Silencing of FaPG1, a Fruit Specific Polygalacturonase Gene, Decreased Strawberry Fruit Fungal Decay during Postharvest †
Abstract
:1. Introduction
2. Materials and Methods
Postharvest Behaviour of Transgenic FaPG1 and Control Fruits
3. Results
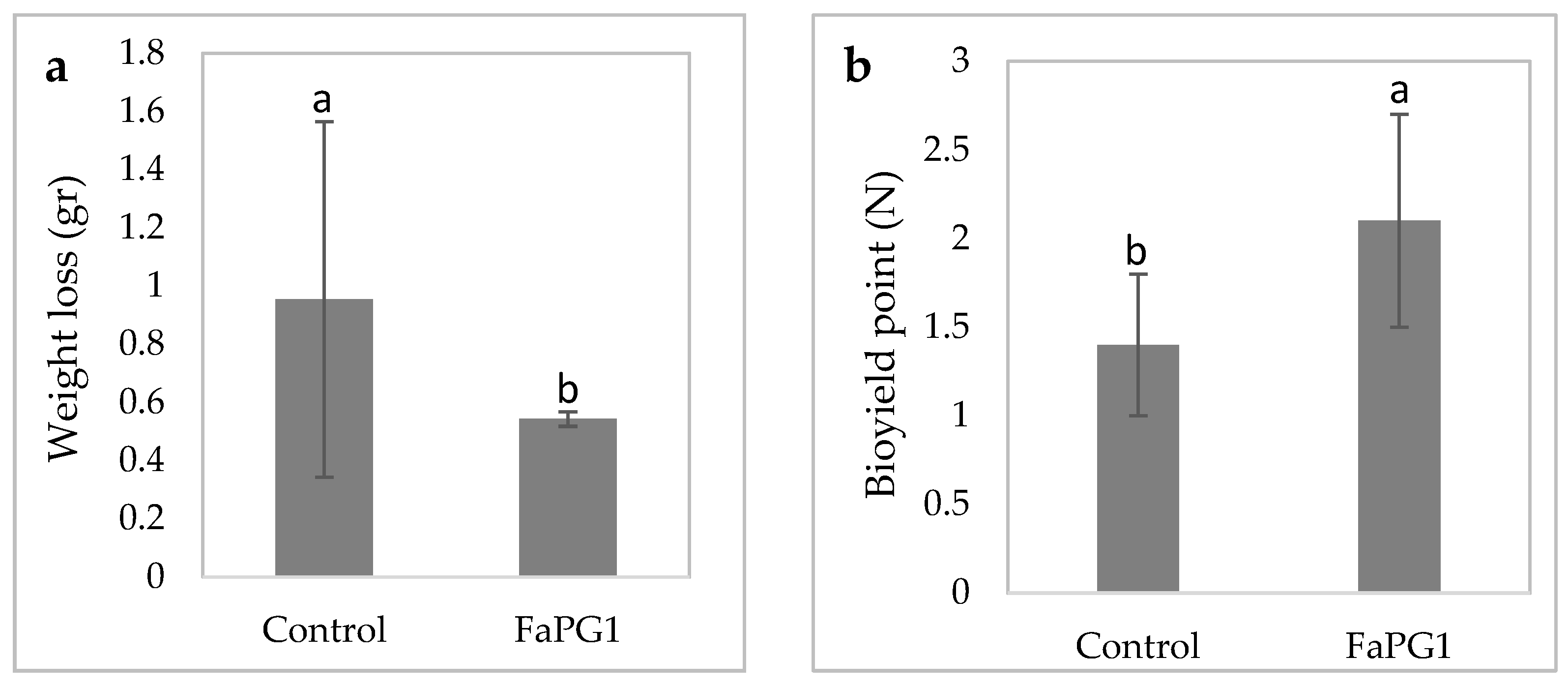
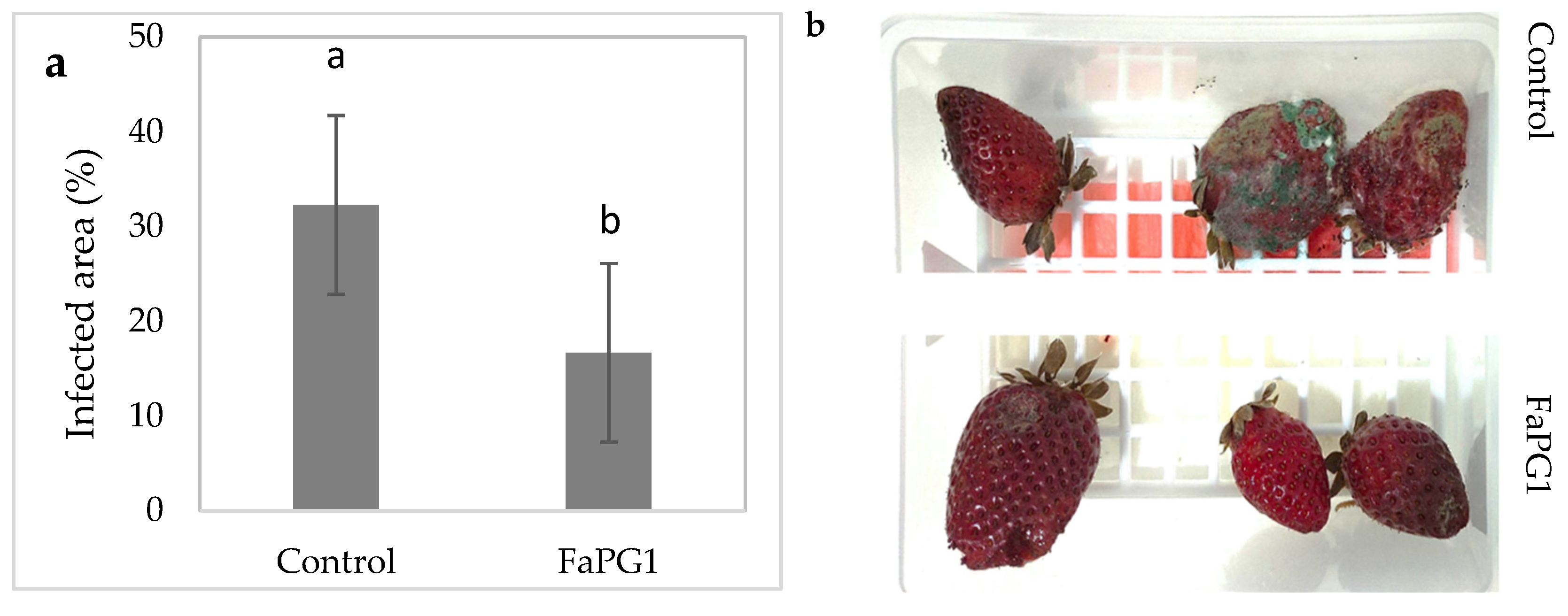
FaPG1 Transgenic Lines Performance during Postharvest Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newton, A.C.; Johnson, S.; Gregory, P. Implications of climate change for diseases, crop yields and food security. Euphytica 2011, 179, 3–18. [Google Scholar] [CrossRef]
- Nejat, N.; Mantri, N. Plant Immune System: Crosstalk Between Responses to Biotic and Abiotic Stresses the Missing Link in Understanding Plant Defence. Curr. Issues Mol. Biol. 2017, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Pontiggia, D.; Raggi, S.; Cheng, Z.; Scaloni, F.; Ferrari, S.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 5533–5538. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Giraldo, L.; Liu, C.; Pose-Albacete, S.; Pattathil, S.; Peralta, A.G.; Young, J.; Westpheling, J.; Hahn, M.G.; Rao, X.; Knox, J.P.; et al. ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE 1 (ADPG1) releases latent defense signals in stems with reduced lignin content. Proc. Natl. Acad. Sci. USA 2020, 117, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Quesada, M.A.; Portales, R.B.; Posé, S.; García-Gago, J.A.; Jiménez-Bermúdez, S.; Muñoz-Serrano, A.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Blanco, J.M. Antisense Down-Regulation of the FaPG1 Gene Reveals an Unexpected Central Role for Polygalacturonase in Strawberry Fruit Softening. Plant Physiol. 2009, 150, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Posé, S.; Paniagua, C.; Cifuentes, M.; Portales, R.B.; Quesada, M.A.; Mercado, J.A. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 2013, 64, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Posé, S.; Kirby, A.R.; Paniagua, C.; Waldron, K.W.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. The nanostructural characterization of strawberry pectins in pectate lyase or polygalacturonase silenced fruits elucidates their role in softening. Carbohydr. Polym. 2015, 132, 134–145. [Google Scholar] [CrossRef]
- Paniagua, C.; Ric-Varas, P.; García-Gago, J.A.; López-Casado, G.; Blanco-Portales, R.; Muñoz-Blanco, J.; Schückel, J.; Knox, J.P.; Matas, A.J.; Quesada, M.A.; et al. Elucidating the role of polygalacturonase genes in strawberry fruit softening. J. Exp. Bot. 2020, 71, 7103–7117. [Google Scholar] [CrossRef]
- Mélida, H.; Bacete, L.; Ruprecht, C.; Rebaque, D.; Del Hierro, I.; López, G.; Brunner, F.; Pfrengle, F.; Molina, A. Arabinoxylan-Oligosaccharides Act as Damage Associated Molecular Patterns in Plants Regulating Disease Resistance. Front. Plant Sci. 2020, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Miedes, E.; Bacete, L.; Rodríguez, T.; Mélida, H.; Denancé, N.; Sánchez-Vallet, A.; Rivière, M.-P.; López, G.; Freydier, A.; et al. Arabidopsis cell wall composition determines disease resistance specificity and fitness. Proc. Natl. Acad. Sci. USA 2021, 118, e2010243118. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paniagua, C.; Sánchez-Raya, C.; Blanco-Portales, R.; Mercado, J.A.; Palomo-Ríos, E.; Posé, S. Silencing of FaPG1, a Fruit Specific Polygalacturonase Gene, Decreased Strawberry Fruit Fungal Decay during Postharvest. Biol. Life Sci. Forum 2022, 11, 96. https://doi.org/10.3390/IECPS2021-12049
Paniagua C, Sánchez-Raya C, Blanco-Portales R, Mercado JA, Palomo-Ríos E, Posé S. Silencing of FaPG1, a Fruit Specific Polygalacturonase Gene, Decreased Strawberry Fruit Fungal Decay during Postharvest. Biology and Life Sciences Forum. 2022; 11(1):96. https://doi.org/10.3390/IECPS2021-12049
Chicago/Turabian StylePaniagua, Candelas, Cristina Sánchez-Raya, Rosario Blanco-Portales, Jose A. Mercado, Elena Palomo-Ríos, and Sara Posé. 2022. "Silencing of FaPG1, a Fruit Specific Polygalacturonase Gene, Decreased Strawberry Fruit Fungal Decay during Postharvest" Biology and Life Sciences Forum 11, no. 1: 96. https://doi.org/10.3390/IECPS2021-12049
APA StylePaniagua, C., Sánchez-Raya, C., Blanco-Portales, R., Mercado, J. A., Palomo-Ríos, E., & Posé, S. (2022). Silencing of FaPG1, a Fruit Specific Polygalacturonase Gene, Decreased Strawberry Fruit Fungal Decay during Postharvest. Biology and Life Sciences Forum, 11(1), 96. https://doi.org/10.3390/IECPS2021-12049






