Abstract
The aim of this study was to the evaluate impact of ethyl acetate extract of Laetiporus sulphureus on the viability of HeLa cells in 2D cell cultures and in a co-culture system with Saccharomyces boulardii. Also, the migratory potential of S. boulardii through agar in this co-culture system was investigated. Cell viability was assessed by trypan blue staining after 12 and 24 h. Tested extract had no cytotoxicity on HeLa cells in the 2D cell cultures. Our results indicate a potential cytotoxic effect of S. boulardii on HeLa cells which could be a consequence of physical contact between yeast and cancer cells, after migration of S. boulardii through agar toward cancer cells, or metabolic activity of S. boulardii. Also, L. sulphureus extract induced strong migration of yeast in co-culture after 12 h, compared to control. Further studies should be conducted regarding this mushroom in a co-culture system with S. boulardii.
1. Introduction
Co-culture systems are widely used in biological research to study interactions between different cell populations. Recently, co-culture systems have been increasingly used in the research of interactions between human cells and certain microorganisms. Many studies indicate the important role of microbiomes in the promotion and inhibition of tumor mass growth [1,2].
Laetiporus sulphureus (Bull.) Murrill is an edible mushroom with large sulfuric-yellow-colored fruiting bodies and is a wood-rotting saprophyte that prefers to grow in deciduous and evergreen forests all over the world [3]. This mushroom is known for its medical properties and proven antioxidant, antimicrobial, and antitumor properties [3]. Although it possesses confirmed anticancer effects [4], its cytotoxicity potential on HeLa cells in a co-culture system is insufficiently investigated.
Saccharomyces boulardii is a probiotic species used effectively in complementing the treatment of acute gastrointestinal diseases such as diarrhea, or chronic diseases such as inflammatory bowel disease [5]. S. cerevisiae can survive in a gastric-like environment and in an intestinal environment (bile salts, pancreatin, pH 7.0), which enables the application of this microorganism in extreme conditions, such as the cancer environment. Also, the advantage of S. boulardii is its very successful penetration through the mucin produced by the epithelial cells and adhesion to these cells. The probiotics express surface adhesins that mediate the attachment to the mucous layer by recognizing host molecules, such as transmembrane proteins (integrins or cadherins), and extracellular matrix components (collagen, fibronectin, laminin, elastin, etc.). Furthermore, a very common mechanism of attachment of S. boulardii to the host epithelium is via mannose residues in the cell wall [6].
Recently, studies showed that the cervical microbiome takes part in inhibition and progress of cancer. Accordingly, the aim of this study was to develop and optimize a co-culture system using specific probiotic species and cancer cells. Furthermore, we examined the effect of ethyl acetate extract of L. sulphureus (EALS) in co-treatment with the probiotic species S. boulardii on the viability of cervical adenocarcinoma (HeLa) cancer cell line in the co-culture system.
2. Materials and Methods
Laetiporus sulphureus was gathered from lying tree trunks of Salix sp. in the Šumadija area, Serbia (43°54′00.32″ N, 20°52′02.90″ E, Adžine Livade, altitude 629 m) in autumn of 2019. Extraction was performed with ethyl acetate solvent according to the standard procedure previously described [4]. The collected material was air-dried in darkness at ambient temperature. A total of 100 g of the examined mushrooms was soaked in 500 mL of the solvent. The finely dried ground mushroom material was macerated three times at room temperature using fresh solvent every 48 h. After every 48 h, the samples were filtered through sterile gauze and the filtrates were collected and evaporated to dryness using a rotary evaporator (IKA, Sindelfingen, Germany) at 40 °C. The dry extracts were stored at −18 °C until they were used in the tests. The obtained amount of crude extract of EALS was 2.47 g.
Cervical adenocarcinoma (HeLa) cancer cell line was obtained from ATCC (Manassas, VA, USA) and was cultured in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and antibiotics (100 U penicillin and 100 U/mL streptomycin). To determine cell viability in 2D culture, HeLa cells were seeded in a 6-well plate. The 2D single cell culture was treated with EALS at a concentration of 10 µg/mL. Untreated cells were used as a control. The viability was determined with trypan blue staining 12 and 24 h after the EALS treatment [7].
The probiotic species S. boulardii was provided by the Microbiology Laboratory, Institute for Information Technologies, University of Kragujevac, Serbia. The bacterial suspension was prepared by the direct colony method (13). The turbidity of the initial suspension was adjusted using a 0.5 McFarland densitometer (Biosan, Riga, Latvia). The initial bacterial suspension contained about 108 colony-forming units (CFU)/mL. The 1:100 dilutions of initial suspension were additionally prepared in sterile 0.85% saline.
The simple co-culture system was formed in 50 mL plastic test tubes. The procedure was as follows: 40 µL of yeast suspension was added into 40 mL of sterile soft Sabouraud dextrose agar (0.7%, wt/vol) (Torlak, Belgrade, Serbia). The soft agar with S. boulardii was poured into plastic tubes and left at room temperature to solidify. The HeLa cells were seeded on the coverslips, and after incubation for 24 h were placed (upside down) on top of the agar and overlaid with 10 mL of DMEM (DMEM, 10% FBS, without antibiotics) or with 10 mL EALS of 10 µg/mL, and test tubes were placed in an incubator at 37 °C without CO2 for 12 and 24 h of incubation. DMEM is food for the cells, and it provides the oxygen necessary for the cells. The HeLa cells’ viability was investigated in 3 experimental conditions: (1) The HeLa cells turned towards the surface of yeast medium (without S. boulardii inoculum) and were overlaid with 10 mL of DMEM-represented negative control (C). (2) The HeLa cells on the surface and S. boulardii were inoculated in agar and overlaid with 10 mL of DMEM; (3) The HeLa cells on the surface and S. boulardii were inoculated in agar and overlaid with 10 mL of EALS treatment. Furthermore, the migratory potential and colony formation of yeast on the top of agar were evaluated. Migration of yeast through soft agar was measured using ImageJ software after 12 and 24 h of incubation. A thin layer on top of the agar indicates migration of S. boulardii, while a thicker layer most likely indicates the proliferation of S. boulardii and colony formation, considering that yeast cells double in number every 100 min [8].
Statistical analyses were performed using a statistical software package (SPSS for Windows, ver. 17, 2008, Chicago, IL, USA): data were analyzed using one-way analysis of variance (ANOVA). p < 0.05 is considered a statistically significant difference between treatments and control and is represented by *# in graphs.
3. Results and Discussion
Our results showed no cytotoxicity effect of EALS extract when it was tested outside the coculture system (control experiment). Moreover, the HeLa cell proliferation is statistically and significantly increased after 12 and 24 h of EALS treatment (Figure 1a). Similar results were confirmed in the research Jovanovic et al., where no significant cytotoxicity of EALS on HeLa cells was obtained [9]; therefore, it was of interest to investigate how the treatment impacted HeLa cells in co-cultures with S. boulardii.
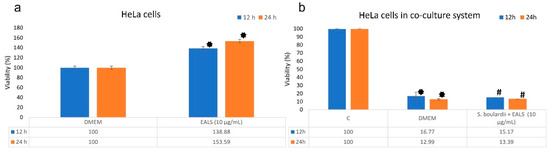
Figure 1.
The effect of EALS on HeLa cells without S. boulardii (a); HeLa cells in a co-culture system with S. boulardii (b); the viability of HeLa cells in control, without EALS and S. boulardii (negative control-C), The viability of HeLa cells with S. boulardii, without treatment (DMEM); and viability of HeLa cells with S. boulardii and EALS (S. boulardii + EALS). The viability of HeLa cells in both experiments is expressed as a percentage ± SE of 3 independent experiments. * A statistical significance difference in incubation between DMEM/EALS (a), and C/S. boulardii (b). # A statistical significance difference in incubation between C/S. boulardii + EALS (10 µg/mL) (b).
On the other hand, the S. boulardii significantly reduces the viability of the HeLa cells in the co-culture system compared to the control. Additionally, the EALS showed no cytotoxicity effect on HeLa cells in the co-cultures with S. boulardii, as well as in the control experiment (Figure 1b). EALS had no negative effect on S. boulardii in the co-culture system, which is a positive result of this investigation. These results indicate a potential cytotoxic effect of S. boulardii on cervical adenocarcinoma cells (HeLa) (Figure 1b). The cytotoxicity could result from the metabolic activity of S. boulardii cells which seems to be further enhanced following the physical contact between yeast and cancer cells. The cytotoxic effect of S. boulardii was also shown on human breast cancer cells in a study by Jovanović et al. [10].
In addition, our study aimed to evaluate the effect of treatment on the migration of S. boulardii in the co-culture system after 12 and 24 h of incubation. Results showed a statistically significant difference in yeast migration after the impact of EALS after 12 h of incubation, compared to DMEM (Figure 2). It is important to examine the influence of treatment on the migration of this yeast, given that physical contact is one of the potential mechanisms of the cytotoxic effect of S. boulardii. More investigations are recommended to be implemented to determine precise mechanisms of cytotoxicity of S. boulardii.
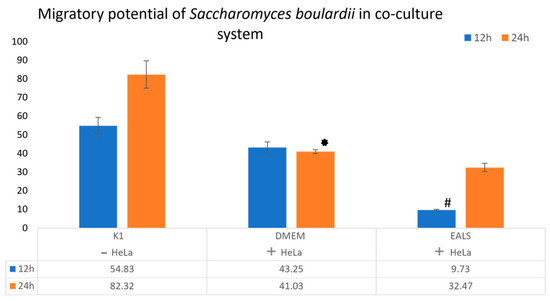
Figure 2.
The S. boulardii migratory potential in the co-culture system after 12 and 24 h of incubation with 10 µg/mL of EALS. S. boulardii in a test tube without HeLa cells, only cover slip (K1), the S. boulardii and HeLa cells in a test tube without treatment (DMEM), and the S. boulardii and HeLa cells in a test tube with EALS. * A statistical significance difference between DMEM and K1 on 24 h of incubation. # A statistical significance difference between EALS and DMEM on 12 h of incubation.
Furthermore, the test tubes with S. boulardii were photographed after 12 and 24 h of incubation, and yeast migration and colony formation on top of the agar after the impact of EALS are shown visually (Figure 3). The colony formation is most probably a consequence of intensive yeast proliferation.
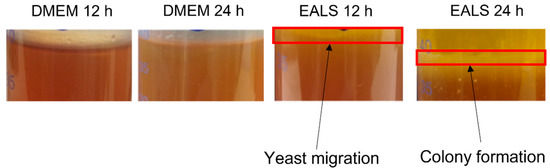
Figure 3.
Visualization of S. boulardii in test tubes and on the cover slip after 12 and 24 h of incubation.
4. Conclusions
In this study, we demonstrated the effect of the probiotic species S. boulardii on the viability of cervical cancer cells (HeLa). Our results indicate a significant reduction in the viability of HeLa cells in the co-culture system with S. boulardii already after 12 h of incubation. This might be a consequence of physical contact or metabolic activity of S. boulardii, that in this study is not investigated. Certainly, additional tests of the cytotoxic effect of this yeast on the viability of HeLa cells are recommended because the physical contact, as well as metabolic activity, might be the cause of cytotoxicity. In addition, the results showed a positive effect of EALS extract on the migration of S. boulardii toward the HeLa cells.
Author Contributions
Conceptualization, D.Š.; methodology, D.A. and K.P.; software, D.A.; validation, D.Š.; formal analysis, D.Š. and D.A.; investigation, D.A.; resources, M.G. and K.M.; data curation, D.Š. and D.A.; writing—original draft preparation, D.A.; writing—review and editing, D.Š. and M.J.; visualization, D.A. and K.P.; supervision, D.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Agreement No. 451-03-68/2022-14/200122 and 451-03-68/2022-14/200378).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA: A Canc. J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Y.; Bao, Y.Y.; Jing, L.; Shu, W.Z.; Liu, L.; Khaled, N.; Xiao, X.X.; Xiao, Y.P.; Jia, P.L. Metabolite secretions of Lactobacillus plantarum YYC-3 may inhibit colon cancer cell metastasis by suppressing the VEGF-MMP2/9 signaling pathway. Microb. Cell Factor. 2020, 19, 1–12. [Google Scholar]
- Younis, A.M.; Yosric, M.; Stewarte, J.K. In vitro evaluation of pleiotropic properties of wild mushroom Laetiporus sulphureus. Ann. Agric. Sci. 2019, 64, 79–87. [Google Scholar] [CrossRef]
- Šeklić, D.S.; Stanković, M.S.; Milutinović, M.G.; Topuzović, M.D.; Štajn, A.Š.; Marković, S.D. Cytotoxic, antimigratory, pro-and antioxidative activities of extracts from medicinal mushrooms on colon cancer cell lines. Arch. Biol. Sci. 2016, 68, 93–105. [Google Scholar] [CrossRef]
- Kelesidis, T.; Pothoulakis, C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Thera. Adve. Gastro. 2012, 5, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Almeida, V.; Yilmaz, M.; Teixeira, C.M. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sadabad, S.M.; Martels, Z.Y.; Khan, T.M.; Blokzijl, T.; Paglia, G.; Dijkstra, G.; Harmsen, M.H.; Faber, N.K. A simple coculture system shows mutualism between anaerobic faecalibacteria and epithelial cells. Sci. Reports 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Herskowitz, I. Life Cycle of the Budding Yeast Saccharomyces cerevisiae. Micr. Rev. 1988, 52, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.M.; Virijević, K.; Grujić, J.; Živanović, M.; Šeklić, S.D. Extract of Edible Mushroom Laetiporus sulphureus Affects the Redox Status and Motility of Colorectal and Cervical Cancer Cell Lines. Biol. Life Sci. Forum 2021, 6, 82. [Google Scholar] [CrossRef]
- Pakbin, B.; Dibazar, P.S.; Allahyari, S.; Javadi, M.; Amani, Z.; Farasat, A.; Darzi, S. Anticancer Properties of Probiotic Saccharomyces boulardii Supernatant on Human Breast Cancer Cells. Probiotics Antimicro. Prot. 2022, 14, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).