Rapid Identification of the Mycotoxin Patulin by Gas Chromatography–Mass Spectrometry †
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Materials
2.2. GC–MS Equipment and Methodology
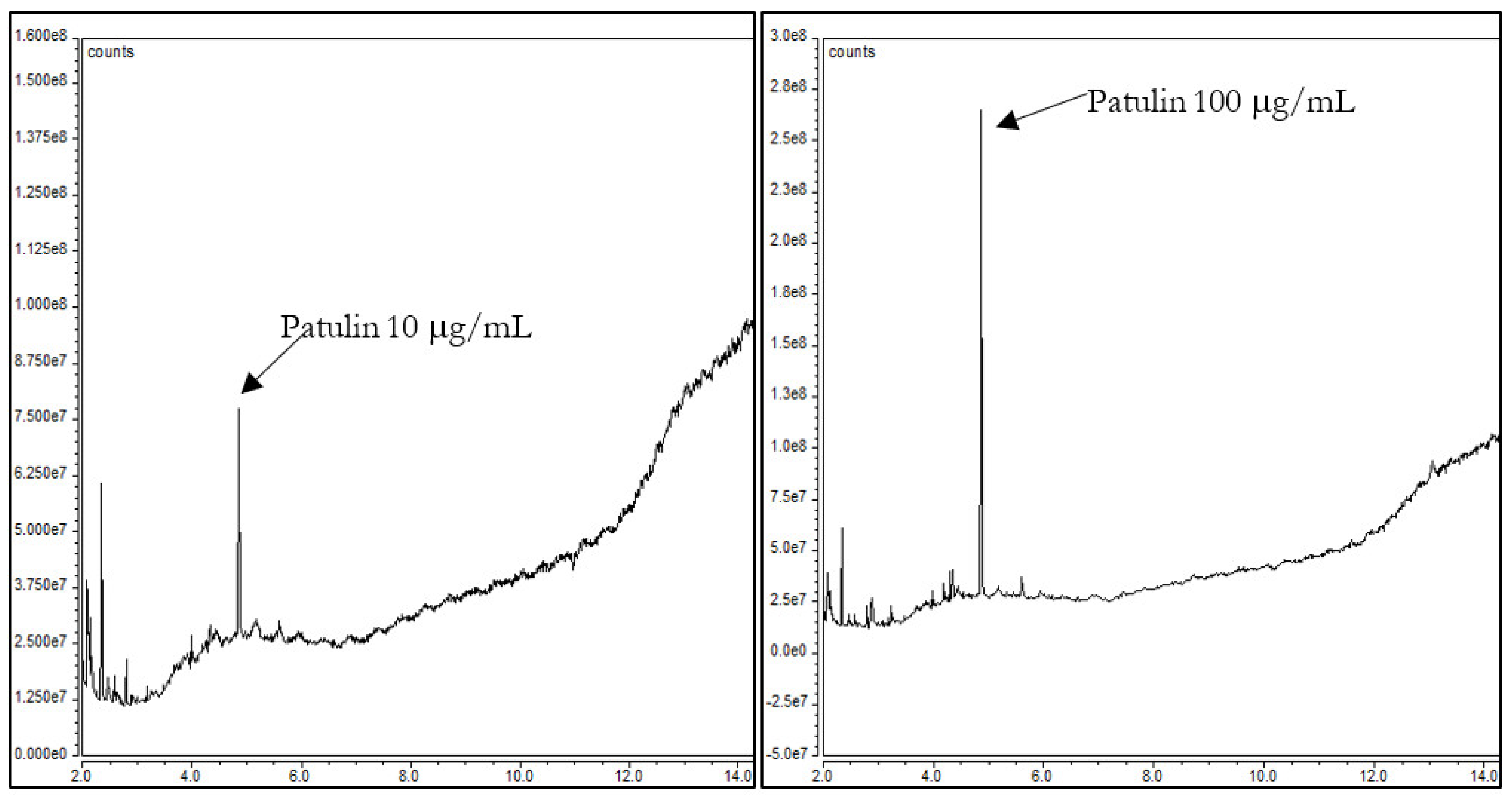
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; Boevre, M. De The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Moake, M.M.; Padilla-Zakour, O.I.; Worobo, R.W. Comprehensive review of patulin control methods in foods. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Faria, M.A.; Fernandes, J.O. Determination of patulin in apple and quince products by GC-MS using 13C5-7 patulin as internal standard. Food Chem. 2009, 115, 352–359. [Google Scholar] [CrossRef]
- Rupp, H.S.; Turnipseed, S.B. Confirmation of patulin and 5-hydroxymethylfurfural in apple juice by gas chromatography/mass spectrometry. J. AOAC Int. 2000, 83, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Iida, K.; Suzuki, J.; Kimura, K.; Ibe, A.; Saito, K. A quantification and confirmation method of patulin in apple juice by GC/MS. J. Food Hyg. Soc. Japan 2004, 45, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Fu, S. A sensitive gas chromatography-mass spectrometry method for the determination of patulin in apple juice. J. AOAC Int. 2012, 95, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Llovera, M.; Viladrich, R.; Torres, M.; Canela, R. Analysis of underivatizated patulin by a GC-MS technique. J. Food Prot. 1999, 62, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.A.; White, K.D.; Trucksess, M.W.; Thomas, F.S. Capillary Gas Chromatography/Mass Spectrometry with Chemical Ionization and Negative Ion Detection for Confirmation of Identity of Patulin in Apple Juice. J. AOAC Int. 2000, 83, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.S. Rapid gas chromatography/mass spectrometry determination and confirmation of patulin in apple juice. J. AOAC Int. 2007, 90, 879–883. [Google Scholar] [PubMed]
- Moukas, A.; Panagiotopoulou, V.; Markaki, P. Determination of patulin in fruit juices using HPLC-DAD and GC-MSD techniques. Food Chem. 2008, 109, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kharandi, N.; Babri, M.; Azad, J. A novel method for determination of patulin in apple juices by GC–MS. Food Chem. 2013, 141, 1619–1623. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Berrada, H.; Font, G.; Mañes, J. Multi-mycotoxin analysis in wheat semolina using an acetonitrile-based extraction procedure and gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 28–40. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H.; Mañes, J. A survey of trichothecenes, zearalenone and patulin in milled grain-based products using GC-MS/MS. Food Chem. 2014, 146, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Marsol-Vall, A.; Balcells, M.; Eras, J.; Canela-Garayoa, R. A rapid gas chromatographic injection-port derivatization method for the tandem mass spectrometric determination of patulin and 5-hydroxymethylfurfural in fruit juices. J. Chromatogr. A 2016, 1453, 99–104. [Google Scholar] [CrossRef] [PubMed]
| Type of Food | Type of Column | Operating Conditions | LODs | LOQs | Reference |
|---|---|---|---|---|---|
| With derivatization | |||||
| Fruit juice | Column 50% phenyl, 50% methyl-polysiloxane, SGE, capillary (30 m × 0.25 mm × 0.25 μm) | Initial T: 100 °C for 2 min, ramped at 10 °C min−1 to 200 °C and then 20 °C min−1 to 300 °C, held for 3 min. Carrier gas: He (constant flow: 1.42 mL/min). | 5.8 μg/kg−1 | 13.8 μg/kg | [12] |
| Apple juice | Three capillary columns (moderate polarity) (1) DB-17 (0.25 μm i.d. × 30 m, 0.25 μm J&W) (2) DB-1701 (0.25 μm i.d. × 30 m, 0.25 μm, J&W) (3) BPX-35 (0.22 μm i.d. × 25 m, 0.25 μm, BSE) | Oven T: 80 °C (2 min) → 150 °C (10 °C/min) → 230 °C (5 °C/min, 15 min) Carrier gas: He at constant pressure of 100 kPa. | 0.1 μg/kg | 1 μg/kg | [7] |
| Apple, Quince | Supelco SLB-5MS Column (30 m × 0.25 mm ID × 0.25 μm film thickness) | Oven T: 100 °C held for 1 min, ramped to 180 °C at 10 °C/min, finally ramped to 280 °C at 30 °C/min and held for 12.67 min (25 min total run time). Carrier gas: He (constant flow: 1 mL min). | 0.4 μg/kg−1 | 1.6 μg/kg | [5] |
| Apple juice | Column HP-5MS, crosslinked 5% phenylmethyl silicone (30 m × 0.25 mm id × 0.25 μm film thickness, Agilent) | Oven T: 100 °C for 2 min, ramped at 10 °C/min to 200 °C, and then 200 to 300 °C, held for 3 min. | 3 μg/L | 10 μg/L | [11] |
| Apple/Pear Juice | Column J&W DB-5MS (30 m × 0.25 mm id; 0.25 μm film thickness) | 70 °C (held for 1 min) to 320 °C at 25 °C/min (held for 2 min) at a constant flow regime of 1 mL/min | n.i. | n.i. | [16] |
| Apple juice | Agilent HP-5MS column (30 m × 0.25 mm × 0.25 μm film thickness) | Oven T initially held at 50 °C (3 min) and programmed to 280 °C at a rate of 10 °C/min, then held for 5 min. Total run time was 31 min. Carrier gas: He (constant flow: 1 mL min). | 0.4 μg/L | 1.3 μg/L | [13] |
| Apple juice | Column HP-5MS 5% phenyl methyl siloxane cross-linked capillary GC column (15 m × 0.25 mm i.d., × 0.25 μm film thickness) | Oven T: initially at 100 °C (2 min) and programmed at 15 °C/min to 210 °C, then at 50 °C/min to a final T of 300 °C, which was held for 2 min. Total run time: 13 min. Inlet: 280 °C. Transfer line: 250 °C. | 2 μg/L | 5 μg/L | [8] |
| Apple juice | HP Ultra 2 crosslinked 5% phenyl methyl silicone (25 m × 0.2 mm), with 0.33 μm film | Oven T: 100 °C (2 min), ramped at 10 °C/min to 200 °C and then 20 °C/min to 300 °C, held for 3 min. Detector T: 300 °C. Injector T: 280 °C. Carrier gas: He (constant flow: 1 mL min). | n.i. | n.i | [6] |
| Without derivatization | |||||
| Apple Juice | Column HP-5MS cross-linked methyl silicone capillary (30 m × 0.25 mm id) | T held at 80 °C for 1 min after injection, programmed to 250 °C at 15 °C/min, and held for 5 min. Carrier gas: He | n.i | n.i | [9] |
| Apple Juice | Capillary column (15 m × 0.53 mm id) with a 1.5 μm film of bonded phase methyl polysiloxane | T held at 60 °C for 1 min after on-column injection, heated at 15 °C/min to 260 °C, and held at 260 °C for 5 min. Transfer lines: 260 °C. Carrier gas: He at gas flow of 25 cm/s. | n.i | n.i | [10] |
| Apple Juice | Capillary column (30 m × 0.25 mm id) with a 0.25 μm film of bonded phase trifluoropropylmethyl polysiloxane | T programmed from 60 °C to 260 °C 1 min after injection at a rate of 20 °C/min. Transfer lines: 260 °C, ion source: 200 °C. Injection: 260 °C. Carrier gas: He at constant flow: 40 cm/s (1.21 mL/min). | n.i | n.i | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteagudo, A.A.; Torres, A.; Chamorro, F.; Prieto, M.A.; Perez-Gregorio, R.; Simal-Gandara, J.; Otero, P. Rapid Identification of the Mycotoxin Patulin by Gas Chromatography–Mass Spectrometry. Biol. Life Sci. Forum 2023, 24, 6. https://doi.org/10.3390/IECT2023-14813
Monteagudo AA, Torres A, Chamorro F, Prieto MA, Perez-Gregorio R, Simal-Gandara J, Otero P. Rapid Identification of the Mycotoxin Patulin by Gas Chromatography–Mass Spectrometry. Biology and Life Sciences Forum. 2023; 24(1):6. https://doi.org/10.3390/IECT2023-14813
Chicago/Turabian StyleMonteagudo, Adrián A., Antía Torres, Franklin Chamorro, Miguel A. Prieto, Rosa Perez-Gregorio, Jesus Simal-Gandara, and Paz Otero. 2023. "Rapid Identification of the Mycotoxin Patulin by Gas Chromatography–Mass Spectrometry" Biology and Life Sciences Forum 24, no. 1: 6. https://doi.org/10.3390/IECT2023-14813
APA StyleMonteagudo, A. A., Torres, A., Chamorro, F., Prieto, M. A., Perez-Gregorio, R., Simal-Gandara, J., & Otero, P. (2023). Rapid Identification of the Mycotoxin Patulin by Gas Chromatography–Mass Spectrometry. Biology and Life Sciences Forum, 24(1), 6. https://doi.org/10.3390/IECT2023-14813










