Development of a UPLC-Q-ToF-MS Method for the Determination of Sulforaphane and Iberin in Cruciferous Vegetables †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Standard Solutions
2.3. Sampling
2.4. Extracts Preparation
2.5. UPLC-Q-ToF-MS
2.6. Chromatographic Study
3. Results and Discussion
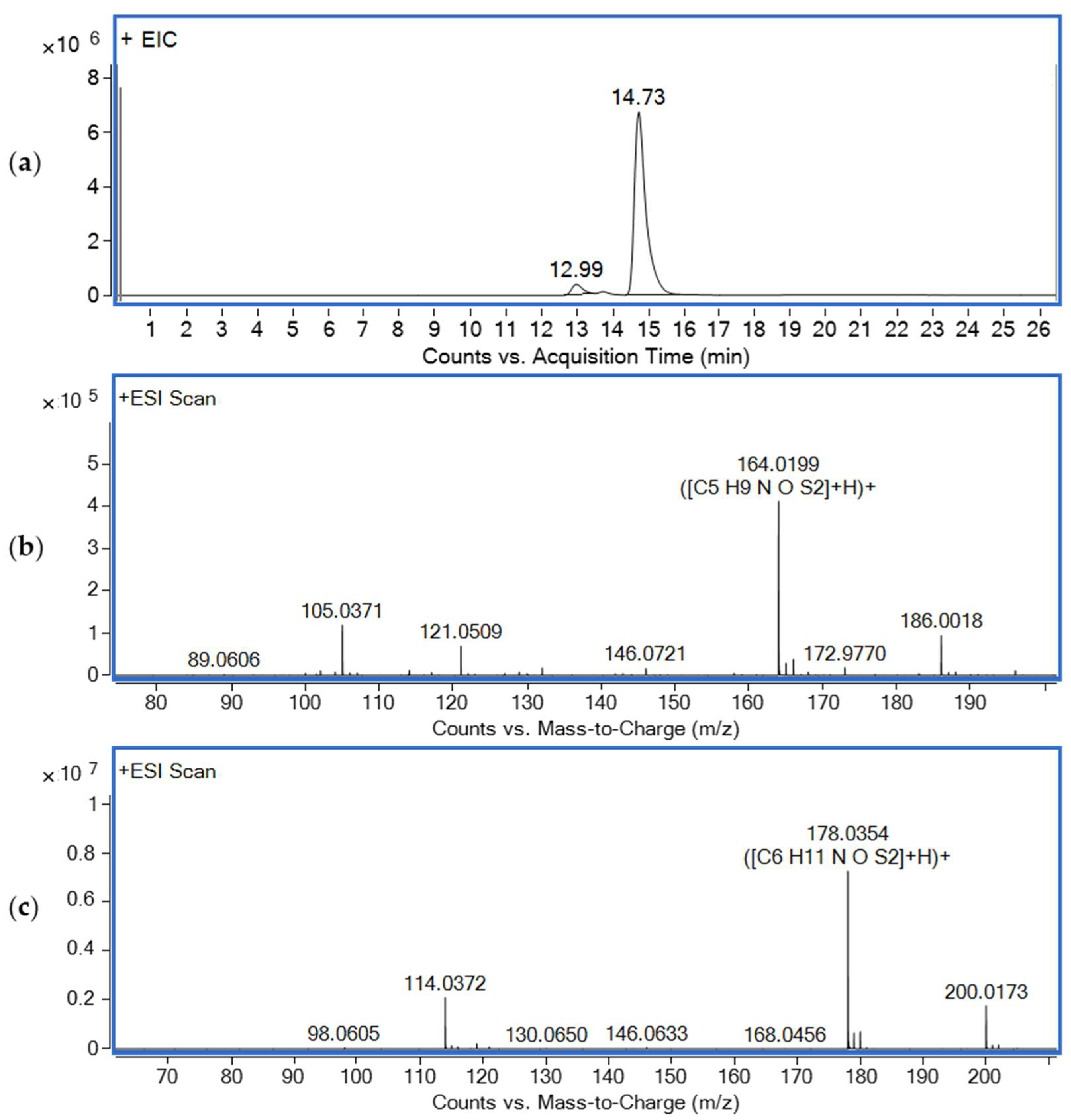
3.1. Mass Spectrometry Study
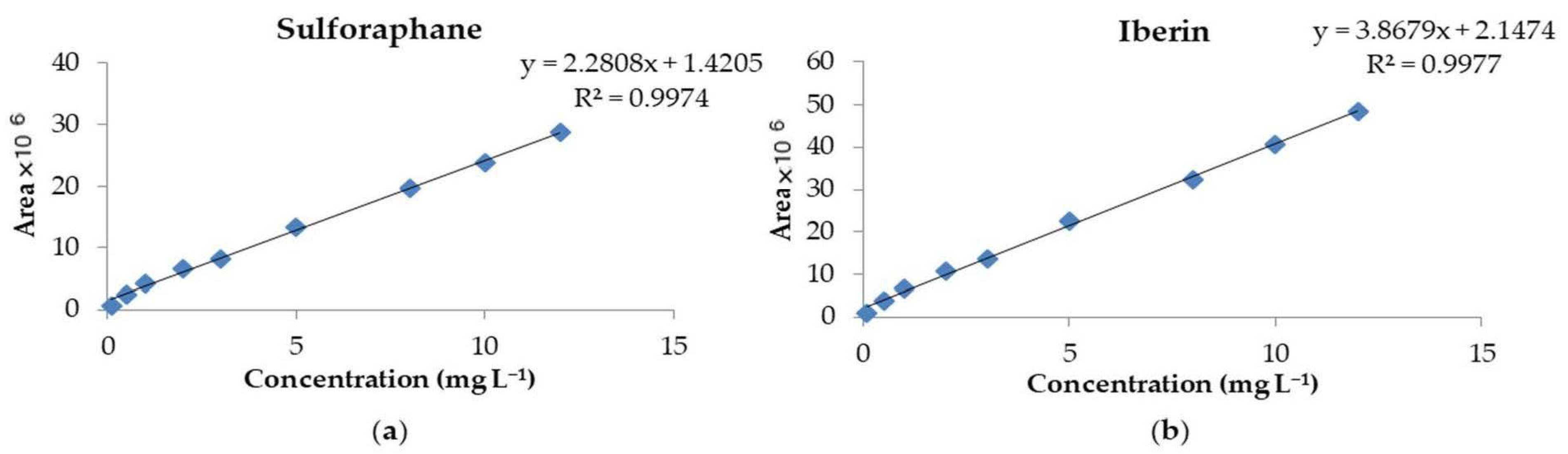
3.2. Method Validation
3.3. Analysis of Extracts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.S. Crucial Facts about Health Benefits of Popular Cruciferous Vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic Modification of Nrf2 by Sulforaphane Increases the Antioxidative and Anti-Inflammatory Capacity in a Cellular Model of Alzheimer’s Disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhäuser, C. Nuclear Factor ΚB Is a Molecular Target for Sulforaphane-Mediated Anti-Inflammatory Mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-ΚB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, T.; Nakashima, F.; Honda, K.; Lu, Y.-J.; Kondo, T.; Ushida, Y.; Aizawa, K.; Suganuma, H.; Oe, S.; Tanaka, H.; et al. Toll-like Receptors as a Target of Food-Derived Anti-Inflammatory Compounds. J. Biol. Chem. 2014, 289, 32757–32772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, S.; Howie, A.F.; Beckett, G.J.; Mithen, R.; Bao, Y. Sulforaphane, Erucin, and Iberin Up-Regulate Thioredoxin Reductase 1 Expression in Human MCF-7 Cells. J. Agric. Food Chem. 2005, 53, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Karanikolopoulou, S.; Revelou, P.-K.; Xagoraris, M.; Kokotou, M.G.; Constantinou-Kokotou, V. Current Methods for the Extraction and Analysis of Isothiocyanates and Indoles in Cruciferous Vegetables. Analytica 2021, 2, 93–120. [Google Scholar] [CrossRef]
- D’Souza, C.A.; Amin, S.; Desai, D. A Facile and Efficient Synthesis of 14C-Labelled Sulforaphane. J. Label. Compd. Radiopharm. 2003, 46, 851–859. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Sánchez-Machado, D.I.; Bueno-Solano, C.; Ramírez-Wong, B.; López-Cervantes, J. HPLC Method Validation for Measurement of Sulforaphane Level in Broccoli By-Products. Biomed. Chromatogr. 2010, 24, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G.; Revelou, P.-K.; Pappas, C.; Constantinou-Kokotou, V. High Resolution Mass Spectrometry Studies of Sulforaphane and Indole-3-Carbinol in Broccoli. Food Chem. 2017, 237, 566–573. [Google Scholar] [CrossRef] [PubMed]


| Compound | Green Broccoli | Purple Broccoli 1 | Purple Broccoli 2 | White Cauliflower | White Cabbage | Red Cabbage | Radish | Watercress |
|---|---|---|---|---|---|---|---|---|
| Sulforaphane | 660.14 ± 34.29 | 15.05 ± 0.43 | 210.11 ± 9.76 | 14.89 ± 1.62 | 73.71 ± 1.27 | 143.83 ± 3.44 | 9.25 ± 0.14 | 4.44 ± 0.53 |
| Iberin | 20.95 ± 0.67 | 144.98 ± 3.56 | <LOD | 47.48 ± 5.07 | 84.57 ± 0.20 | 30.12 ± 0.13 | 0.83 ± 0.09 | <LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revelou, P.-K.; Xagoraris, M.; Michail, A.; Kokotou, M.G.; Constantinou-Kokotou, V. Development of a UPLC-Q-ToF-MS Method for the Determination of Sulforaphane and Iberin in Cruciferous Vegetables. Biol. Life Sci. Forum 2022, 12, 24. https://doi.org/10.3390/IECN2022-12389
Revelou P-K, Xagoraris M, Michail A, Kokotou MG, Constantinou-Kokotou V. Development of a UPLC-Q-ToF-MS Method for the Determination of Sulforaphane and Iberin in Cruciferous Vegetables. Biology and Life Sciences Forum. 2022; 12(1):24. https://doi.org/10.3390/IECN2022-12389
Chicago/Turabian StyleRevelou, Panagiota-Kyriaki, Marinos Xagoraris, Alexandros Michail, Maroula G. Kokotou, and Violetta Constantinou-Kokotou. 2022. "Development of a UPLC-Q-ToF-MS Method for the Determination of Sulforaphane and Iberin in Cruciferous Vegetables" Biology and Life Sciences Forum 12, no. 1: 24. https://doi.org/10.3390/IECN2022-12389
APA StyleRevelou, P.-K., Xagoraris, M., Michail, A., Kokotou, M. G., & Constantinou-Kokotou, V. (2022). Development of a UPLC-Q-ToF-MS Method for the Determination of Sulforaphane and Iberin in Cruciferous Vegetables. Biology and Life Sciences Forum, 12(1), 24. https://doi.org/10.3390/IECN2022-12389









