fertility [2,10]. There is a pressing need to boost maize yield on existing farmland due to the rising demand for maize as a staple crop [11].
Nitrogen (N) is one of the most critical nutrient elements in maize production [2], with sub-optimal and target dosages of <10 kg ha−1 and 50 kg ha−1, respectively, in sub-Saharan Africa [12]. It is essential for several enzymatic, metabolic, and physiological processes. N deficiency can inhibit chlorophyll formation, photosynthesis, protein synthesis, cell division and elongation, and overall grain yield and biomass [13]. It is the essence of vitality for maize. Its availability in the soil can be the difference between a thriving crop and a stunted one, and significant growth responses are highest when N is applied at an optimal rate [2]. Changing climatic conditions, unprecedented rainfall patterns, and evolving agricultural practices have intensified the challenges farmers face in ensuring their maize crops receive sufficient amounts of this vital nutrient [6]. Deficiency symptoms manifest initially as a mild yellowing of the leaves, which is a faint whisper of distress that can go unnoticed until it is too late. The consequences of such oversight are dire, not only in terms of diminished yields but also in the larger context of food security.
Detecting N deficiency early in the plant’s development before visual symptoms become pronounced and before irreparable damage is done is crucial to maintaining optimum grain yield and biomass production. Chlorophyll fluorescence technology (CFT) is a quick, non-invasive, non-destructive, and informative technique that can be used to study the physiological status of a photosynthesizing material at any given stage of plant development [14]. It offers a mechanism through which nitrogen stress can be detected early in the growth stage before considerable damage is done. The objective of the study was to determine the photosynthetic and growth responses of an N-deficient maize plant in contrast to a healthy plant.
2. Materials and Methods
2.1. Experimental Site
The experiment was carried out at the greenhouse of the Institute of Bio- and Geosciences (IBG-2), Plant Sciences, Forschungszentrum Julich GmbH, 50.9224° N, 6.4111° E, Germany, between January and February 2022.
2.2. Materials
Saatmals maize variety, Tetry Basisdunger (0%-14%-38%-5% of N-P-K-Ca), and Harnstoff (46% N) commercial fertilizers were used for the experiment. The seeds were grown in pots of size 3 L in a growth medium composed of quartz sand and NullErde substrate (zero nutrients) in a 1:1 ratio.
2.3. Pot Establishment, Treatments, and Experimental Design
Plants were seeded in germination trays, and uniform and vigorous-looking seedlings were transplanted to the pots containing the growth medium 1 week after planting (WAP). Two N-fertilizer rates, 10 kg N ha−1 and 50 kg N ha−1, were evaluated in a completely randomized design with 10 replications following a uniform application of 50 kg ha−1 of Tetry Basisdunger fertilizer (0%-14%-38%-5% of N-P-K-Ca) a week after transplanting (WAT).
2.4. Data Collection
Data were collected on plant height, chlorophyll content, and photosynthesis parameters such as maximum quantum yield of photosystem II (PSII) photochemistry (Fv/Fm), effective PSII photochemical yield [Y(II)], non-photochemical stress due to down-regulation of nitric oxide production in PSII [Y(NPQ)], non-photochemical stress due to up regulation of nitric oxide production in PSII [Y(NO)], and electron transport rate (ETR) at 3 and 4 WAT.
2.5. Measurement of Plant Height, Chlorophyll, and Photosynthesis Parameters
Plant height was measured as the vertical distance from the base to the top of the canopy. Relative chlorophyll value was measured using the SPAD device (KONICA MINOLTA). Plants were dark adapted for 30 min in a dark room to measure Fv/Fm. Measurements of light-acclimated leaves were made on the third leaf from the top per plant between 9.00 and 9.30 am., and intensity of 120 and 150 µmol m−2 s−1 under flood lamps using a portable pulse amplitude-modulated chlorophyll fluorometer (Mini-PAM II) at a measuring light intensity of about 0.04 µmol m−2 s−1 and a saturating light pulse of about 5000 µmol m−2 s−1. The parameters Fv/Fm, Y(II), Y(NPQ), Y(NO), and ETR were computed following the procedures of Genty et al. [15,16] as follows:
Fv/Fm = (Fm − Fo)/Fm; Y(II) = (Fm’ − F’)/Fm’; Y(NO) = F’/Fm; Y(NPQ) = (F’/Fm’) − (F’/Fm); ETR = PAR × ETR factor × (PPS2/PPS1+2) × Y(II). Where PAR is photosynthetic active radiation, ETR factor and (PPS2/PPS1+2) are constants with values of 0.84 and 0.5, respectively.
2.6. Statistical Analysis
Data collected were subjected to analysis of variance using Genstat 18th edition, and significant treatment means were separated using the least significant difference. Graphs were constructed using GraphPad Prism 6.
3. Results
3.1. Effect of N-Stress on Plant Height, Leaf Color, and Chlorophyll Value
The effect of N-stress on the plant height of maize was negligible (p > 0.05). Both 10 kg ha−1 N and 50 kg ha−1 N were statistically similar at 3 and 4 weeks after fertilizer application (WAFA) (Figure 1). However, obvious variation was observed in leaf color as the N-stressed plants tended towards pale yellow while the unstressed plants remained green (Scheme 1). N-stress significantly reduced the chlorophyll value of leaves from 45.14 in unstressed plants to 28.98 in stressed plants at 3 WAFA and from 45.70 to 29.42 at 4 WAFA in unstressed and stressed plants, respectively (Figure 2).
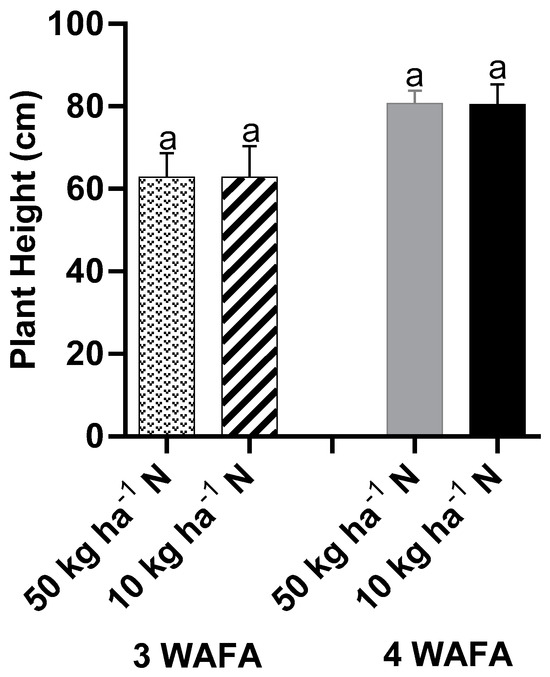
Figure 1.
Effect of Nstress on plant height of maize. WAFA: weeks after fertilizer application. Bars with same letters are not significantly different at p < 0.05.

Scheme 1.
N-stressed and unstressed maize plants growing in the greenhouse at 3 weeks after fertilizer application.
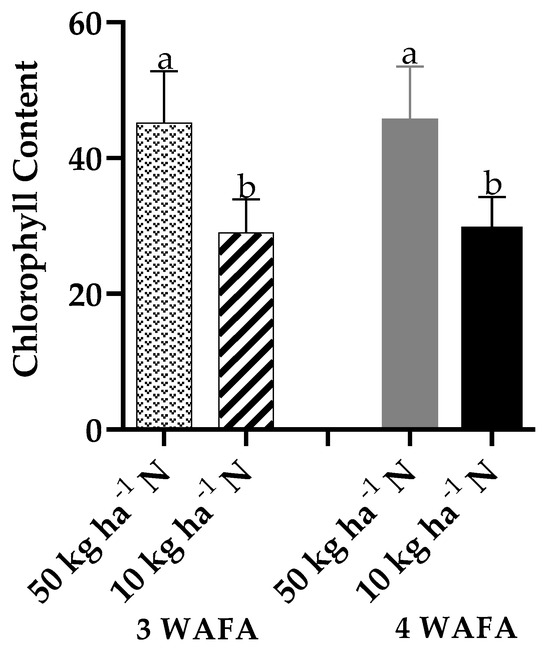
Figure 2.
Effect of N-stress on chlorophyll content of maize. WAFA: weeks after fertilizer application; bars with different letters are significantly different at p < 0.05.
3.2. Effect of N-Stress on Photosynthetic Traits
Photosynthetic variables showed clear-cut variations between the two treatments. Unstressed plants were significantly higher in maximum quantum yield of PSII photosynthesis, effective PSII photochemical yield, and electron transport rate. Non-photochemical stress due to down-regulation of nitric oxide production was not significant, while non-photochemical stress due to up-regulation of nitric oxide production was significantly higher (p < 0.05) in N-deficit stressed seedlings (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).
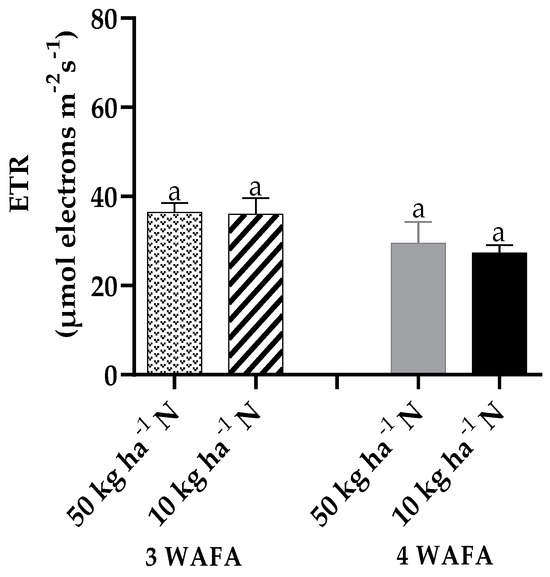
Figure 3.
Effect of N-stress on ETR of maize. WAFA: weeks after fertilizer application; bars with the same letters are not significantly different at p < 0.05.
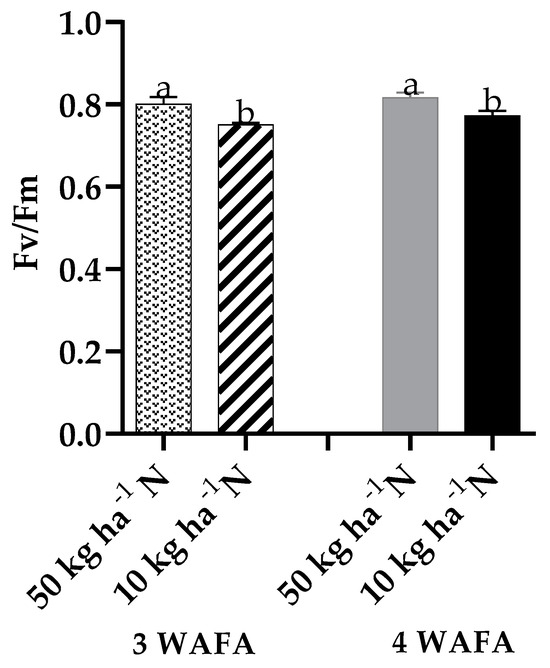
Figure 4.
Effect of N-stress on maximum PSII photochemistry of maize. WAFA: weeks after fertilizer application; bars with different letters are significantly different at p < 0.05.
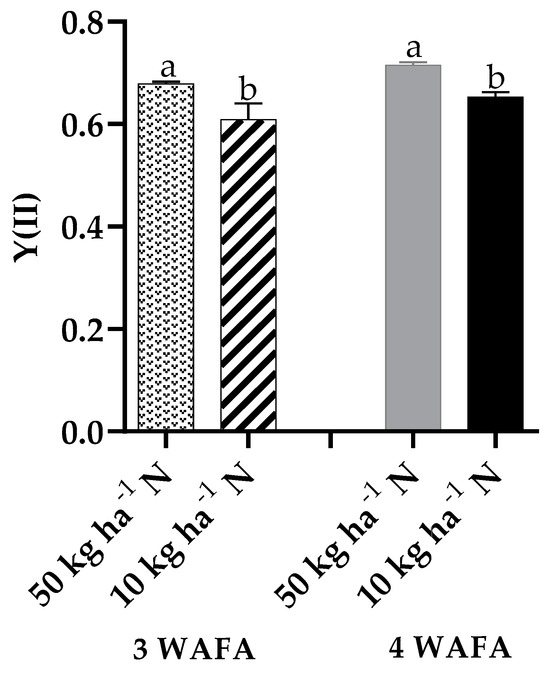
Figure 5.
Effect of N-stress on Y(II) of maize. WAFA: weeks after fertilizer application; bars with different letters are significantly different at p < 0.05.
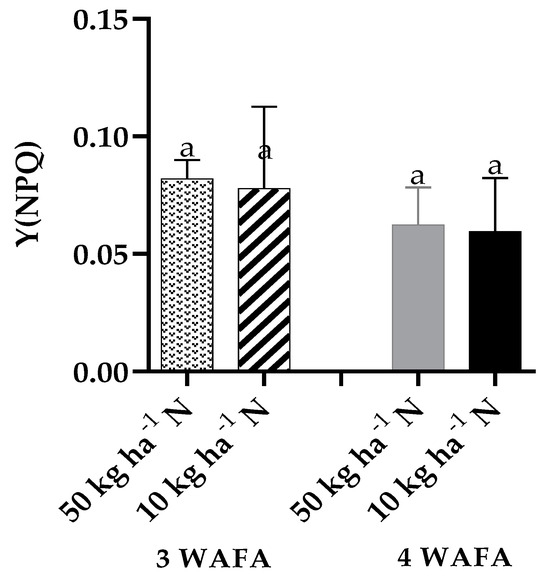
Figure 6.
Effect of N-stress on Y(NPQ) photochemistry of maize. WAFA: weeks after fertilizer application; bars with the same letters are significantly not different at p < 0.05.
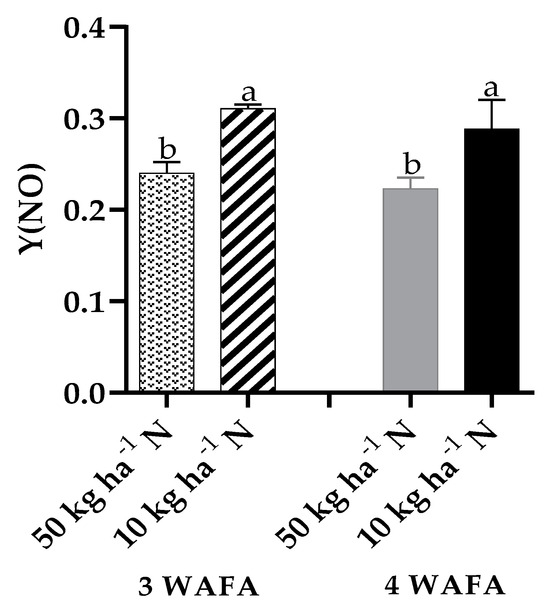
Figure 7.
Effect of N-stress on Y(NO) of maize. WAFA: weeks after fertilizer application; bars with different letters are significantly different at p < 0.05.
4. Discussion
This study recorded comparable responses of maize seedlings to N-stress in plant height at 3 and 4 WAFA. Early N-stress detection was evident in terms of chlorophyll decline in unstressed plants compared to stressed plants. This decline is a result of the significant role of nitrogen in chlorophyll synthesis [13,17], which was evident in the visual discoloration of the stressed leaves from green to pale yellow.
Maximum quantum yield of PSII photochemistry, CO2 assimilation rate, and photosynthetic efficiency were significantly higher in unstressed plants essentially because of their higher chlorophyll value. Chlorophyll molecules are indispensable light-harvesting pigments in plants that are responsible for intercepting light energy for use in photochemical processes. In contrast, N-stressed plants recorded lower values for those photosynthetic variables due to reduced chlorophyll value, which inhibited photosynthesis and other metabolic processes. The findings of this study are consistent with the reports of Meng et al. [18] and Wen et al. [13]. The significantly higher levels of Y(NO) in plants on 10 kg ha−1 N against plants on 50 kg ha−1 N suggest an alteration in nitrogen metabolism and an increased production of reactive nitrogen species which may potentially cause cellular damage if not diagnosed early and managed adequately before irreparable damage is done.
5. Conclusions
The study investigated the growth and photosynthesis responses of maize seedlings to N-deficit stress in a greenhouse. A non-significant increase in plant height was recorded in N-deficient seedlings compared to the unstressed seedlings. A noticeable change in color from green to pale yellow was observed in N-deficit plants, which could be explained by its significantly reduced chlorophyll content. In addition, N-deficit plants also recorded lower values for maximum quantum yield of PSII photochemistry, effective PSII photochemical yield, and electron transport rate, which are critical for efficient photosynthesis. Furthermore, up-regulation of nitric acid production was significantly higher in N-deficit plants, which highlights the importance of adequate and timely nitrogen management to avoid the production of reactive nitrogen species that could potentially cause damage to plants. Early N-deficit detection and management are therefore indispensable components for crop health and sustainable maize production.
Author Contributions
Conceptualization, methodology, investigation, U.N.U. and J.U.A.; formal analysis, data curation, writing—original draft preparation, U.N.U. and I.A.U.; writing—review and editing, I.A.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Ministry of Education and Research (BMBF) in the framework of YESPVNIGBEN project, grant number 03SF0576A.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated in this research are included in the manuscript.
Acknowledgments
The authors are grateful to the BMBF for funding. The YESPVNIGBEN project coordinator, Solomon Agbo, and the entire staff of the Institute of Bio- and Geosciences (IBG-2), Plant Sciences, Forschungszentrum Julich, Germany, are acknowledged for their contributions. Special thanks to Onno Muller, Ladislav Nedbal, Silvia Schrey, and Michael Uguru for their guidance and mentorship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bedeke, S.; Vanhove, W.; Gezahegn, M.; Natarajan, K.; Van Damme, P. Adoption of climate change adaptation strategies by maize-dependent smallholders in Ethiopia. NJAS-Wagening. J. Life Sci. 2019, 88, 96–104. [Google Scholar] [CrossRef]
- Gotosa, J.; Kodzwa, J.; Nyamangara, J.; Gwenzi, W. Effect of Nitrogen Fertiliser Application on Maize Yield Across Agro-Ecological Regions and Soil Types in Zimbabwe: A Meta-analysis Approach. Int. J. Plant Prod. 2019, 13, 251–266. [Google Scholar] [CrossRef]
- Abate, T.; Shiferaw, B.; Menkir, A.; Wegary, D.; Kebede, Y.; Tesfaye, K.; Kassie, M.; Bogale, G.; Tadesse, B.; Keno, T. Factors that transformed maize productivity in Ethiopia. Food Secur. 2015, 7, 965–981. [Google Scholar] [CrossRef]
- Adimassu, Z.; Kessler, A.; Stroosnijder, L. Farmers’ strategies to perceived trends of rainfall and crop productivity in the Central Rift Valley of Ethiopia. Environ. Dev. 2014, 11, 123–140. [Google Scholar] [CrossRef]
- Kamara, A.Y.; Ekeleme, F.; Chikoye, D.; Omoigui, L.O. Planting date and cultivar effects on grain yield in dryland corn production. Agron. J. 2009, 101, 91–98. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Roberts, D.C.; Adams, H.; Adelekan, I.; Adler, C.; Adrian, R.; Okem, A. Technical Summary. In Climate Change 2022: Impacts, Adaptation, and Vulnerability; Pörtner, H.O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Rama, B., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Di Falco, S. Adaptation to climate change in sub-Saharan agriculture: Assessing the evidence and rethinking the drivers. Eur. Rev. Agric. Econ. 2014, 41, 405–430. [Google Scholar] [CrossRef]
- Ngetich, F.K.; Diels, J.; Shisanya, C.A.; Mugwe, J.N.; Mucheru-Muna, M.; Mugendi, D.N. Effects of selected soil and water conservation techniques on runoff, sediment yield, and maize productivity under sub-humid and semi-arid conditions in Kenya. Catena 2014, 121, 288–296. [Google Scholar] [CrossRef]
- Mulwa, C.; Marenya, P.; Bahadur, D.; Kassie, M. Response to climate risks among smallholder farmers in Malawi: A multivariate probit assessment of the role of information, household demographics, and farm characteristics. Clim. Risk Manag. 2017, 16, 208–221. [Google Scholar] [CrossRef]
- Akinnifesi, F.K.; Ajayi, O.C.; Sileshi, G.; Chirwa, P.W.; Chianu, J. Fertilizer trees for sustainable food security in the maize-based production systems of east and southern Africa: A review. Agron. Sustain. Dev. 2010, 30, 615–629. [Google Scholar] [CrossRef]
- Adamtey, N.; Musyoka, M.W.; Zundel, C.; Cobo, J.G.; Karanja, E.; Fiaboe, K.K.M.; Muriuki, A.; Mucheru-Muna, M.; Vanlauwe, B.; Berset, E.; et al. Productivity, profitability, and partial nutrient balance in maize-based conventional and organic farming systems in Kenya. Agric. Ecosyst. Environ. 2016, 235, 61–79. [Google Scholar] [CrossRef]
- AU, NEPAD/NPCA. The Abuja Declaration on fertilizers for an African Green Revolution: Status of implementation at regional and national levels. Policy Brief, African Union and NEPAD Planning and Coordinating Agency. Addis Ababa Midrand. 2006. Available online: https://www.inter-reseaux.org/wp-content/uploads/Seventh_Progress_Report_Abuja_Declaration_FINAL_June_2011.pdf (accessed on 20 August 2023).
- Wen, B.; Li, C.; Fu, X.; Li, D.; Li, L.; Chen, X.; Wu, H.; Cui, X.; Zhang, X.; Shen, H.; et al. Effects of nitrate deficiency on nitrate assimilation and chlorophyll synthesis of detached apple leaves. Plant Physiol. Biochem. 2019, 142, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R.; Rosenqvist, E. Applications of Chlorophyll Fluorescence Can Improve Crop Production Strategies: An Examination of Future Possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Genty, B.; Harbinson, J.; Cailly, A.L.; Rizza, F. Fate of excitation at PS II in leaves: The non-photochemical side. In Proceedings of the Third BBSRC Robert Hill Symposium of Photosynthesis, University of Sheffield, Department of Molecular Biology and Biotechnology, Western Bank, Sheffield, UK, 31 March–3 April 1996. Abstract P28. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Meng, L.; Fan, Z.; Zhang, Q.; Wang, C.; Gao, Y.; Deng, Y.; Fu, D.Q. Bel1-like homeodomain 11 regulates chloroplast development and chlorophyll synthesis in tomato fruit. Plant J. 2018, 94, 1126–1140. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).