Efficacy of Fumonisin B1 Removal from Various Simulated Water Types Using UV and UV/H2O2 Treatments †
Abstract
:1. Introduction
2. Material and Methods
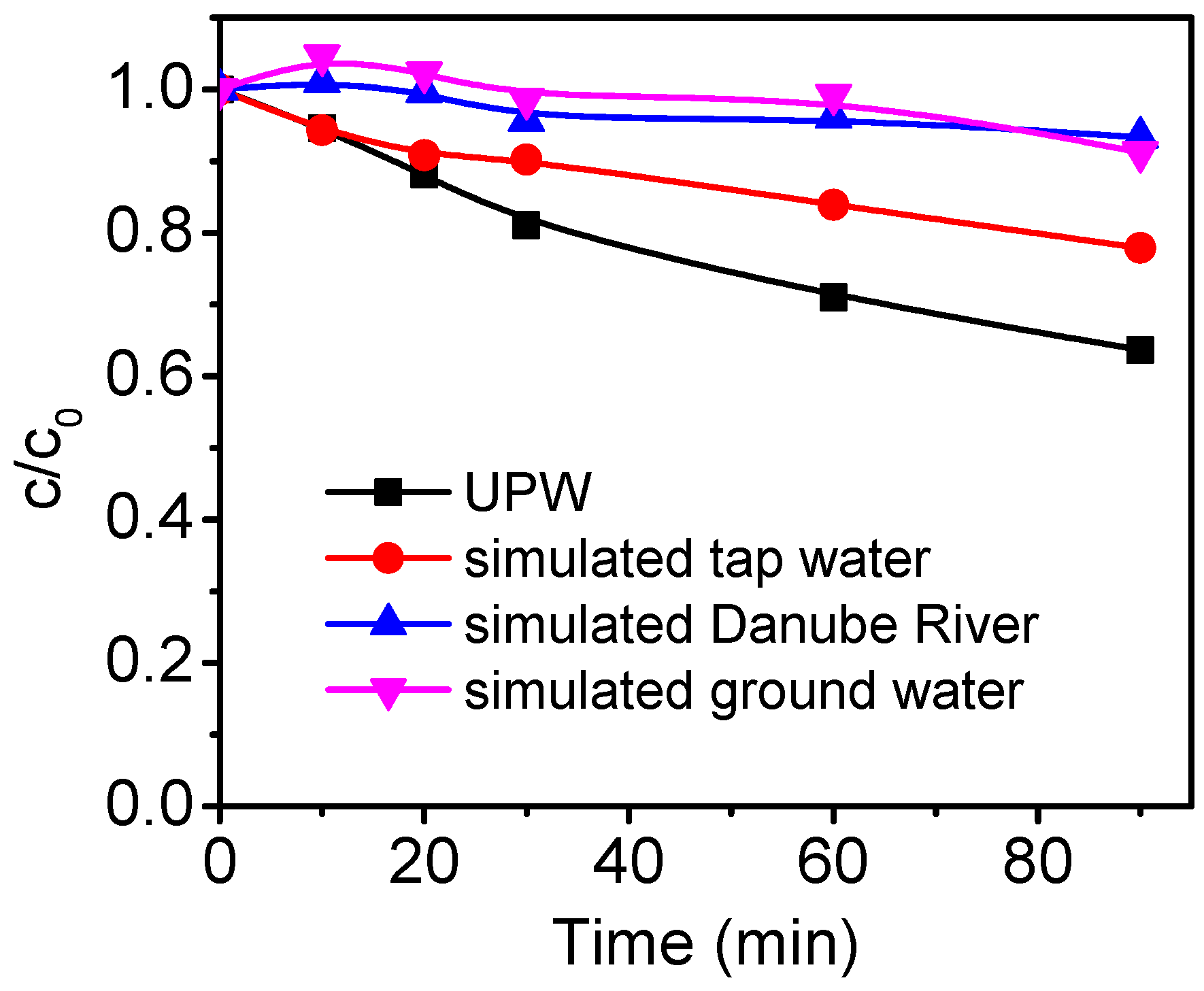
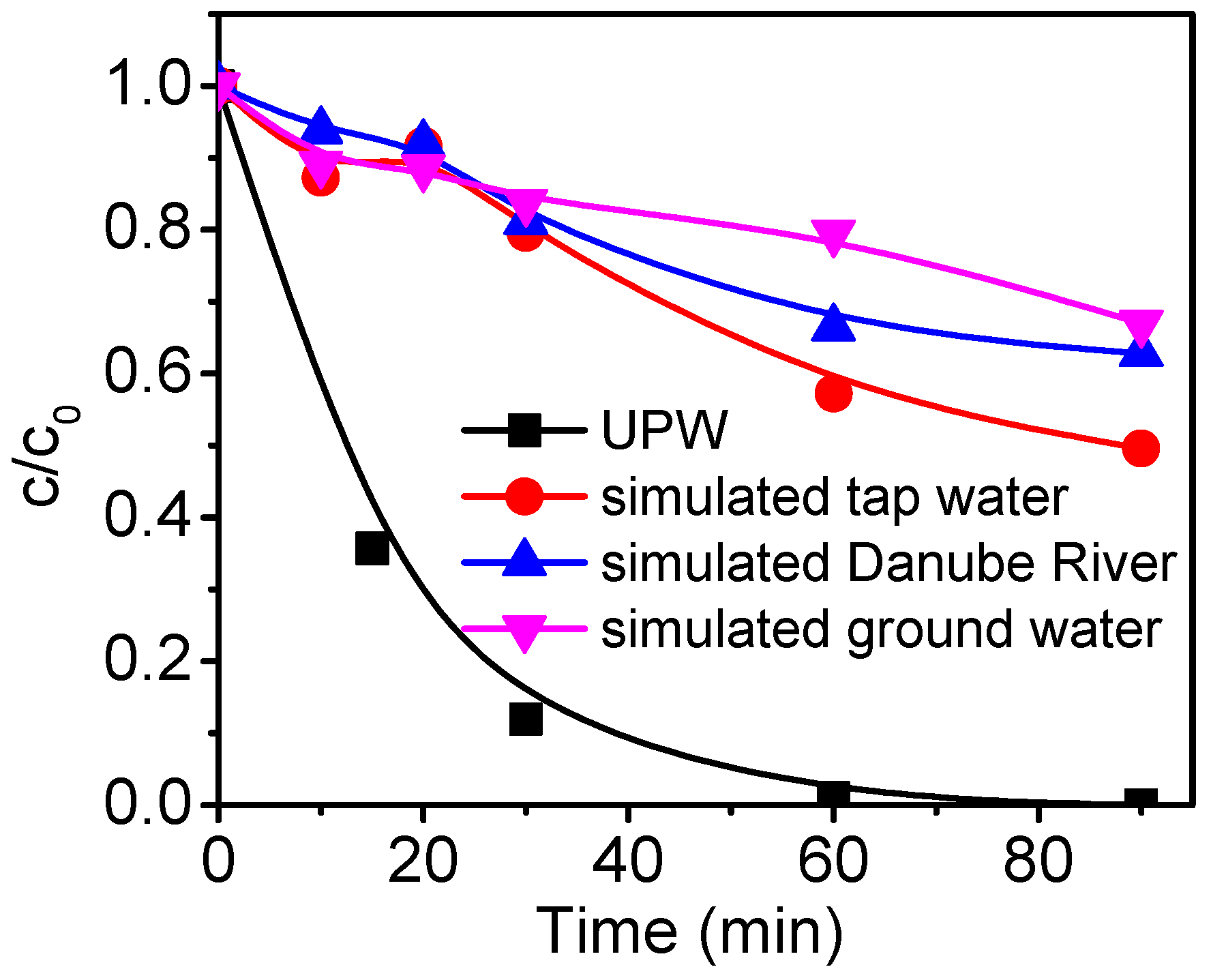
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schenzel, J.; Hungerbühler, K.; Bucheli, T.D. Mycotoxins in the environment: II. Occurrence and origin in Swiss river waters. Environ. Sci. Technol. 2012, 46, 13076–13084. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Al-gabr, H.M.; Zheng, T.; Yu, X. Occurrence and quantification of fungi and detection of mycotoxigenic fungi in drinking water in Xiamen City, China. Sci. Total Environ. 2014, 466–467, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Erbs, M.; Wettstein, F.E.; Schwarzenbach, R.P.; Bucheli, T.D. Quantification of estrogenic mycotoxins at the ng/L level in aqueous environmental samples using deuterated internal standards. J. Chromatogr. A 2007, 1138, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.; Mata, A.T.; Ferreira, J.P.; Barreto Crespo, M.T.; Pereira, V.J.; Bronze, M.R. Production of mycotoxins by filamentous fungi in untreated surface water. Environ. Sci. Pollut. Res. Int. 2018, 25, 17519–17528. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, I. Direct and Indirect Photolysis of Fumonisins in the Aquatic Environment as well as Assessment of their Toxicity. Ph.D. Thesis, University in Novi Sad, Faculty of Sciences, Novi Sad, Serbia, 2022. Available online: https://nardus.mpn.gov.rs/handle/123456789/19078 (accessed on 25 May 2022). (In Serbian).
- Gromadzka, K.; Waśkiewicz, A.; Goliński, P.; Świetlik, J. Occurrence of estrogenic mycotoxin—Zearalenone in aqueous environmental samples with various NOM content. Water Res. 2009, 43, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Bucheli, T.D.; Wettstein, F.E.; Hartmann, N.; Erbs, M.; Vogelgsang, S.; Forrer, H.-R.; Schwarzenbach, R.P. Fusarium mycotoxins: Overlooked aquatic micropollutants? Agric. Food Chem. 2008, 56, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Hoerger, C.C.; Meyer, M.T.; Wettstein, F.E.; Hubbard, L.E.; Bucheli, T.D. Phytoestrogens and mycotoxins in Iowa streams: An examination of under investigated compounds in agricultural basins. J. Environ. Qua. 2010, 39, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.; Bacaloni, A.; De Leva, I.; Faberi, A.; Fago, G.; Marino, A. Analytical methodologies for determining the occurrence of endocrine disrupting chemicals in sewage treatment plants and natural waters. Anal. Chim. Acta 2004, 501, 79–88. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Maia, A.; Santos, M.; Tiritan, M.E.; Ribeiro, C.M.R. Occurrence of natural contaminants of emerging concern in the Douro River estuary, Portugal. Arch. Environ. Contam. Toxicol. 2015, 70, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.T.; Ferreira, J.P.; Oliveira, B.R.; Batoréu, M.C.; Barreto Crespo, M.T.; Pereira, V.J.; Bronze, M.R. Bottled water: Analysis of mycotoxins by LC–MS/MS. Food Chem. 2015, 176, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Banu, N.; Malaikumar, B.; Pavithra, S. Enumeration of terrestrial mycobiota and aflatoxin in drinking water. Asian Jr. Microbiol. Biotech. Env. Sc. 2016, 18, 207–217. [Google Scholar]
- Waśkiewicz, A.; Bocianowski, J.; Perczak, A.; Goliński, P. Occurrence of fungal metabolites—Fumonisins at the ng/L level in aqueous environmental samples. Sci. Total Environ. 2015, 524–525, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, I.; Jakšić, S.; Šojić Merkulov, D.; Bognár, S.; Abramović, B.; Ivetić, T. Matrix effects of different water types on the efficiency of fumonisin B1 removal by photolysis and photocatalysis using ternary-and binary-structured ZnO-based nanocrystallites. Catalysts 2023, 13, 375. [Google Scholar] [CrossRef]
- Jevtić, I.; Jakšić, S.; Simin, D.Č.; Uzelac, M.; Abramović, B. UV-induction of photolytic and photocatalytic degradation of fumonisins in water: Reaction kinetics and toxicity. Environ. Sci. Pollut. Res. 2021, 28, 53917–53925. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Water Type | |||
|---|---|---|---|---|
| Danube River | Ground Water | Tap Water | UPW 1 | |
| pH | 7.70 | 7.62 | 7.30 | 6.56 |
| Conductivity at 25 °C (μS/cm) | 333 | 466 | 516 | 0.055 |
| TOC (mg/dm3) 2 | 2.30 | 0.78 | 1.80 | <DL 3 |
| Hydrogen carbonate (mg/dm3) | 209 | 768 | 238 | <DL |
| Chloride (mg/dm3) | 44.02 | 61.39 | 16.50 | <DL |
| Sulphate (mg/dm3) | 15.52 | 0.486 | 35.0 | <DL |
| Nitrate (mg/dm3) | 3.86 | 0.099 | 1.87 | <DL |
| Calcium (mg/dm3) | 0.136 | <DL | 70.49 | <DL |
| Magnesium (mg/dm3) | 0.078 | 0.129 | 20.3 | <DL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevtić, I.; Jakšić, S.; Šojić Merkulov, D.; Bognár, S.; Abramović, B. Efficacy of Fumonisin B1 Removal from Various Simulated Water Types Using UV and UV/H2O2 Treatments. Biol. Life Sci. Forum 2023, 24, 7. https://doi.org/10.3390/IECT2023-14802
Jevtić I, Jakšić S, Šojić Merkulov D, Bognár S, Abramović B. Efficacy of Fumonisin B1 Removal from Various Simulated Water Types Using UV and UV/H2O2 Treatments. Biology and Life Sciences Forum. 2023; 24(1):7. https://doi.org/10.3390/IECT2023-14802
Chicago/Turabian StyleJevtić, Ivana, Sandra Jakšić, Daniela Šojić Merkulov, Szabolcs Bognár, and Biljana Abramović. 2023. "Efficacy of Fumonisin B1 Removal from Various Simulated Water Types Using UV and UV/H2O2 Treatments" Biology and Life Sciences Forum 24, no. 1: 7. https://doi.org/10.3390/IECT2023-14802
APA StyleJevtić, I., Jakšić, S., Šojić Merkulov, D., Bognár, S., & Abramović, B. (2023). Efficacy of Fumonisin B1 Removal from Various Simulated Water Types Using UV and UV/H2O2 Treatments. Biology and Life Sciences Forum, 24(1), 7. https://doi.org/10.3390/IECT2023-14802









