Molecular and Functional Characterization of Human SW 872 Adipocytes as a Model System for Testing Nutraceutical Products †
Abstract
1. Introduction
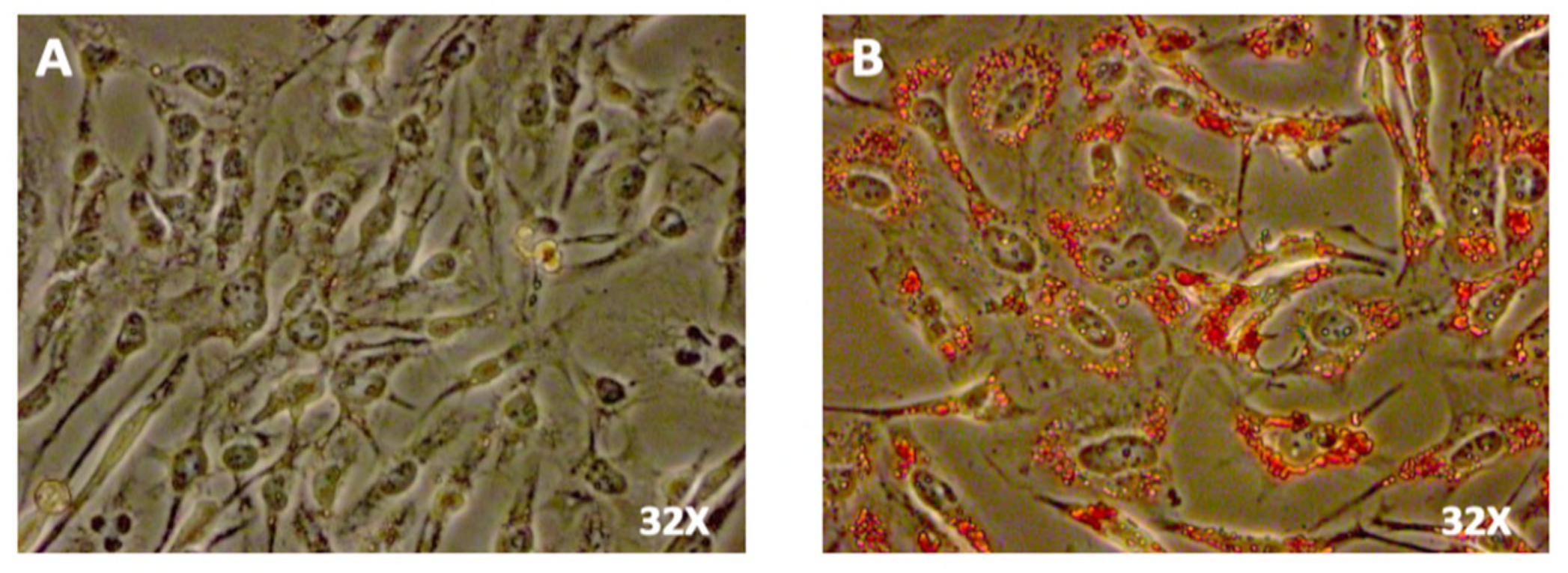
2. SW 872 Adipocyte Differentiation
3. Glucometabolic Studies in SW 872 Cells
4. Inflammation
5. Modulation of Dysfunctional Aspects of SW 872 Cell Line by Plant Extracts
6. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A consensus statement from the IAS and ICCR working group on visceral obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Atchan Nwakiban, A.P.; Passarelli, A.; Da Dalt, L.; Olivieri, C.; Demirci, T.N.; Piazza, S.; Sangiovanni, E.; Carpentier-Maguire, E.; Martinelli, G.; Shivashankara, S.T.; et al. Cameroonian spice extracts modulate molecular mechanisms relevant to cardiometabolic diseases in SW 872 human liposarcoma cells. Nutrients 2021, 13, 4271. [Google Scholar] [CrossRef] [PubMed]
- Cicolari, S.; Dacrema, M.; Tsetegho Sokeng, A.J.; Xiao, J.; Atchan Nwakiban, A.P.; Di Giovanni, C.; Santarcangelo, C.; Magni, P.; Daglia, M. Hydromethanolic extracts from adansonia digitata L edible parts positively modulate pathophysiological mechanisms related to the metabolic syndrome. Molecules 2020, 25, 2858. [Google Scholar] [CrossRef] [PubMed]
- Fève, B. Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Wassef, H.; Bernier, L.; Davignon, J.; Cohn, J.S. Synthesis and secretion of apoC-I and apoE during maturation of human SW872 liposarcoma cells. J. Nutr. 2004, 134, 2935–2941. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carmel, J.F.; Tarnus, E.; Cohn, J.S.; Bourdon, E.; Davignon, J.; Bernier, L. High expression of apolipoprotein E impairs lipid storage and promotes cell proliferation in human adipocytes. J. Cell. Biochem. 2009, 106, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Stratford, E.W.; Castro, R.; Daffinrud, J.; Skårn, M.; Lauvrak, S.; Munthe, E.; Myklebost, O. Characterization of liposarcoma cell lines for preclinical and biological studies. Sarcoma 2012, 2012, 148614. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; De Matteis, R.; Canonico, B.; Blandino, G.; Mazzoli, A.; Montanari, M.; Guidarelli, A.; Cantoni, O. Temporal correlation of morphological and biochemical changes with the recruitment of different mechanisms of reactive oxygen species formation during human SW872 cell adipogenic differentiation. BioFactors 2021, 47, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, A.; Kazantzis, M.; Thomas, R.; Wabitsch, M.; Tews, D.; Seetharama Sastry, K.; Abdelkarim, M.; Zilberfarb, V.; Strosberg, A.D.; Chouchane, L. Comprehensive molecular characterization of human adipocytes reveals a transient brown phenotype. J. Transl. Med. 2015, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- He, Y.H.; He, Y.; Liao, X.L.; Niu, Y.C.; Wang, G.; Zhao, C.; Wang, L.; Tian, M.J.; Li, Y.; Sun, C.H. The calcium-sensing receptor promotes adipocyte differentiation and adipogenesis through PPARc pathway. Mol. Cell. Biochem. 2012, 361, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, M.; Gao, Y.; Wang, H.; Dai, L.; Wen, Y.; Na, L. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol. Med. Rep. 2015, 12, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Pedersen, S.B.; Richelsen, B. Interleukin-8 production in human adipose tissue. inhibitory effects of anti-diabetic compounds, the thiazolidinedione ciglitazone and the biguanide metformin. Horm. Metab. Res. 2000, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Azhar, S. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 2010, 6, 657–691. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, V.; Passaretti, F.; Hammarstedt, A.; Liguoro, D.; Terracciano, D.; Molea, G.; Canta, L.; Miele, C.; Smith, U.; Beguinot, F.; et al. Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia 2012, 55, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Nwakiban, A.P.A.; Cicolari, S.; Piazza, S.; Gelmini, F.; Sangiovanni, E.; Martinelli, G.; Bossi, L.; Carpentier-Maguire, E.; Tchamgoue, A.D.; Agbor, G.; et al. Oxidative stress modulation by cameroonian spice extracts in HepG2 cells: Involvement of Nrf2 and improvement of glucose uptake. Metabolites 2020, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef] [PubMed]


| Triglyceride Reduction | Glucose Uptake Stimulation | ROS Production | IL-6 Reduction | IL-8 Reduction | |
|---|---|---|---|---|---|
| Xylopia aethiopica | −14.5% | +55.8% | −21.1% | ||
| Xylopia parviflora | −13.8% | −50.5% | −36.8% | ||
| Scorodophloeus zenkeri | −18.5% | ||||
| Monodora myristica | −15.3% | −40% | −24.3% | ||
| Tetrapleura tetraptera | −13.8% | +40.8% | −27.4% | −29.7% | |
| Echinops giganteus | −11.3% | −43.6% | −29% | ||
| Afrostyrax lepidophyllus | −16.5% | −24.6% | |||
| Dichrostachys glomerata | −17.4% | −40% | |||
| Aframomum melegueta | −13% | +41.7% | −43.1% | ||
| Aframomum citratum | −16% | −58.6% | |||
| Zanthoxylum leprieurii | −13.4% | +56.6% | −32.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivieri, C.; Ruzza, M.; Tolaj, F.; DaDalt, L.; Magni, P. Molecular and Functional Characterization of Human SW 872 Adipocytes as a Model System for Testing Nutraceutical Products. Biol. Life Sci. Forum 2022, 12, 19. https://doi.org/10.3390/IECN2022-12370
Olivieri C, Ruzza M, Tolaj F, DaDalt L, Magni P. Molecular and Functional Characterization of Human SW 872 Adipocytes as a Model System for Testing Nutraceutical Products. Biology and Life Sciences Forum. 2022; 12(1):19. https://doi.org/10.3390/IECN2022-12370
Chicago/Turabian StyleOlivieri, Chiara, Marco Ruzza, Fationa Tolaj, Lorenzo DaDalt, and Paolo Magni. 2022. "Molecular and Functional Characterization of Human SW 872 Adipocytes as a Model System for Testing Nutraceutical Products" Biology and Life Sciences Forum 12, no. 1: 19. https://doi.org/10.3390/IECN2022-12370
APA StyleOlivieri, C., Ruzza, M., Tolaj, F., DaDalt, L., & Magni, P. (2022). Molecular and Functional Characterization of Human SW 872 Adipocytes as a Model System for Testing Nutraceutical Products. Biology and Life Sciences Forum, 12(1), 19. https://doi.org/10.3390/IECN2022-12370







