Bladder Cancer and Fluorescence Cystoscopy †
Abstract
:1. Introduction
2. Methods
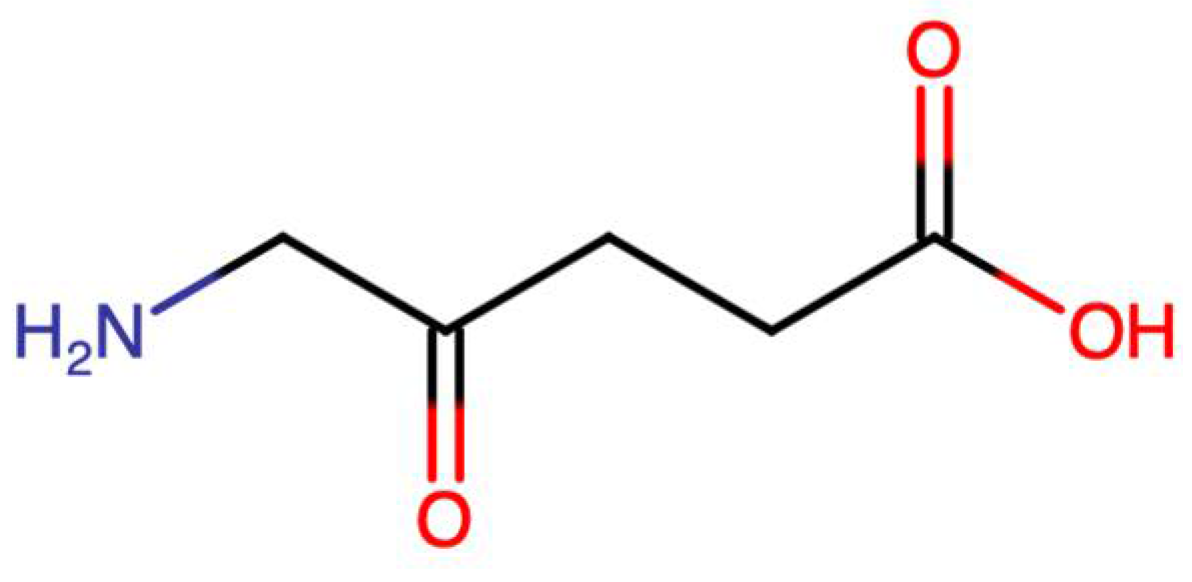
2.1. 5-Aminolevulinic Acid
2.2. 5-Aminolevulinic Acid Hexyl
2.3. Hypericin and Other Photosensitizers
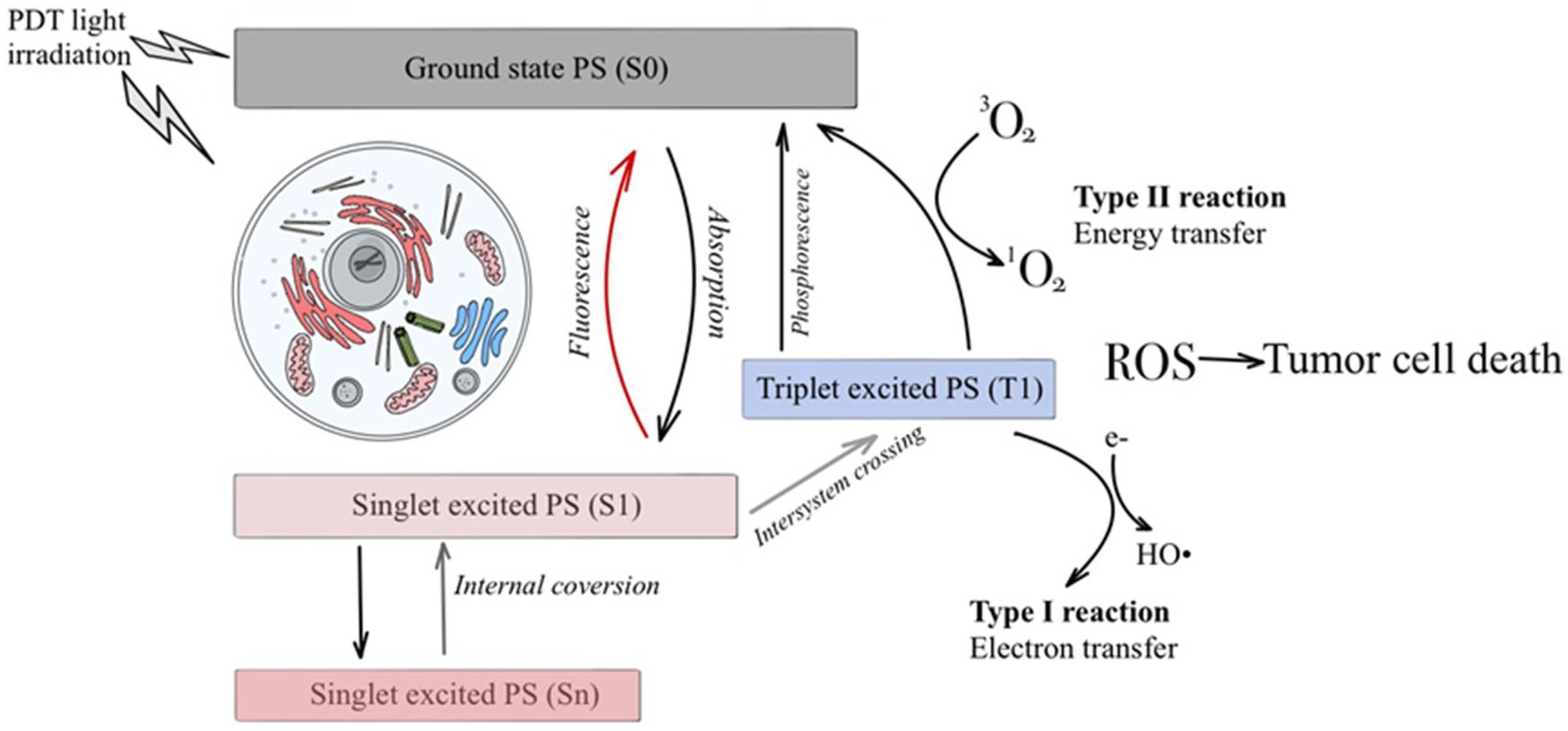
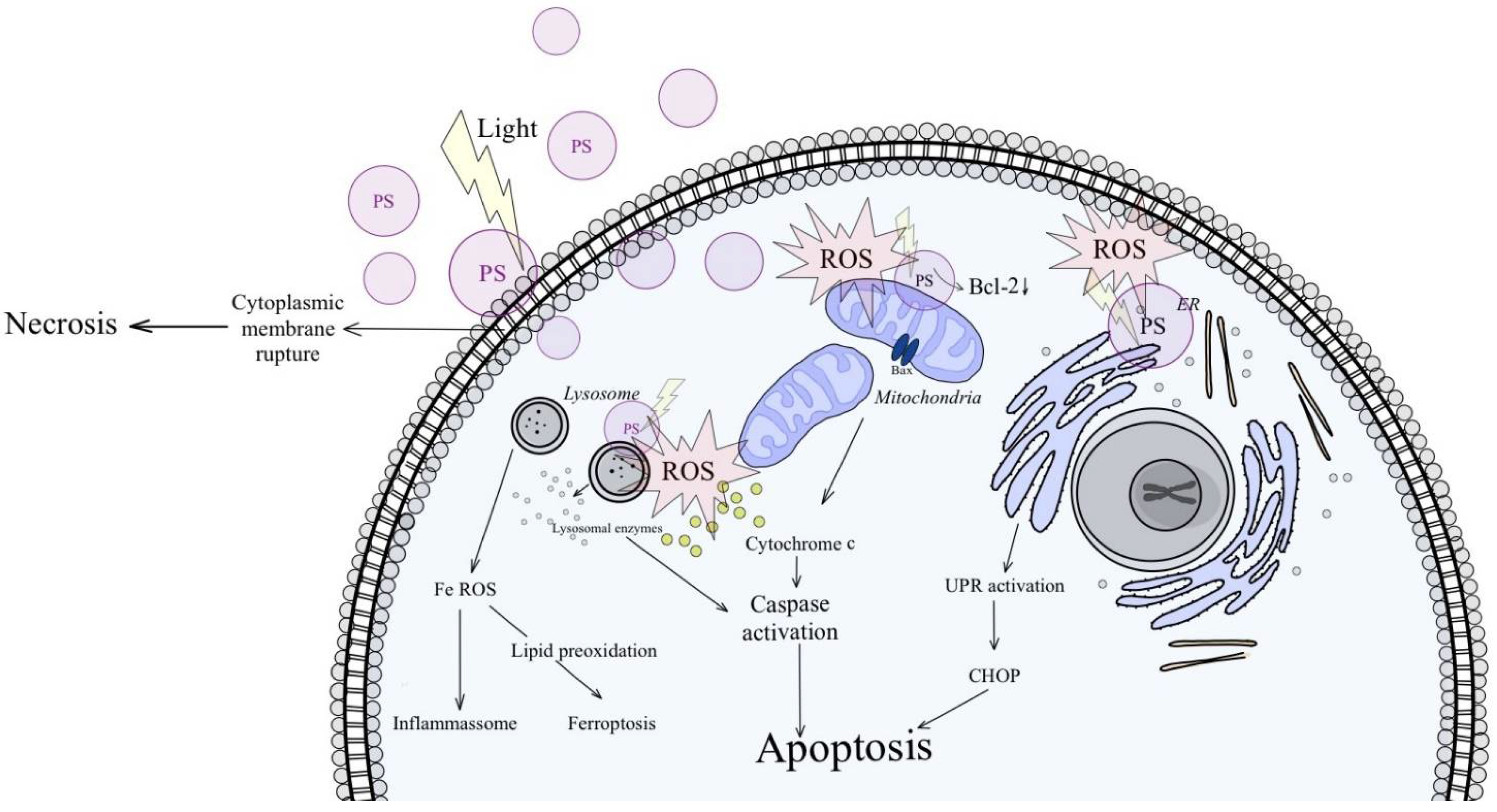
3. Discussion
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.; Kiemeney, L.A. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Koenig, F.; McGovern, F.J.; Larne, R.; Enquist, H.; Schomacker, K.T.; Deutsch, T.F. Diagnosis of bladder carcinoma using protoporphyrin IX fluorescence induced by 5-aminolaevulinic acid. BJU Int. 1999, 83, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Hallewin, M.A.; Bezdetnaya, L.; Guillemin, F. Fluorescence Detection of Bladder Cancer: A Review. Eur. Urol. 2002, 42, 417–425. [Google Scholar] [CrossRef]
- Makito, M.; Yasushi, N.; Satoshi, A.; Yoshihiro, T.; Masaomi, K.; Sayuri, O.; Yoshitomo, C.; Nobumichi, T.; Yoshihiko, H.; Kiyohide, F. Diagnostic approach for cancer cells in urine sediments by 5-aminolevulinic acid-based photodynamic detection in bladder cancer. Cancer Sci. 2014, 105, 616–622. [Google Scholar]
- Fradet, Y.; Grossman, H.B.; Gomella, L.; Lerner, S.; Cookson, M.; Albala, D.; Droller, M.J.; PC B302/01 Study Group. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: A phase III, multicenter study. J. Urol. 2007, 178, 68–73. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; MSHS, M. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Lipiński, M. Diagnostyka fluorescencyjna nowotworów pęcherza moczowego. Przegląd Urol. 2013, 2, 78. [Google Scholar]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Chatterton, K.; Ray, E.; O’Brien, T.S. Fluorescence diagnosis of bladder cancer. Br. J. Nurs. 2006, 15, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Lerner, S.P.; Goh, A. Novel endoscopic diagnosis for bladder cancer. Cancer 2015, 121, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jichlinski, P.; Leisinger, H. Fluorescence Cystoscopy in the Management of Bladder Cancer: A Help for the Urologist! Urol. Int. 2005, 74, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Stief, C.G.; Zaak, D.; Stenzl, A.; Wieland, W.F.; Jocham, D.; Otto, W.; Denzinger, S. Hexaminolevulinate is equal to 5-aminolevulinic acid concerning residual tumor and recurrence rate following photodynamic diagnostic assisted transurethral resection of bladder tumors. Urology 2009, 74, 1282–1286. [Google Scholar] [CrossRef]
- Zaak, D.; Kriegmair, M.; Stepp, H.; Stepp, H.; Baumgartner, R.; Oberneder, R.; Schneede, P.; Corvin, S.; Frimberger, D.; Knuchel, R.; et al. Endoscopic detection of transitional cell carcinoma with 5-aminolevulinic acid: Results of 1012 fluorescence endoscopies. Urology 2001, 57, 690–694. [Google Scholar] [CrossRef]
- Zumbragel, A.; Bichler, K.; Stenzl, A. 5-ALA induced fluorescence cystoscopy for superficial bladder cancer: Five year results. Eur. Urol. 2003, 1 (Suppl 2), 111. [Google Scholar] [CrossRef]
- Batlle, A.M. Porphyrins, porphyrias, cancer and photodynamic therapy—A model for carcinogenesis. J. Photochem. Photobiol. B 1993, 20, 5–22. [Google Scholar] [CrossRef]
- Lange, N.; Jichlinski, P.; Zellweger, M.; Forrer, M.; Marti, A.; Guillou, L.; Kucera, P.; Wagnières, G.; van den Bergh, H. Photodetection of early human bladder cancer based on the fluorescence of 5-aminolevulinic acid hexyl ester-induced protoporphyrin IX: A pilot study. Br. J. Cancer 1999, 80, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Jichlinski, P.; Guillou, L.; Karlsen, S.J.; Malmstrom, P.U.; Jocham, D.; Brennhovd, B.; Johansson, E.; Gartner, T.; Lange, N.; van den Bergh, H.; et al. Hexaminolevulinate fluorescence cystoscopy: New diagnostic tool for photodiagnosis of superficial bladder cancer—A multicenter study. J. Urol. 2003, 170, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Daneshmand, S.; Bazargani, S.T.; Bivalacqua, T.J.; Holzbeierlein, J.M.; Willard, B.; Taylor, J.M.; Liao, J.C.; Pohar, K.; Tierney, J.; Konety, B. Blue Light Cystoscopy with Cysview Registry Group. Blue light cystoscopy for the diagnosis of bladder cancer: Results from the US prospective multicenter registry. Urol. Oncol. 2018, 36, 361.e1–361.e6. [Google Scholar] [CrossRef]
- Jocham, D.; Witjes, F.; Wagner, S.; Zeylemaker, B.; Van Moorselaar, J.; Grimm, M.O.; Muschter, R.; Popken, G.; König, F.; Knüchel, R.; et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: A prospective, phase III multicenter study. J. Urol. 2005, 174, 862–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, H.B.; Gomella, L.; Fradet, Y.; Morales, A.; Presti, J.; Ritenour, C.; Nseyo, U.; Droller, M.J.; PC B302/01 Study Group. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J. Urol. 2007, 178, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hermann, G.G.; Mogensen, K.; Carlsson, S.; Marcussen, N.; Duun, S. Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients: A randomized two-centre study. BJU Int. 2011, 108, E297–E303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Hallewin, M.A.; De Witte, P.; Waelkens, E.; Merlevede, W.; Baert, L. Fluorescence detection of flat bladder carcinoma in situ after intravesical instillation of hypericin. J. Urol. 2000, 164, 349–351. [Google Scholar] [CrossRef]
- D’Hallewin, M.A.; Kamuhabwa, A.R.; Roskams, T.; de Witte, P.A.; Baert, L. Hypericin-based fluorescence diagnosis of bladder carcinoma. BJU 2002, 89, 760–763. [Google Scholar] [CrossRef]
- Kubin, A.; Meissner, P.; Wierrani, F.; Burner, U.; Bodenteich, A.; Pytel, A.; Schmeller, N. Fluorescence diagnosis of bladder cancer with new water soluble hypericin bound to polyvinylpyrrolidone: PVP-hypericin. Photochem. Photobiol. 2008, 84, 1560–1563. [Google Scholar] [CrossRef]
- Vandepitte, J.; Van Cleynenbreugel, B.; Hettinger, K.; Van Poppel, H.; de Witte, P.A. Biodistribution of PVP-hypericin and hexaminolevulinate-induced PpIX in normal and orthotopic tumor-bearing rat urinary bladder. Cancer Chemother. Pharmacol. 2011, 67, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fang, R.; Wang, L.; Chen, G.; Wang, H.; Wang, Z.; Zhao, D.; Pavlov, V.N.; Kabirov, I.; Wang, Z.; et al. Identification of Carbonic Anhydrase IX as a Novel Target for Endoscopic Molecular Imaging of Human Bladder Cancer. Cell Physiol. Biochem. 2018, 47, 1565–1577. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Mang, T.S. Lasers and light sources for PDT: Past, present and future. Photodiagnosis Photodyn. Ther. 2004, 1, 43–48. [Google Scholar] [CrossRef]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Olsen, C.E.; Weyergang, A.; Edwards, V.T.; Berg, K.; Brech, A.; Weisheit, S.; Høgset, A.; Selbo, P.K. Development of resistance to photodynamic therapy (PDT) in human breast cancer cells is photosensitizer-dependent: Possible mechanisms and approaches for overcoming pdt-resistance. Biochem. Pharmacol. 2017, 144, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Buytaert, E.; Breyssens, H.; Hendrickx, N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004, 3, 721–729. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. Role of Bcl-2 Family Proteins in Photodynamic Therapy Mediated Cell Survival and Regulation. Molecules 2020, 25, 5308. [Google Scholar] [CrossRef]
- Mayer, B.; Oberbauer, R. Mitochondrial regulation of apoptosis. Physiology 2003, 18, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [Green Version]
- Maiuri, M.C.; Criollo, A.; Kroemer, G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 2010, 29, 515–516. [Google Scholar] [CrossRef] [Green Version]
- Mohammad-Hadi, L.; MacRobert, A.J.; Loizidou, M.; Yaghini, E. Photodynamic therapy in 3D cancer models and the utilisation of nanodelivery systems. Nanoscale 2018, 10, 1570–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, F.; Ohgari, Y.; Yamaki, N.; Kitajima, S.; Shimokawa, O.; Matsui, H.; Taketani, S. The role of nitric oxide in δ-aminolevulinic acid (ALA)-induced photosensitivity of cancerous cells. Biochem. Biophys. Res. Commun. 2007, 353, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Stringer, M.; Moghissi, K. Photodiagnosis and fluorescence imaging in clinical practice. Photodiagn. Photodyn. Ther. 2004, 1, 9–12. [Google Scholar] [CrossRef]
- Sieroń, A.; Stręk, W.; Podbielska, H. Diagnostyka i Terapia Fotodynamiczna; Wydawnictwo Medyczne Urban & Partner: Wrocław, Poland, 2004; pp. 185–278. [Google Scholar]
- Lotan, Y. Promises and challenges of fluorescence cystoscopy. Urol. Oncol. 2015, 33, 261–264. [Google Scholar] [CrossRef]
- Kriegmair, M.C.; Rother, J.; Grychtol, B.; Theuring, M.; Ritter, M.; Günes, C.; Michel, M.S.; Deliolanis, N.C.; Bolenz, C. Multiparametric Cystoscopy for Detection of Bladder Cancer Using Real-time Multispectral Imaging. Eur. Urol. 2020, 77, 251–259. [Google Scholar] [CrossRef]
- Steinbach, P.; Weingandt, H.; Baumgartner, R.; Kriegmair, M.; Hofstadter, F.; Knuchel, R. Cellular fluorescence of the endogenous photosensitizer protoporphyrin IX following exposure to 5-aminolevulinic acid. Photochem. Photobiol. 1995, 62, 887–895. [Google Scholar] [CrossRef]
- Avritscher, E.B.; Cooksley, C.D.; Grossman, H.B.; Sabichi, A.L.; Hamblin, L.; Dinney, C.P.; Elting, L.S. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology 2006, 68, 549–553. [Google Scholar] [CrossRef]
- Konety, B.R.; Joyce, G.F.; Wise, M. Bladder and upper tract urothelial cancer. J. Urol. 2007, 177, 1636–1645. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przygoda, M.; Aebisher, D. Bladder Cancer and Fluorescence Cystoscopy. Biol. Life Sci. Forum 2022, 12, 4. https://doi.org/10.3390/IECN2022-12402
Przygoda M, Aebisher D. Bladder Cancer and Fluorescence Cystoscopy. Biology and Life Sciences Forum. 2022; 12(1):4. https://doi.org/10.3390/IECN2022-12402
Chicago/Turabian StylePrzygoda, Maria, and David Aebisher. 2022. "Bladder Cancer and Fluorescence Cystoscopy" Biology and Life Sciences Forum 12, no. 1: 4. https://doi.org/10.3390/IECN2022-12402
APA StylePrzygoda, M., & Aebisher, D. (2022). Bladder Cancer and Fluorescence Cystoscopy. Biology and Life Sciences Forum, 12(1), 4. https://doi.org/10.3390/IECN2022-12402






