Abstract
Vitamin D deficiency is associated with increased levels of oxidative stress. Oxidative stress is the mechanism by which light radiation (e.g., ultraviolet, UV) has a damaging effect on cells. At the same time, regardless of the data on the negative impact of light radiation and oxidative stress on carcinogenesis, both of these factors are used in the treatment of, e.g., skin cancer, breast cancer, etc. We wish to provide an overview of several systems that have been recently reported that employ both radiative and non-radiative chemiluminescent energy transfer for photosensitizer excitation that have been developed in the hope of achieving “dark” photodynamic therapy. This presentation reviews several of these important new developments in the design of therapeutic systems that utilize chemiluminescence. Thus, oxidative stress causes a condition in which cellular components, including DNA, proteins, and lipids, are oxidized and damaged. The anti-tumor effects result from a combination of direct photodamage to tumor cells, destruction of the tumor vasculature, and activation of the immune response. In this review, we will present how Vitamin D affects oxidative stress. The effect of vitamin D administration on the markers of oxidative stress was observed in people with a high-fat diet. A high fat diet is a potent inducer of oxidative stress by altering oxygen metabolism. The topics discussed in this speech will also concern the relationship of Vitamin D with PDT-treated tissue (skin and breast) by enabling accumulation of photosynthetizers. We will present an overview of the published research to date and our own research.
1. Introduction
Neoplastic diseases are an increasingly common cause of death. Especially in developed countries. In 2020, the number of deaths from neoplastic diseases amounted to nearly 10 million [1]. It is estimated that the number of new cases may increase by as much as 70% over the next two decades [2]. The classic methods of cancer treatment include: surgery, chemotherapy, radiotherapy, and, more recently, immunotherapy. We also consider the combination of these techniques to be a classic approach to the treatment of neoplastic diseases [3]. Unfortunately, all of these techniques have serious side effects. After incomplete surgical removal, local disease recurrence may occur [4]. Radiotherapy causes damage to areas not affected by the neoplastic process. [5]. Chemotherapy is associated with numerous side effects, such as alopecia or bone marrow suppression [6]. Therefore, new forms of therapy are sought which could, if not replace the described techniques, at least be used together with traditional forms of treatment. For 25 years, photodynamic therapy (PDT) has been considered one of the modern methods [7]. The main advantage of PDT compared to standard ones is its high selectivity for cancer cells and thus minimal impact on healthy tissues.
PDT is a treatment using a combination of light-absorbing photosensitizers and dissolved oxygen to kill cancer. One specific limitation of photodynamic therapy is that the visible light used for photosensitizer excitation has a short tissue penetration depth of several millimeters. This limits the application of PDT to surface cancers in the absence of a technique to illuminate deeper tissue. Efforts to extend tissue depth to which PDTT can be applied have been attempted with use of up-conversion and persistent-luminescent nanoparticles that absorb near infrared light and emit visible light for photosensitizer excitation, yet an initial excitation with an external light source is still required. More recently, systems employing chemiluminescence as an excitation energy source designed to bypass the use of external light have been developed and investigated as potential agents that could overcome the problem of achieving photodynamic therapy in deep tissue.
2. Natural Sources of Vitamin D
Vitamin D is a group of fat-soluble steroidal organic compounds whose main role in the body is to regulate the calcium and phosphate metabolism and maintain the proper structure of the skeleton (Figure 1). Vit. D2 (ergocalciferol) is found in plants and yeast cells and D3 (cholycalciferol) is found in animal cells. Its main source is sea fish, fish oil and, to a much lesser extent, dairy products. It is also possible to naturally biosynthesize these compounds in human skin, but it requires long-term exposure to UV radiation. Currently, in many countries, some products are obligatorily enriched with vitamin D to prevent its deficiency [1,2,3]. In the EU, these are breakfast cereals and margarine, and in the USA, in addition to the previously mentioned items, milk, yoghurt, or even orange juice are additionally enriched. Vitamin D occurs in nature in the form of vitamin D2-ergocalciferol and D3-cholecalciferol. Ergocalciferol is found in products of plant origin, and cholecalciferol in products of animal origin, including trout. Fish oil is an oil obtained from the liver of cod fish and the fatty tissue of some marine mammals (Figure 2). Its homeland is Norway, where it has been produced for over a thousand years. The use of fish oil has varied greatly over time. Initially, it was used for tanning leather and preserving wood—e.g., floors or walls. Later it was used in street oil lamps. The healing properties of cod liver oil were not discovered until the mid-17th century, but doctors only used it as a remedy against rickets and rheumatic diseases. Only at the end of the 19th century, the famous Norwegian pharmacist Peter Moller was the first to appreciate and describe other properties of fish oil and patent the process of its production [1,2,3].
Figure 1.
Vitamin D.
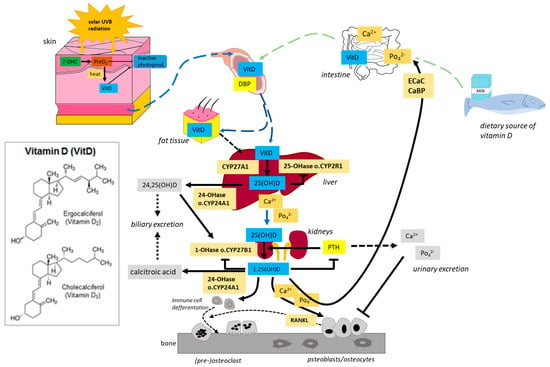
Figure 2.
Generation and metabolism of Vitamin D. Vitamin D is produced in the skin by exposure to UVB radiation or is ingested in the diet. In the liver, vitamin D is metabolized by vitamin D 25-hydroxylase (CYP2R1 and CYP27A1) to 25(OH)D (calcidiol). 25(OH)D is further metabolized by 25(OH)D 1α-hydroxylase (CYP27B1) mainly in the proximal tubule of the kidney to 1α,25-dihydroxyvitamin D [1α,25(OH)2D, calcitriol. Calcitriol is delivered target tissues such as intestine, bone, and kidney. After being produced, the levels of both calcidiol and calcitriol are tightly regulated by 25(OH)D 24-hydroxylase (CYP24A1).
One of the many sources of vitamin D is red meat, which includes, among others beef, pork, veal, lamb, lamb, and goat meat. It owes its name to the high content of myoglobin, which gives the meat its red color [4]. The composition of avocado oil includes fatty acids: monounsaturated fatty acids, omega-3 and omega-6 polyunsaturated fatty acids and vitamins: A, B, B3, D, E, H, K, Mg, Fe, P, Si, Me, and squalene [5].
3. The Link between Vitamin D and Oxidative Stress
Vitamin D deficiency is associated with increased levels of oxidative stress. An accumulation of oxygen reactive species (ROS) occurs during oxidative stress, which is a critical function within the body. In comparison thiol/disulfide (T/DS) homeostasis is the balance between thiols, which have antioxidant and anti-inflammatory properties, and their oxidized forms DS’s. Ts regulate intracellular redox metabolism and are the first antioxidants to be consumed in diverse biological settings when oxidants are present. Ts are oxidized and form DS’s bond structures. Ts were found to be significantly higher in the vitamin D deficiency group when compared to the control group. Those with sufficient vitamin D levels are more likely to possess a healthy balance of antioxidant activity, while those with subpar vitamin D levels (vitamin D deficiency) are more likely to experience increased levels of oxidative stress (yet another reason to obtain enough vitamin D daily and throughout life. DS’s can also be cut down again into T structures, which successfully restores balance [6].
The first records of attempts to treat diseases with the help of light and plant-derived ingredients come from the times of ancient civilization [7,8]. However, the beginning of PDT is considered to be the description by Hermann von Tappeiner of the photosensitivity reaction in the presence of oxygen. It was at the beginning of the 20th century. He called this reaction “photodynamic action” [9]. He noted that the presence of a light source and a dye was not sufficient to kill cells. For the phototoxicity reaction to occur, oxygen is still needed. Describing by von Tappeiner is the culmination of earlier observations. In the 1890s, Raab described the death of a slipper (paramecia) to which acridine was administered after being exposed to visible light. He described that the key element of this reaction is the transfer of light energy to the acridine red [10]. In 1900, Jean Prime made the first clinical attempt to use the effect observed by Raab in the treatment of epilepsy by administering eosin to patients [11]. The first attempt to treat cancer with PDT was an experiment by von Tappeiner and Jodlbauer. They used topically applied eosin and a light source to treat skin tumors [12]. On the basis of this experiment, von Tappeiner developed his theory about the crucial importance of oxygen. Unfortunately, despite promising results, these experiments were forgotten for decades [13]. It was not until the 1950s and 1960s that PDT became interested again. The main reason for the renewed interest in PDT was the use of porphyrin as a photosensitizer (PS) [14]. The use of porphyrin solved many problems and enabled PDT to be effective in the treatment of many neoplastic diseases, including lung, urinary and nervous system tumors [15]. The introduction of PDT to therapy, not only to clinical trials, took place in 1990, when Kennedy and colleagues published a paper in which they managed to achieve a 90% complete response rate after one dose [16]. In their work they used 5-aminolevulinic acid (5ALA) and visible light in the treatment of Basal cell carcinoma. 5ALA easily reaches tumor cells and is quickly washed out of the body, thus reducing the toxicity of the entire therapy [17]. Research is currently underway to address further PDT problems. The aim is to increase the specificity of the therapy or reduce phototoxicity. During clinical trials, PDT is combined with electroporation [18], nanoparticles are attached to PS molecules [19], or the use of PS that are excited by two photons [20]. Figure 3 showed the generation of ROS and singlet oxygen.
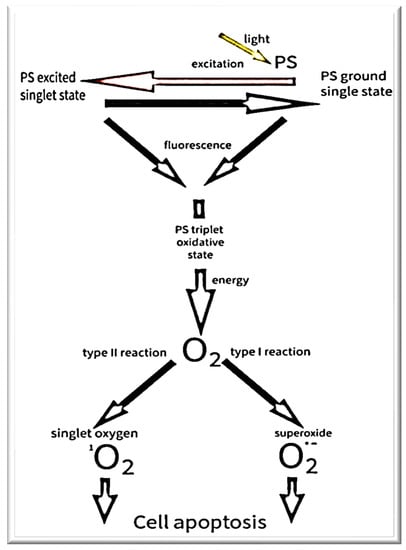
Figure 3.
The mechanism of PDT.
4. Current PDT
Neoplastic diseases are an increasingly common cause of death, especially in developed countries. In 2020, the number of deaths from neoplastic diseases amounted to nearly 10 million [21]. It is estimated that the number of new cases may increase by as much as 70% over the next two decades [22]. The classic methods of cancer treatment include: surgery, chemotherapy, radiotherapy, and, more recently, immunotherapy. We also consider the combination of these techniques to be a classic approach to the treatment of neoplastic diseases [23]. Unfortunately, all of these techniques have serious side effects. After incomplete surgical removal, local disease recurrence may occur [24]. Radiotherapy causes damage to areas not affected by the neoplastic process [25]. Chemotherapy is associated with numerous side effects, such as alopecia or bone marrow suppression [25]. Therefore, new forms of therapy are sought which could, if not replace the described techniques, at least be used together with traditional forms of treatment. For 25 years, photodynamic therapy (PDT) has been considered one of the modern methods [25]. The main advantage of PDT compared to standard ones is its high selectivity for cancer cells and thus minimal impact on healthy tissues.
5. Vitamin D and PDT
The study showed that Vitamin D enhanced the effects of PDT with the use of ALA [26]. PDT with the use of vitamin D supplementation has been used, e.g., to treat actinic keratosis, basal cell carcinoma, and squamous cell carcinoma in situ [27]. Anand et al. in clinical trials with PDT, used vitamin D to initiate cell differentiation. Treatment of lesions with vitamin D leads to increased activity of the heme synthesis pathway. As a consequence, after the administration of the photosensitizer ALA, there was an increased generation of free radicals and thus a faster initiation of the apoptosis process [28]. Vitamin D is the so-called biological PDT amplifier. It enhances photodynamic cell death through alternative pathways (e.g., necrosis) [28,29].
6. Conclusions
The literature shows proof that the combination of active forms of vitamin D with PDT improves the PDT treatment efficacy and enhances the apoptosis process of neoplastic cells, thus initiating increased ROS production.
Author Contributions
Conceptualization, A.M., K.K., K.D. and D.A. investigation, A.M., K.K., K.D. and D.A.; writing—original draft preparation, A.M., K.K., K.D. and D.A.; writing—review and editing, A.M., K.K., K.D. and D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study is available by request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohr, S.B. A brief history of vitamin d and cancer prevention. Ann. Epidemiol. 2009, 19, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Hernigoum, P.; Sitbon, J.; Dubory, A.; Auregan, J.C. Vitamin D history part III: The “modern times”—New questions for orthopaedic practice: Deficiency, cell therapy, osteomalacia, fractures, supplementation, infections. Int. Orthop. 2019, 43, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Mishra, S.K.; Mithal, A. Vitamin D toxicity resulting from overzealous correction of vitamin D deficiency. Clin. Endocrinol. 2015, 83, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Hayes, A. Red meat’s role in addressing ‘nutrients of public health concern’. Meat Sci. 2017, 132, 196–203. [Google Scholar] [CrossRef]
- Zanobini, A.; Firenzuoli, A.M.; Bianchi, A. Isolamento e dosaggio della vitamina D in avocado (Persea gratissima). Isolation and determination of vitamin D in avocado (Persea gratissima). Boll. Soc. Ital. Biol. Sper. 1974, 50, 887–891. [Google Scholar]
- Gemcioglu, E.; Baser, S.; Yilmaz Cakmak, N.; Erel, Ö.; Akman, B.T.; Ahmadova, P.; Ersoy, O. Assessing Oxidative Stress by Thiol/Disulfide Homeostasis Among Vitamin D-Deficient Patients. Cureus 2021, 13, e20400. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta—Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Munavalli, G.S. Photodynamic Therapy. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Drager, J.; Abels, C.; Landthaler, M. History of Photodynamic Therapy in Dermatology. In Photodynamic Therapy and Fluorescence Diagnosis in Dermatology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, p. 16. [Google Scholar]
- Raab, O. Uber die Wirkung fluoreszierender Stoffe auf Infusorien. Z. Biol. 1900, 39, 524–546. [Google Scholar]
- Prime, J. Des Accidents Toxiques Prodult par l’Eosinate se Sodium, 2nd ed.; Jouve et Boyer: Paris, France, 1900. [Google Scholar]
- Tappeiner, H.; Jodlbauer, A. Über die Wirkung der photodynamischen (fluoreszierenden) Stoffe auf Infusorien. Dtsch. Arch. Klin. Med. 1904, 80, 427–487. [Google Scholar]
- Taub, A.F. Photodynamic therapy in dermatology: History and horizons. J. Drugs Dermatol. 2004, 3, 8–25. [Google Scholar]
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. The use of a derivative of hematoporphyrin in tumor detection. J. Natl. Cancer Inst. 1961, 26, 1–11. [Google Scholar] [PubMed]
- Rkein, A.M.; Ozog, D.M. Photodynamic Therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamictherapy with endogenous protoporphyrin IX: Basicprinciples and present clinical experience. J. Photochem. Photobiol. B 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Babilas, P.; Schreml, S.; Landthaler, M. Photodynamic therapy in dermatology: State-of-the-art. Photodermatol. Photoimmunol. Photomed. 2010, 26, 118–132. [Google Scholar] [CrossRef]
- Weżgowiec, J.; Kulbacka, J.; Saczko, J.; Rossowska, J.; Chodaczek, G.; Kotulska, M. Biological effects in photodynamic treatment combined with electropermeabiliza-tion in wild and drug resistant breast cancer cells. Bioelectrochemistry 2018, 123, 9–18. [Google Scholar] [CrossRef]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.-L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Johnsen, M.; Arnbjerg, J.; Pittelkow, M.; McIlroy, S.P.; Ogilby, P.R.; Jørgensen, M. Synthesis and characterization of water-soluble phenylene-vinylene-based singlet oxygen sensitizers for two-photon excitation. J. Org. Chem. 2005, 70, 7065–7079. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 27 January 2022).
- Zhang, C.; Jiang, J.P.; Figueiró Longo, R.B.; Azevedo, H.; Zhang, L.A. Muehlmann, An updated overview on the development of new photosensitizers for anticancerphotodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [PubMed]
- Liu, G.; Zhang, S.; Ma, Y.; Wang, Q.; Chen, X.; Zhang, L.; Ma, F. Effects of error on dose of target region and organs at risk in treating nasopharynx cancer with intensity modulated radiation therapy. Pak. J. Med. Sci. 2016, 32, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollakanti, K.R.; Anand, S.; Maytin, E.V. Vitamin D enhances the efficacy of photodynamic therapy in a murine model of breast cancer. Cancer Med. 2015, 4, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, C.; Bedocs, P.M. Disseminated Superficial Actinic Porokeratosis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Anand, S.; Ortel, B.J.; Pereira, S.P.; Hasan, T.; Maytin, E.V. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 2012, 326, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Moore, B.W.; Keevey, S.; Drazba, J.A.; Hasan, T.; Maytin, E.V. Vitamin D enhances ALA-induced protoporphyrin IX production and photodynamic cell death in 3-D organotypic cultures of keratinocytes. J. Investig. Dermatol. 2007, 127, 925–934. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).