Abstract
Oil-in-water (O/W) nanoemulsions (d < 200 nm) are systems with considerable potential for protecting and delivering sensible ingredients such as chia seed oil rich in ω-3 fatty acids (~64% α-linolenic acid). These systems can be formed by applying either low- or high-energy methods. High-pressure homogenization, microfluidization and sonication are included within the latter. The main aim of this research work was to obtain and characterize chia oil-in-water nanoemulsions by microfluidization. Therefore, O/W nanoemulsions with 10% (w/w) chia oil and 2% (w/w) sodium caseinate were prepared at three levels of microfluidization pressure: 600, 1000 and, 1200 bar. Droplet sizes of the nanoemulsions expressed as the Sauter mean diameter, were found between 108 to 125 nm. Additionally, the resulting superficial droplet charge was between −37 to −41 mV. The global stability of the different systems was evaluated through the evolution of their backscattering for 50 days. In this sense, nanoemulsions obtained at 1000 and 1200 bar recorded high global stability, while those obtained at 600 bar showed some signs of destabilization. In terms of oxidative stability, all systems studied recorded low values of primary and secondary oxidation products as a function of storage, as determined by peroxide value index (PV) and thiobarbituric acid reactive substances (TBARs) assays, respectively. The omega-3 fatty acid content of the nanosystems was also evaluated, without significant changes during the storage period. Thus, chia O/W nanoemulsions obtained by microfluidization proved to be suitable delivery systems for bioactive compounds of chia seed, with potential applications in the development of functional food.
1. Introduction
The lower content of saturated fatty acids, the adequate concentration of linoleic acid (18–20%), and the high content of alpha-linolenic acid (55–60%) make chia oil an appealing option for healthy food and cosmetic applications [1]. Consumption of sufficiently high levels of ω-3 fatty acids has been linked to reduced risk of certain chronic diseases, such as inflammation, cardiovascular disease, immune response, and mental disorders [2]. However, due to the high content of unsaturated fatty acids, chia oil is susceptible to lipid oxidation and needs to be protected for its potential incorporation into foods. In this sense, emulsion-based delivery systems have proved to be an effective strategy to protect and deliver sensitive ingredients. Nanoemulsions can be used as delivery systems due to their extended stability and improved bioavailability [3]. In these systems, the smaller sizes of droplets give them more stability against gravitational separation and droplet aggregation than conventional emulsions [4]. Among the different approaches available for forming nanoemulsions, microfluidization has been shown to be efficient at producing small droplets with uniform particle size distributions. During the microfluidization process, a combination of high disruptive forces (cavitation, turbulence, and shear) leads to the formation of very fine oil droplets [5].
In this context, the aim of this work was to study the incorporation of chia oil into functional O/W nanoemulsions and to evaluate the effect of the homogenizer pressure level on its physicochemical properties.
2. Materials and Methods
2.1. Materials
Commercial chia oil extracted by cold pressing was purchased by SDA S.A. (Lobos, Argentina) and stored at 4 ± 1 °C until use in an amber bottle. Sodium caseinate (NaCas) was purchased from Sigma Chemical Company (St. Louis, MO, USA). All reagents used were of analytical grade.
2.2. Preparation of O/W Emulsions
Chia oil-in-water (O/W) nanoemulsions with an oil:water ratio of 10:90, and 2% NaCas as an emulsifying agent, were obtained. In the first homogenization step, the aqueous phase and the chia oil were mixed using an Ultraturrax T-25 (Janke & Kunkel GmbH, Staufen, Germany) at 13,500 rpm for 2 min. A second step was carried out using a Microfluidizer (LM10, Microfluidics, Westwood, CA, USA) by applying three different pressures (600, 1000 and 1200 bar) and three passes. Nisin and potassium sorbate were added to all systems to prevent microbial growth. The O/W nanoemulsions were stored at 4 °C for 50 days protected from light.
2.3. Emulsions Characterization
2.3.1. Droplet Size
Droplet size was determined by laser diffraction with a particle size analyzer (Mastersize 2000E, Malvern Instrument Ltd., Worcestershire, UK). For each measurement, approximately 1 mL of sample was diluted directly in the water bath of the dispersion system with a pump speed of 1700 rpm (Hydro 2000G), reaching an obscuration of 10–12%. Measurements were performed in sextuplicate.
2.3.2. Emulsion Stability
The physical stability of the emulsions was monitored by periodic measurements of dispersed light using a Vertical Scan Analyzer Quick Scan (Coulter Corp., Miami, FL, USA) according to Pan et al. (2002) [6].
2.3.3. ζ-Potential
The ζ-potential was determined at room temperature using a Zeta Potential Analyzer (Brookhaven 90Plus/Bi-MAS, New York, NY, USA) according to Julio et al. (2015) [7]. For each determination, 0.05 g of the sample was dispersed in 100 mL of milli-Q water. Measurements were performed in duplicate.
2.3.4. Rheological Properties
Rheological measurements were performed in an AR-G2 stress-controlled oscillatory rheometer (TA instrument, New Castle, DE, USA) at 25 ± 0.3 °C with a cone-plate sensor system. Samples were subjected to a shear rate increase (1 to 500 s−1) for 3 min, maintained at 500 s−1 for 1 min, and a decrease in shear rate (500 to 1 s−1) for 3 min.
2.4. Oxidative Stability of Emulsions
To evaluate the oxidative stability of chia O/W nanoemulsions during the storage period, primary and secondary oxidative products were determined through peroxide value (PV) and 2-thiobarbituric acid reactive substances (TBARs), respectively. The PV was carried out according to the spectrophotometric method described by Diaz et al. (2003) [8], while the TBARs assay was according to Hu and Zhong (2010) [9]. In addition, chia oil was extracted from the different nanoemulsions and its fatty acid profile was obtained using Agilent Technologies 7890A equipment at the initial time and then 50 days of storage.
2.5. Statically Analysis
Analysis of variance (ANOVA) was performed on the data set using Statgraphics Centurion XV (StatPoint Technologies, Warrenton, VA, USA). II. Fisher’s test (LSD) was also applied at a significance level of 5%.
3. Results and Discussion
3.1. Emulsion Characterization
The droplet size distribution (DSD) of chia O/W emulsions obtained at 600, 1000 and 1200 bar presented similar bimodal curves (data not shown) with a major peak between ~0.040 to 0.500 µm and a small shoulder ranging from ~0.500 to 1.010 µm. In addition, it was observed that droplet size distribution curves were narrower at higher pressure levels, denoting a higher degree of uniformity for Cas1000 and Cas1200 systems; the droplet sizes (D3.2) of the systems are presented in Table 1. It could be observed that there was a significant influence (p ≤ 0.05) of the microfluidization pressure on the droplet size, resulting in the following increasing order Cas1000 < Cas1200 < Cas600. Although a decrease in particle size would be expected as a function of pressure increase [10], this did not occur for samples Cas1000 and Cas1200. This could be because the emulsifying agent was not sufficient to fully stabilize the droplet interface, favoring the formation of larger particles during homogenization.

Table 1.
De Sauter mean diameter (D3.2) and ζ-potential of chia O/W nanoemulsions.
Regarding ζ-potential, all systems recorded negatively charged oil droplets ranging from −35 to −41 mV (Table 1). Cas1200 recorded the smallest (p ≤ 0.05) surface charge between them, possibly because of the high-pressure treatment, which could have resulted in the unfolding of the protein structure affecting their ionic and hydrophobic interactions (Xinfa Ma et al. 2021) [11].
The global stability of the emulsions was studied through the evolution of their backscattering profiles (%BS) during storage (Figure 1). As can be seen, Cas1000 and Cas1200 presented high global stabilities, maintaining their BS profiles without significant changes throughout the whole period studied. The emulsion prepared at 600 bar exhibited slight signs of destabilization by creaming, showing a simultaneous decrease of the %BS at the bottom of the tube and an increase of this parameter at the top of it.
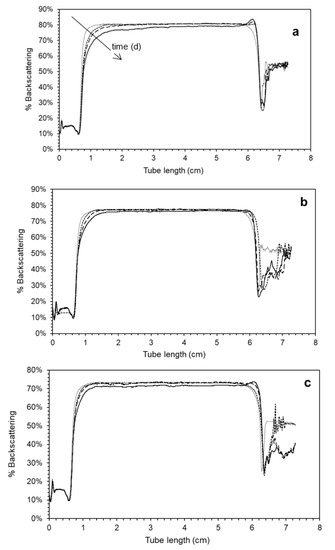
Figure 1.
Backscattering patterns of chia O/W emulsions prepared at three levels of homogenization pressure (a) 600 (b) 1000 and (c) 1200 bar as a function of time ( ) 1 d, (
) 1 d, ( ) 21 d, (
) 21 d, ( ) 30 d, and (
) 30 d, and ( ) 50 d of storage. Average values (n = 2).
) 50 d of storage. Average values (n = 2).
 ) 1 d, (
) 1 d, ( ) 21 d, (
) 21 d, ( ) 30 d, and (
) 30 d, and ( ) 50 d of storage. Average values (n = 2).
) 50 d of storage. Average values (n = 2).
The rheological data from emulsions measurements were fitted to the power-law model obtaining the K (consistency coefficient) and n (flow behavior index) parameters. All systems recorded values of n~1, denoting Newtonian behavior. Besides, K values resulted between 1.77–1.64 × 10−3 Pa.sn. In this sense, C600 samples had lower (p ≤ 0.05) K values, which could be associated with their larger droplet size.
3.2. Oxidative Stability of Emulsions
The evaluation of the primary lipid oxidation was carried out through emulsions PV assays during the storage period. Initially, all emulsions recorded low PV values indicating that the applied emulsification process did not affect the emulsified chia oil. After storage, the primary oxidation products of all systems remained low without exceeding the maximum limit of 10 meq of hydroperoxides/kg oil established by the Codex Alimentarius (Figure 2). Regarding the secondary oxidation monitored through TBARS measurements, the three systems studied presented low values of this parameter. In addition, the omega-3 fatty acid content of the nanosystems was also evaluated, without significant changes during the storage period. Based on these results, chia O/W nanoemulsions exhibited higher oxidative stability after 50 days of refrigerated storage.
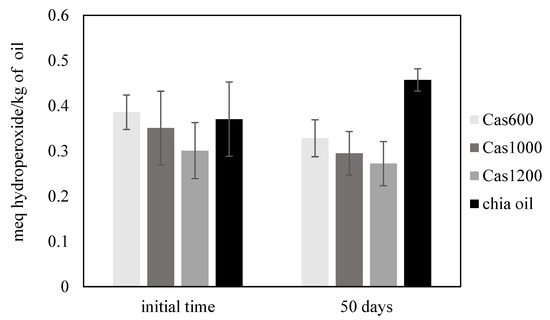
Figure 2.
Peroxide value (meq of hydroperoxide/kg of oil) of chia O/W nanoemulsions at the initial time and after 50 days of refrigerated storage.
4. Conclusions
From the concentration of the emulsifying agent and the conditions of the microfluidization applied in the present study, it was possible to obtain stable chia O/W emulsions with nanoscale droplet sizes.
It was found that microfluidization pressure had a significant effect on the properties of chia O/W nanoemulsions. In this sense, emulsions obtained at 1000 and 1200 bar recorded smaller droplet sizes and higher global stability than those prepared at 600 bar. Regarding the oxidative stability of the nanoemulsions, all systems recorded low values of primary and secondary oxidation products after 50 days at 4 °C. Thus, chia nanoemulsions obtained by microfluidization technology proved to be suitable delivery systems that can protect chia seed oil.
Author Contributions
N.C.: Conceptualization, Methodology, Investigation, Formal analysis, Writing—original draft preparation; L.M.J.: Conceptualization, Methodology, Investigation, Formal analysis, Writing—review & editing, Supervision, Project administration; M.C.T.: Conceptualization, Methodology, Writing—review & editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants La ValSe-Food-CYTED (119RT0567) Spain, Universidad Nacional de La Plata (X907), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2019-01775; 2020-01274), CONICET PIP 2007, Argentina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors wish to thank Mariela Fernandez (CETMIC) and Mariana Pennisi (CIDCA) for their technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Imran, M.; Nadeem, M.; Manzoor, M.F.; Javed, A.; Ali, Z.; Akhtar, M.N.; Muhammad, A.; Yasir, H. Fatty acids characterization, oxidative perspectives and consumer acceptability of oil extracted from pre-treated chia (Salvia hispanica L.) seeds. Lipids Health Dis. 2016, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tur, J.A.; Bibiloni, M.M.; Sureda, A.; Pons, A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. Br. J. Nutr. 2012, 107, S23–S52. [Google Scholar] [CrossRef]
- Bai, L.; McClements, D.J. Development of microfluidization methods for efficient production of concentrated nanoemulsions: Comparison of single-and dualchannel microfluidizers. J. Colloid Interf. Sci. 2016, 466, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Komaiko, J.S.; Mcclements, D.J. Formation of food-grade nanoemulsions using low-energy preparation methods: A review of available methods. Compr. Rev. Food Sci. Food Saf. 2016, 16, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Rodríguez, I.; Monroy-Villagrana, A.; Cornejo-Mazon, M.; García-Pinilla, S.; Hernandez-Sánchez, H.; Gutiérrez-Lopez, G.F. Microfluidization in nanofood engineering. In Food Engineering Series; Hebbar, U., Ranjan, S., Dasgupta, N., Kumar Mishra, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 153–175. [Google Scholar] [CrossRef]
- Pan, L.G.; Tomás, M.C.; Añón, M.C. Effect of sunflower lecithins on the stability of water-in-oil and oil-in-water emulsions. J. Surfactants Deterg. 2002, 5, 135–143. [Google Scholar] [CrossRef]
- Julio, L.M.; Ixtaina, V.Y.; Fernández, M.A.; Torres Sánchez, R.M.; Wagner, J.R.; Nolasco, S.M.; Tomás, M.C. Chia seed oil-in-water emulsions as potential delivery systems of ω-3 fatty acids. J. Food Eng. 2015, 162, 48–55. [Google Scholar] [CrossRef]
- Díaz, M.; Dunn, C.M.; McClements, D.J.; Decker, E.A. Use of caseinophosphopeptides as natural antioxidants in oil-in-water emulsions. J. Agric. Food Chem. 2003, 51, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhong, Q. Determination of thiobarbituric acid reactive substances in microencapsulated products. Food Chem. 2010, 123, 794–799. [Google Scholar] [CrossRef]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chatterton, D.E. Strategies to improve the physical stability of sodium caseinate stabilized emulsions: A literature review. Food Hydrocoll. 2021, 119, 1–14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).