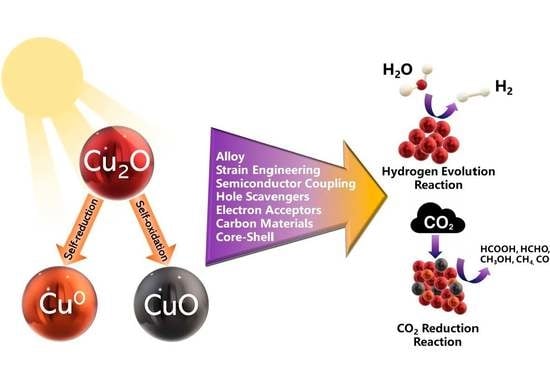
Cu-Based Materials as Photocatalysts for Solar Light Artificial Photosynthesis: Aspects of Engineering Performance, Stability, Selectivity
Abstract
1. Introduction
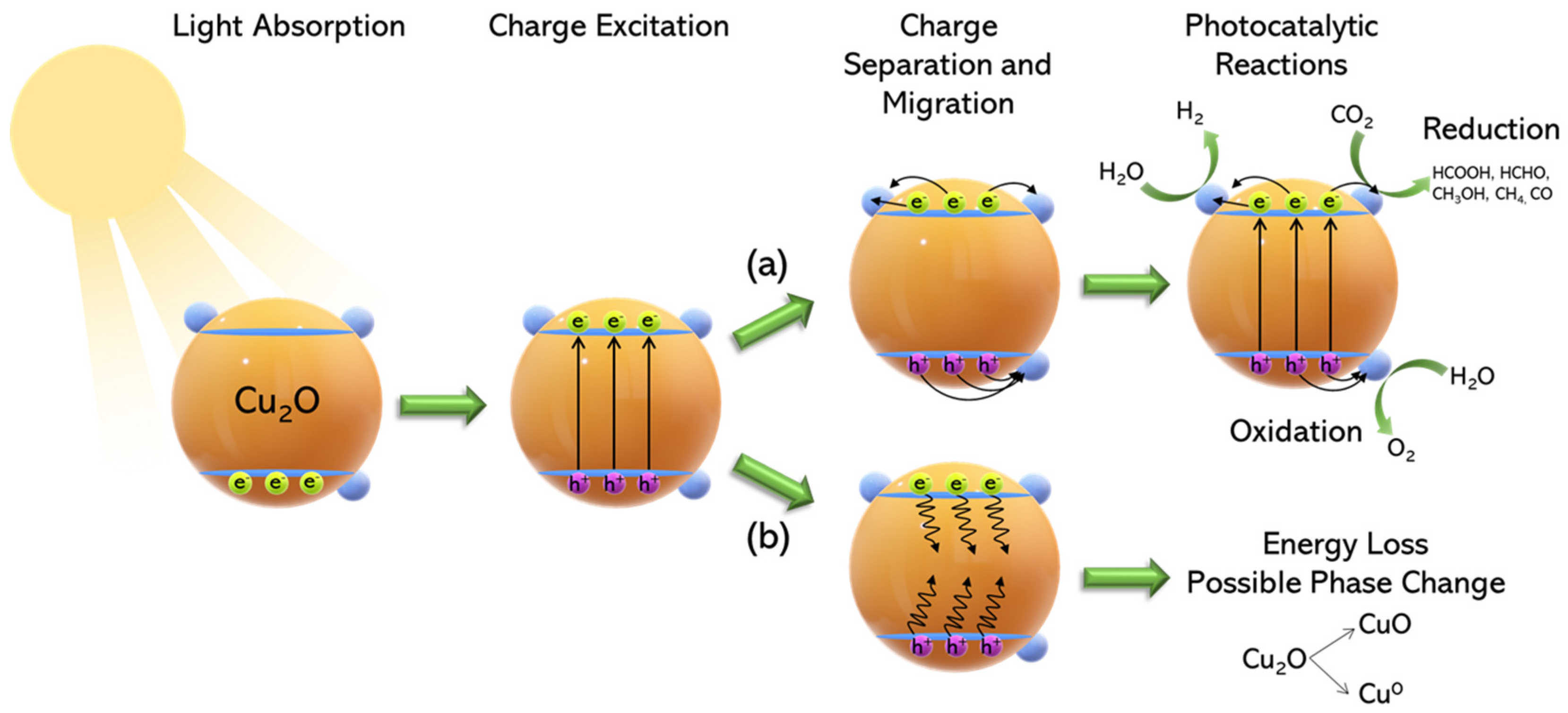
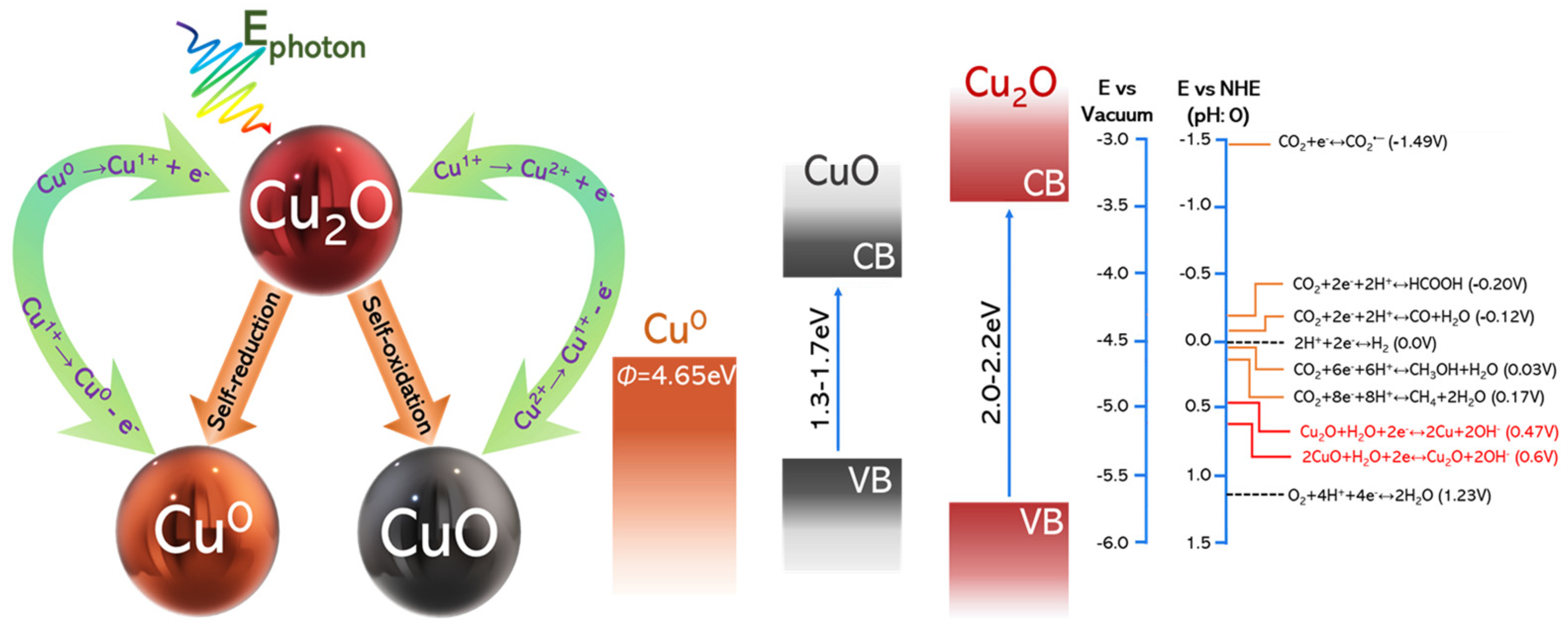
2. The Problem of Photocorrosion and Some Specific Approaches to Prevent It
- [i]
- by the oxidation of Cu1+ to Cu2+, by photogenerated holes. Formally, this tends to convert Cu2O to CuO.
- [ii]
- by the reduction of Cu1+ to Cu0 by photogenerated or electrochemically provided electrons. Formally, this tends to convert Cu2O to metallic Cu0.
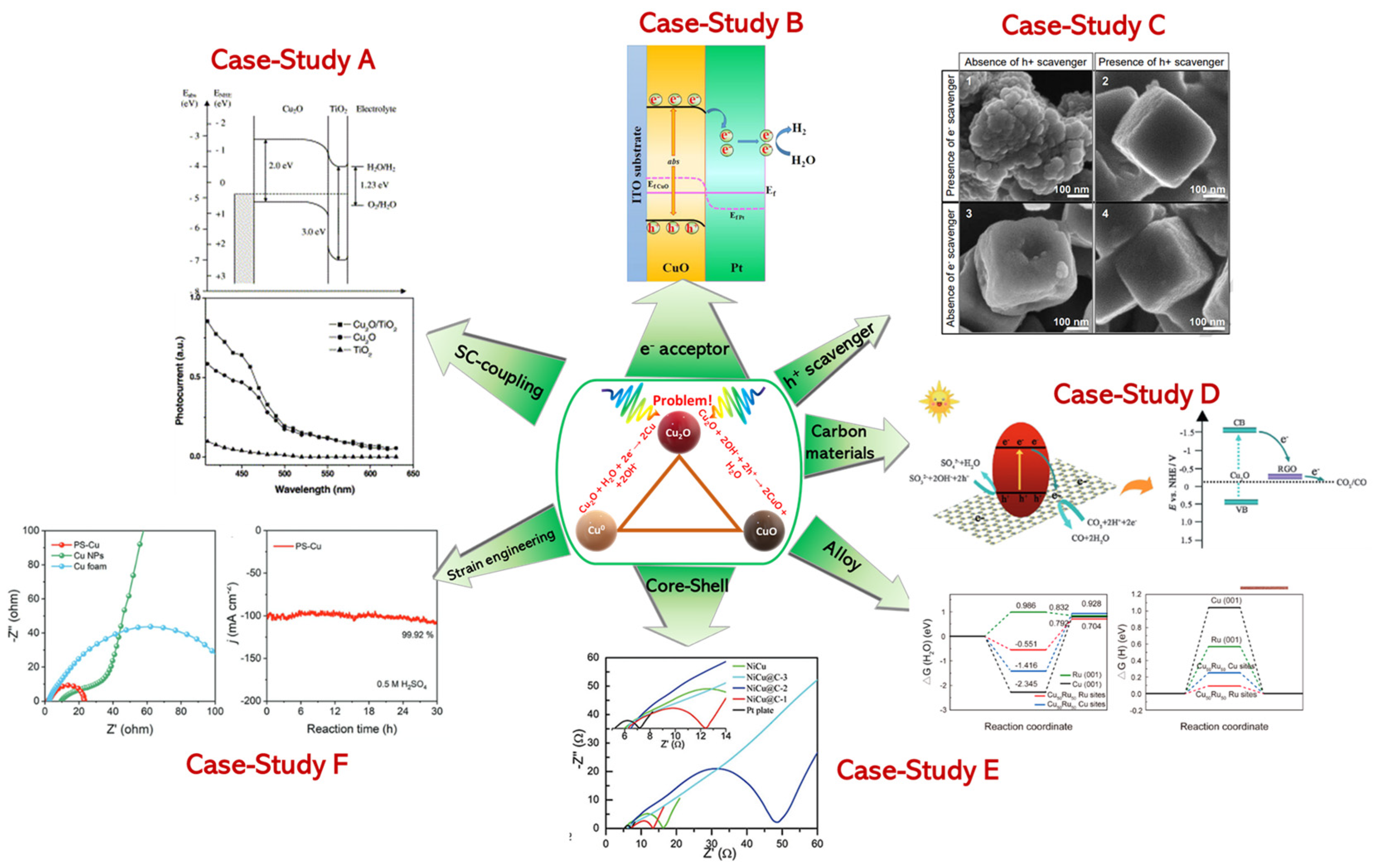
2.1. Case−Study A: Photocathode-Heterojunctions (e.g., CuxO/TiO2, CuO/BiVO4, CuO/Cu2O)
2.2. Case−Study B: The Case of e− Capture/Acceptor (CuO/Pt and CuO/Pd-Au)
2.3. Case−Study C: The Use of Hole Scavengers
2.4. Case−Study D: The Effect of Carbonaceous Materials
2.5. Case−Study E: Core-Shell and Alloys
2.6. Case−Study F: Lattice-Size-Shape-Facets
3. Hydrogen Evolution by Cu-Oxide Based Materials
| Photocatalytic Hydrogen Production | ||||
|---|---|---|---|---|
| Catalyst | Hole Scavenger | Irradiation Source | H2 Evolution Rate | Ref. |
| Cubic-Cu2O | Pure H2O (Water Splitting) | 300 W Xe-Lamp | Not detected | [54] |
| Octahedra-Cu2O | >0.4 μmol g−1 h−1 | |||
| Rhombic Dodecahedra-Cu2O | ~1.6 μmol g−1 h−1 | |||
| Cu2O/TiO2 (C-1.5/T-2) | 30%MeOH (H2O/MeOH) | Full-arc Xe Lamp 100 mW cm−2 | 11 mmol g−1 h−1 | [58] |
| 30%MeOH (Seawater/MeOH) | 5.1 mmol g−1 h−1 | |||
| CuO (Later Cu2O)-TiO2 | 10% Glycerol (H2O/Glycerol) | 300 W Xe-Lamp | 336 μmol g−1 h−1 | [59] |
| Cu0-TiO2 | ~867 μmol g−1 h−1 | |||
| Cu2O/TiO2 | 10% MeOH (H2O/MeOH) | 300 W Xe-Lamp | 70 μmol g−1 h−1 | [60] |
| 3 wt.% Cu-TiO2 | ~8% MeOH (H2O/MeOH 11:1) | 125 W Hg-Lamp (325 & 365nm) | 2.07 mmol g−1 h−1 | [61] |
| 13.5 wt.% Cu-TiO2 | 2.48 mmol g−1 h−1 | |||
| Electrodeposited Cu2O-WO3 | Pure H2O (Water Splitting) | 400 W Hg-Lamp | ~7 μmol g−1 | [29] |
| Cu@Cu2O/ZnO | 0.75M Na2S and 1.05M Na2SO3 | 300 W Xe-Lamp | 1.47 mmol g−1 h−1 | [62] |
| ZnO/Cu2O-CuO | 60mM Na2S | 150 W Xe-Lamp | 1092.5 μmol g−1 h−1 | [63] |
| CuSA-TiO2 (~1.5 wt%) | ~70% MeOH (H2O/MeOH 1:2) | 500 W Xe-Lamp | 101.74 mmol g−1 h−1 | [40] |
| 0.75% Cu atom-TiO2 | 25% MeOH (H2O/MeOH 3:1) | 100 W Xe-Lamp | 16.6 mmol g−1 h−1 | [64] |
| NDs-Cu2O | 20% EtOH (H2O/EtOH) | 300 W Xe-Lamp (100 mW cm−2) | 1597 μmol g−1 h−1 | [65] |
| Visible Light 420–760 nm 77.5 mW cm−2 | 824 μmol g−1 h−1 | |||
| Cu2O@g-C3N4 (CN5 5wt.%) | Pure H2O | 300 W Xe-Lamp (≥420 nm) | 795 μmol g−1 h−1 | [66] |
3.1. Photocatalytic Hydrogen Production
3.1.1. Facet Engineering
3.1.2. Coupling with Semiconductors
- The case of Cu-TiO2
- Coupling of Cu with Non-TiO2 Semiconductors
3.1.3. Carbon-Based Materials and Core-Shell Structures
3.1.4. The Case of Cu-Single Atoms
3.2. Electrocatalytic Hydrogen Production
- [i]
- exploring the intrinsic activity of catalysts by measuring the capacitance of double layer Cdl to detect the electrochemical active surface area (ESCA) [71],
- [ii]
- overpotential needs to be the lowest possible, at 10 mA/cm2 [72],
- [iii]
- the Tafel slope shows the rate of adsorption–desorption kinetics, where the electrochemical desorption step is rate-determining according to the Volmer–Heyrovsky mechanism [73].
- [iv]
- through EIS measurements the charge transfer resistance can easily be found [56].
- [v]
- Mott–Schottky plots can provide information about flat band potential and the populations of donors and acceptors [74].
3.3. Photoelectrochemical Hydrogen Evolution Reaction
| Photoelectrochemical Hydrogen Production | ||||
|---|---|---|---|---|
| Catalyst | Environment | Light Source | J @ Applied Potential | Ref. |
| CuO/TiO2 | 1 M KOH | 500 W Xe-Lamp | −0.54 mA/cm2 @ −0.55 V vs. Ag/AgCl | [26] |
| CuO/Pt | 1 M KOH | −0.57 mA/cm2 @ −0.55 V vs. Ag/AgCl | ||
| CuO/TiO2/Pt | 1 M KOH | −0.75 mA/cm2 @ −0.55 V vs. Ag/AgCl | ||
| Cu2O/CuO Bilayered composite | 0.5 M Na2SO4 + 1 M KOH | 300 W Xe-Lamp (1000 mW m−2) | −3.15 mA/cm2 @ 0.40V vs. RHE | [18] |
| Cu2O/Ga2O3/TiO2/Rux | 0.5 M Na2SO4 + 0.1 M phosphate solution | 300 W Xe-Lamp (1000 mW m−2) | −10 mA/cm2 @ 0V vs. RHE | [87] |
| 3D CuO | 150 W solar simulator (1000 mW m−2) | −3.15 mA/cm2 @ 0.42V vs. RHE | [88] | |
| CuO/thin film | 0.1 M Na2SO4 | Sunlight (1000 mW m−2) | −3.1 mA/cm2 @ 0V vs. RHE | [23] |
| Au-Pd decorated CuO thin film | 0.1 M Na2SO4 | Sunlight (1000 mW m−2) | −3.88 mA/cm2 @ 0V vs. RHE | |
| CuSA-TiO2 | 0.2 M Na2SO4 | 150 W Xe-Lamp | −10 mA/cm2 @ −0.72V vs. NHE | [40] |
4. CO2 Reduction by Cu-Based Materials
4.1. Photocatalytic CO2 Reduction
4.1.1. Facet Dependency of CO2 Reduction
4.1.2. Coupling with Semiconductors
- The Case of Cu-TiO2
- The Case of Cu Coupling with Non-TiO2 Semiconductors
4.1.3. Carbon-Based Materials and Core-Shell Cu-Oxide Structures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barber, J.; Tran, P.D. From Natural to Artificial Photosynthesis. J. R. Soc. Interface 2013, 10, 20120984. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, E.; Tang, J. Insight on Reaction Pathways of Photocatalytic CO2 Conversion. ACS Catal. 2022, 12, 7300–7316. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Liu, G.; Du, K.; Haussener, S.; Wang, K. Charge Transport in Two-Photon Semiconducting Structures for Solar Fuels. ChemSusChem 2016, 9, 2878–2904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting Materials for Photoelectrochemical Energy Conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Rej, S.; Bisetto, M.; Naldoni, A.; Fornasiero, P. Well-Defined Cu2O Photocatalysts for Solar Fuels and Chemicals. J. Mater. Chem. A 2021, 9, 5915–5951. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Caudillo-Flores, U.; Barba-Nieto, I.; Fernández-García, M. Towards Full-Spectrum Photocatalysis: Successful Approaches and Materials. Appl. Catal. A Gen. 2021, 610, 117966. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly Active Oxide Photocathode for Photoelectrochemical Water Reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Zhang, Z.; Dua, R.; Zhang, L.; Zhu, H.; Zhang, H.; Wang, P. Carbon-Layer-Protected Cuprous Oxide Nanowire Arrays for Efficient Water Reduction. ACS Nano 2013, 7, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Golden, T.D.; Shumsky, M.G.; Zhou, Y.; VanderWerf, R.A.; Van Leeuwen, R.A.; Switzer, J.A. Electrochemical Deposition of Copper(I) Oxide Films. Chem. Mater. 1996, 8, 2499–2504. [Google Scholar] [CrossRef]
- Guo, X.; Diao, P.; Xu, D.; Huang, S.; Yang, Y.; Jin, T.; Wu, Q.; Xiang, M.; Zhang, M. CuO/Pd Composite Photocathodes for Photoelectrochemical Hydrogen Evolution Reaction. Int. J. Hydrog. Energy 2014, 39, 7686–7696. [Google Scholar] [CrossRef]
- Yu, J.; Hai, Y.; Jaroniec, M. Photocatalytic Hydrogen Production over CuO-Modified Titania. J. Colloid Interface Sci. 2011, 357, 223–228. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef]
- Siripala, W.; Ivanovskaya, A.; Jaramillo, T.F.; Baeck, S.-H.; McFarland, E.W. A Cu2O/TiO2 Heterojunction Thin Film Cathode for Photoelectrocatalysis. Sol. Energy Mater. Sol. Cells 2003, 77, 229–237. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Chen, Q.; Zhou, Y.-J.; Li, H.-M.; Fu, J.-W.; Liu, M. Cu-Based Bimetallic Catalysts for CO2 Reduction Reaction. Adv. Sens. Energy Mater. 2022, 1, 100023. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; Kim, H.; In, S.-I. Activity, Selectivity, and Stability of Earth-Abundant CuO/Cu2O/Cu0-Based Photocatalysts toward CO2 Reduction. Chem. Eng. J. 2022, 429, 131579. [Google Scholar] [CrossRef]
- Toe, C.Y.; Scott, J.; Amal, R.; Ng, Y.H. Recent Advances in Suppressing the Photocorrosion of Cuprous Oxide for Photocatalytic and Photoelectrochemical Energy Conversion. J. Photochem. Photobiol. C: Photochem. Rev. 2019, 40, 191–211. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Siavash Moakhar, R.; Chua, C.S.; Kushwaha, A.; Dalapati, G.K. Stable and Efficient CuO Based Photocathode through Oxygen-Rich Composition and Au–Pd Nanostructure Incorporation for Solar-Hydrogen Production. ACS Appl. Mater. Interfaces 2017, 9, 27596–27606. [Google Scholar] [CrossRef] [PubMed]
- Masudy-Panah, S.; Eugene, Y.-J.K.; Khiavi, N.D.; Katal, R.; Gong, X. Aluminum-Incorporated p-CuO/n-ZnO Photocathode Coated with Nanocrystal-Engineered TiO2 Protective Layer for Photoelectrochemical Water Splitting and Hydrogen Generation. J. Mater. Chem. A 2018, 6, 11951–11965. [Google Scholar] [CrossRef]
- McKone, J.R.; Pieterick, A.P.; Gray, H.B.; Lewis, N.S. Hydrogen Evolution from Pt/Ru-Coated p-Type WSe2 Photocathodes. J. Am. Chem. Soc. 2013, 135, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; E, L.; Guo, Z.; Zhao, D.; Li, X.; Liu, Z. Exposing the Photocorrosion Mechanism and Control Strategies of a CuO Photocathode. Inorg. Chem. Front. 2019, 6, 2488–2499. [Google Scholar] [CrossRef]
- Kang, W.; Feng, Y.; Li, Z.; Yang, W.; Cheng, C.; Shi, Z.; Yin, P.; Shen, G.; Yang, J.; Dong, C.; et al. Strain-Activated Copper Catalyst for PH-Universal Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2112367. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, M.; Han, J.; Peng, W.; Zhao, Y.; Chen, D.; Peng, M.; Liu, J.; de Groot, F.M.F.; Tan, Y. Identifying Electrocatalytic Sites of the Nanoporous Copper–Ruthenium Alloy for Hydrogen Evolution Reaction in Alkaline Electrolyte. ACS Energy Lett. 2020, 5, 192–199. [Google Scholar] [CrossRef]
- Hu, C.-C.; Nian, J.-N.; Teng, H. Electrodeposited P-Type Cu2O as Photocatalyst for H2 Evolution from Water Reduction in the Presence of WO3. Sol. Energy Mater. Sol. Cells 2008, 92, 1071–1076. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalysis for Hydrogen Production and CO2 Reduction: The Case of Copper-Catalysts. ChemCatChem 2019, 11, 368–382. [Google Scholar] [CrossRef]
- Toe, C.Y.; Zheng, Z.; Wu, H.; Scott, J.; Amal, R.; Ng, Y.H. Photocorrosion of Cuprous Oxide in Hydrogen Production: Rationalising Self-Oxidation or Self-Reduction. Angew. Chem. Int. Ed. 2018, 57, 13613–13617. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Li, K.; Tang, J. Cu2O/Reduced Graphene Oxide Composites for the Photocatalytic Conversion of CO2. ChemSusChem 2014, 7, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, Y.; Wang, D.; Wu, X.; Li, J.; Xi, J. Nickel-Copper Alloy Encapsulated in Graphitic Carbon Shells as Electrocatalysts for Hydrogen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1701759. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.-V. Photocatalytic Activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 Heterojunctions. Catal. Today 2005, 101, 315–321. [Google Scholar] [CrossRef]
- Chen, J.-L.; Liu, M.-M.; Xie, S.-Y.; Yue, L.-J.; Gong, F.-L.; Chai, K.-M.; Zhang, Y.-H. Cu2O-Loaded TiO2 Heterojunction Composites for Enhanced Photocatalytic H2 Production. J. Mol. Struct. 2022, 1247, 131294. [Google Scholar] [CrossRef]
- Wei, T.; Zhu, Y.-N.; An, X.; Liu, L.-M.; Cao, X.; Liu, H.; Qu, J. Defect Modulation of Z-Scheme TiO2/Cu2O Photocatalysts for Durable Water Splitting. ACS Catal. 2019, 9, 8346–8354. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Jeong, H.; Ryu, H.; Bae, J.-S. Improvement of CuO Photostability with the Help of a BiVO4 Capping Layer by Preventing Self-Reduction of CuO to Cu2O. J. Ind. Eng. Chem. 2021, 104, 416–426. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, H.; Xiao, B.; Zhang, W.; Zhao, X.; Lv, T.; Thangamuthu, M.; Zhang, J.; Guo, Y.; et al. Single-Atom Cu Anchored Catalysts for Photocatalytic Renewable H2 Production with a Quantum Efficiency of 56%. Nat Commun 2022, 13, 58. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.-T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The Roles of Metal Co-Catalysts and Reaction Media in Photocatalytic Hydrogen Production: Performance Evaluation of M/TiO2 Photocatalysts (M=Pd, Pt, Au) in Different Alcohol–Water Mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Hussain, N.; Alawadhi, H.; Rahman, S.M.A.; Abdelkareem, M.A. Facile Synthesis of Novel Cu2O-g-C3N4/Vulcan Carbon Composite as Anode Material with Enhanced Electrochemical Performances in Urea Fuel Cell. Sustain. Energy Technol. Assess. 2021, 45, 101107. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, W.; Zhao, L.; Chen, H.; He, X.; Li, W.; Tian, P.; Huang, Z. g-C3N4 Foam/Cu2O QDs with Excellent CO2 Adsorption and Synergistic Catalytic Effect for Photocatalytic CO2 Reduction. Environ. Int. 2019, 130, 104898. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wen, Z. Self-Supported Three-Dimensional Cu/Cu2O–CuO/RGO Nanowire Array Electrodes for an Efficient Hydrogen Evolution Reaction. Chem. Commun. 2018, 54, 6388–6391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lei, H.; Lu, S.; Yang, Z.; Xu, B.B.; Xing, L.; Liu, T.X. Cu2O Nano-Flowers/Graphene Enabled Scaffolding Structure Catalyst Layer for Enhanced CO2 Electrochemical Reduction. Appl. Catal. B: Environ. 2022, 305, 121022. [Google Scholar] [CrossRef]
- Zhang, S.-N.; Li, M.; Hua, B.; Duan, N.; Ding, S.; Bergens, S.; Shankar, K.; Luo, J.-L. A Rational Design of Cu2O−SnO2 Core-Shell Catalyst for Highly Selective CO2-to-CO Conversion. ChemCatChem 2019, 11, 4147–4153. [Google Scholar] [CrossRef]
- Jansonius, R.P.; Reid, L.M.; Virca, C.N.; Berlinguette, C.P. Strain Engineering Electrocatalysts for Selective CO2 Reduction. ACS Energy Lett. 2019, 4, 980–986. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.-Y.; Feng, Y.; Dong, C.-K.; Liu, H.; Du, X.-W. A Silver Catalyst Activated by Stacking Faults for the Hydrogen Evolution Reaction. Nat. Catal. 2019, 2, 1107–1114. [Google Scholar] [CrossRef]
- Khorshidi, A.; Violet, J.; Hashemi, J.; Peterson, A.A. How Strain Can Break the Scaling Relations of Catalysis. Nat. Catal. 2018, 1, 263–268. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.; Gao, W.; Maxson, T.; Raciti, D.; Giroux, M.; Pan, X.; Wang, C.; Greeley, J. Tunable Intrinsic Strain in Two-Dimensional Transition Metal Electrocatalysts. Science 2019, 363, 870–874. [Google Scholar] [CrossRef]
- Farinazzo Bergamo Dias Martins, P.; Papa Lopes, P.; Ticianelli, E.A.; Stamenkovic, V.R.; Markovic, N.M.; Strmcnik, D. Hydrogen Evolution Reaction on Copper: Promoting Water Dissociation by Tuning the Surface Oxophilicity. Electrochem. Commun. 2019, 100, 30–33. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Q.; Liang, X.; Wang, Z.; Zheng, Z.; Wang, P.; Liu, Y.; Dai, Y.; Whangbo, M.-H.; Huang, B. Cu2O Nanoparticles with Both 100 and 111 Facets for Enhancing the Selectivity and Activity of CO2 Electroreduction to Ethylene. Adv. Sci. 2020, 7, 1902820. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. Oxide Nanocrystal Model Catalysts. Acc. Chem. Res. 2016, 49, 520–527. [Google Scholar] [CrossRef]
- Kwon, Y.; Soon, A.; Han, H.; Lee, H. Shape Effects of Cuprous Oxide Particles on Stability in Water and Photocatalytic Water Splitting. J. Mater. Chem. A 2014, 3, 156–162. [Google Scholar] [CrossRef]
- Lasia, A. Mechanism and Kinetics of the Hydrogen Evolution Reaction. Int. J. Hydrog. Energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design Principles for Hydrogen Evolution Reaction Catalyst Materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Y.; Zhou, Y.; Liu, Q.; Song, C.; Wang, D. Oxygen Vacancy Stimulated Direct Z-Scheme of Mesoporous Cu2O/TiO2 for Enhanced Photocatalytic Hydrogen Production from Water and Seawater. J. Alloy. Compd. 2021, 868, 159144. [Google Scholar] [CrossRef]
- Jung, M.; Hart, J.N.; Scott, J.; Ng, Y.H.; Jiang, Y.; Amal, R. Exploring Cu Oxidation State on TiO2 and Its Transformation during Photocatalytic Hydrogen Evolution. Appl. Catal. A: Gen. 2016, 521, 190–201. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, X.L.; Scott, J.; Ng, C.; Amal, R. TiO2 -Supported Copper Nanoparticles Prepared via Ion Exchange for Photocatalytic Hydrogen Production. J. Mater. Chem. A 2014, 2, 6432–6438. [Google Scholar] [CrossRef]
- Kubacka, A.; Muñoz-Batista, M.J.; Fernández-García, M.; Obregón, S.; Colón, G. Evolution of H2 Photoproduction with Cu Content on CuO-TiO2 Composite Catalysts Prepared by a Microemulsion Method. Appl. Catal. B: Environ. 2015, 163, 214–222. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, Y.; Cheng, L.; Chen, J.; Zhao, Y. A Stable Plasmonic Cu@Cu2O/ZnO Heterojunction for Enhanced Photocatalytic Hydrogen Generation. ChemSusChem 2018, 11, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kahng, S.; Hyeun Kim, J. Z-Scheme Assisted ZnO/Cu2O-CuO Photocatalysts to Increase Photoactive Electrons in Hydrogen Evolution by Water Splitting. Sol. Energy Mater. Sol. Cells 2020, 204, 110211. [Google Scholar] [CrossRef]
- Lee, B.-H.; Park, S.; Kim, M.; Sinha, A.K.; Lee, S.C.; Jung, E.; Chang, W.J.; Lee, K.-S.; Kim, J.H.; Cho, S.-P.; et al. Reversible and Cooperative Photoactivation of Single-Atom Cu/TiO2 Photocatalysts. Nat. Mater. 2019, 18, 620–626. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, J.; Li, L.; Liu, P.; Wang, C.; Yang, G. Nanodiamond-Embedded p-Type Copper(I) Oxide Nanocrystals for Broad-Spectrum Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1501865. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Y.; Hu, J.; Liang, Y.; Cui, W. Efficient Visible-Light Photocatalytic Hydrogen Evolution and Enhanced Photostability of Core@shell Cu2O@g-C3N4 Octahedra. Appl. Surf. Sci. 2015, 351, 1146–1154. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 Heterostructures for CO2 Reduction through a Direct Z-Scheme: Protecting Cu2O from Photocorrosion. Appl. Catal. B: Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, J.; Li, J.; Li, Y.; Wang, X.; Zhang, Y.; Jiang, J.; Chen, S.; Zhao, C.; Qian, D. Designed Synthesis of a Novel BiVO4–Cu2O–TiO2 as an Efficient Visible-Light-Responding Photocatalyst. J. Colloid Interface Sci. 2015, 444, 58–66. [Google Scholar] [CrossRef]
- Han, P.; Martens, W.; Waclawik, E.R.; Sarina, S.; Zhu, H. Metal Nanoparticle Photocatalysts: Synthesis, Characterization, and Application. Part. Part. Syst. Charact. 2018, 35, 1700489. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Khademi, M.; Barz, D.P.J. Structure of the Electrical Double Layer Revisited: Electrode Capacitance in Aqueous Solutions. Langmuir 2020, 36, 4250–4260. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Li, S.; Du, Y.; Han, X.; Xu, P. How to Reliably Report the Overpotential of an Electrocatalyst. ACS Energy Lett. 2020, 5, 1083–1087. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed]
- Adán-Más, A.; Silva, T.M.; Guerlou-Demourgues, L.; Montemor, M.F. Application of the Mott-Schottky Model to Select Potentials for EIS Studies on Electrodes for Electrochemical Charge Storage. Electrochim. Acta 2018, 289, 47–55. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, H.; Zhou, X.; Liu, K.; Zhang, C.; Zhou, Z.; Wang, C.; Lin, W. Electrocatalytic Reduction of CO2 to CO with 100% Faradaic Efficiency by Using Pyrolyzed Zeolitic Imidazolate Frameworks Supported on Carbon Nanotube Networks. J. Mater. Chem. A 2017, 5, 24867–24873. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Liu, C.; Pandey, S.; Woo Joo, S.; Son, N.; Kang, M. Achieving a Long-Term Stability by Self-Redox Property between Fe and Mn Ions in the Iron-Manganese Spinel Structured Electrode in Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 546, 149124. [Google Scholar] [CrossRef]
- Manikandan, A.; Lee, L.; Wang, Y.-C.; Chen, C.-W.; Chen, Y.-Z.; Medina, H.; Tseng, J.-Y.; Wang, Z.M.; Chueh, Y.-L. Graphene-Coated Copper Nanowire Networks as a Highly Stable Transparent Electrode in Harsh Environments toward Efficient Electrocatalytic Hydrogen Evolution Reactions. J. Mater. Chem. A 2017, 5, 13320–13328. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Chen, Y.-Y.; Zhao, L.; Huang, L.-B.; Luo, H.; Jiang, W.-J.; Zhang, X.; Niu, S.; Gao, D.; et al. Encased Copper Boosts the Electrocatalytic Activity of N-Doped Carbon Nanotubes for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 36857–36864. [Google Scholar] [CrossRef]
- Parvin, S.; Kumar, A.; Ghosh, A.; Bhattacharyya, S. An Earth-Abundant Bimetallic Catalyst Coated Metallic Nanowire Grown Electrode with Platinum-like PH-Universal Hydrogen Evolution Activity at High Current Density. Chem. Sci. 2020, 11, 3893–3902. [Google Scholar] [CrossRef]
- Hodes, G. Photoelectrochemical Cell Measurements: Getting the Basics Right. J. Phys. Chem. Lett. 2012, 3, 1208–1213. [Google Scholar] [CrossRef]
- Steinmiller, E.M.P.; Choi, K.-S. Photochemical Deposition of Cobalt-Based Oxygen Evolving Catalyst on a Semiconductor Photoanode for Solar Oxygen Production. Proc. Natl. Acad. Sci. USA 2009, 106, 20633–20636. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Diao, P.; Xu, D.; Wu, Q. High-Aspect-Ratio WO3 Nanoneedles Modified with Nickel-Borate for Efficient Photoelectrochemical Water Oxidation. Electrochim. Acta 2013, 114, 271–277. [Google Scholar] [CrossRef]
- Berglund, S.P.; Flaherty, D.W.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Photoelectrochemical Oxidation of Water Using Nanostructured BiVO4 Films. J. Phys. Chem. C 2011, 115, 3794–3802. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Q.; Xiao, X. Hydrogen Evolution from Pt Nanoparticles Covered P-Type CdS:Cu Photocathode in Scavenger-Free Electrolyte. J. Phys. Chem. C 2014, 118, 2306–2311. [Google Scholar] [CrossRef]
- Gao, L.; Cui, Y.; Wang, J.; Cavalli, A.; Standing, A.; Vu, T.T.T.; Verheijen, M.A.; Haverkort, J.E.M.; Bakkers, E.P.A.M.; Notten, P.H.L. Photoelectrochemical Hydrogen Production on InP Nanowire Arrays with Molybdenum Sulfide Electrocatalysts. Nano Lett. 2014, 14, 3715–3719. [Google Scholar] [CrossRef]
- Fareza, A.R.; Nugroho, F.A.; Abdi, F.; Fauzia, V. Nanoscale Metal Oxides–2D Materials Heterostructures for Photoelectrochemical Water Splitting—A Review. J. Mater. Chem. A 2022, 10, 8656–8686. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.-K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the Performance of Cu2O Photocathodes for Unassisted Solar Water Splitting Devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Epstein, J.; Brown, A.; Munday, J.N.; Culver, J.N.; Ehrman, S. Biological Templates for Antireflective Current Collectors for Photoelectrochemical Cell Applications. Nano Lett. 2012, 12, 6005–6011. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic Reduction of Carbon Dioxide in Aqueous Suspensions of Semiconductor Powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Wu, Y.A.; McNulty, I.; Liu, C.; Lau, K.C.; Liu, Q.; Paulikas, A.P.; Sun, C.-J.; Cai, Z.; Guest, J.R.; Ren, Y.; et al. Facet-Dependent Active Sites of a Single Cu2O Particle Photocatalyst for CO2 Reduction to Methanol. Nat Energy 2019, 4, 957–968. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lu, J.-S.; Pu, Y.-C.; Fan, H.-C. Enhanced Photoreduction of CO2 into Methanol by Facet-Dependent Cu2O/Reduce Graphene Oxide. J. CO2 Util. 2019, 33, 171–178. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Kuang, C.-C.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C.S. Selective Photocatalytic Reduction of CO2 into CH4 over Pt-Cu2O TiO2 Nanocrystals: The Interaction between Pt and Cu2O Cocatalysts. Appl. Catal. B Environ. 2017, 202, 695–703. [Google Scholar] [CrossRef]
- Liu, L.; Gao, F.; Zhao, H.; Li, Y. Tailoring Cu Valence and Oxygen Vacancy in Cu/TiO2 Catalysts for Enhanced CO2 Photoreduction Efficiency. Appl. Catal. B Environ. 2013, 134–135, 349–358. [Google Scholar] [CrossRef]
- Paulino, P.N.; Salim, V.M.M.; Resende, N.S. Zn-Cu Promoted TiO2 Photocatalyst for CO2 Reduction with H2O under UV Light. Appl. Catal. B Environ. 2016, 185, 362–370. [Google Scholar] [CrossRef]
- Bae, K.-L.; Kim, J.; Lim, C.K.; Nam, K.M.; Song, H. Colloidal Zinc Oxide-Copper(I) Oxide Nanocatalysts for Selective Aqueous Photocatalytic Carbon Dioxide Conversion into Methane. Nat. Commun. 2017, 8, 1156. [Google Scholar] [CrossRef]
- Xiang, T.; Xin, F.; Zhao, C.; Lou, S.; Qu, W.; Wang, Y.; Song, Y.; Zhang, S.; Yin, X. Fabrication of Nano Copper Oxide Evenly Patched on Cubic Sodium Tantalate for Oriented Photocatalytic Reduction of Carbon Dioxide. J. Colloid Interface Sci. 2018, 518, 34–40. [Google Scholar] [CrossRef]
- Li, H.; Lei, Y.; Huang, Y.; Fang, Y.; Xu, Y.; Zhu, L.; Li, X. Photocatalytic Reduction of Carbon Dioxide to Methanol by Cu2O/SiC Nanocrystallite under Visible Light Irradiation. J. Nat. Gas Chem. 2011, 20, 145–150. [Google Scholar] [CrossRef]
- Kim, C.; Cho, K.M.; Al-Saggaf, A.; Gereige, I.; Jung, H.-T. Z-Scheme Photocatalytic CO2 Conversion on Three-Dimensional BiVO4 /Carbon-Coated Cu2O Nanowire Arrays under Visible Light. ACS Catal. 2018, 8, 4170–4177. [Google Scholar] [CrossRef]
- Adekoya, D.O.; Tahir, M.; Amin, N.A.S. g-C3N4/(Cu/TiO2) Nanocomposite for Enhanced Photoreduction of CO 2 to CH3OH and HCOOH under UV/Visible Light. J. CO2 Util. 2017, 18, 261–274. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced Graphene Oxide–CuO Nanocomposites for Photocatalytic Conversion of CO2 into Methanol under Visible Light Irradiation. Appl. Catal. B Environ. 2016, 181, 352–362. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Tseng, I.-H. Photocatalytic Conversion of Gas Phase Carbon Dioxide by Graphitic Carbon Nitride Decorated with Cuprous Oxide with Various Morphologies. J. CO2 Util. 2018, 26, 511–521. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Zhou, J.; Chen, W.; Liu, M. Encapsulating CuO Quantum Dots in MIL-125(Ti) Coupled with g-C3N4 for Efficient Photocatalytic CO2 Reduction. Chem. Eng. J. 2020, 399, 125782. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Vanmaekelbergh, D.; Kelly, J.J. Cu2O: A Catalyst for the Photochemical Decomposition of Water? Chem. Commun. 1999, 12, 1069–1070. [Google Scholar] [CrossRef]
- Slamet Nasution, H.W.; Purnama, E.; Kosela, S.; Gunlazuardi, J. Photocatalytic Reduction of CO2 on Copper-Doped Titania Catalysts Prepared by Improved-Impregnation Method. Catal. Commun. 2005, 6, 313–319. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene-Based Nanoassemblies for Energy Conversion. J. Phys. Chem. Lett. 2011, 2, 242–251. [Google Scholar] [CrossRef]
- Lightcap, I.V.; Kosel, T.H.; Kamat, P.V. Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Catalyst Mat. Storing and Shuttling Electrons with Reduced Graphene Oxide. Nano Lett 2010, 10, 577–583. [Google Scholar] [CrossRef]
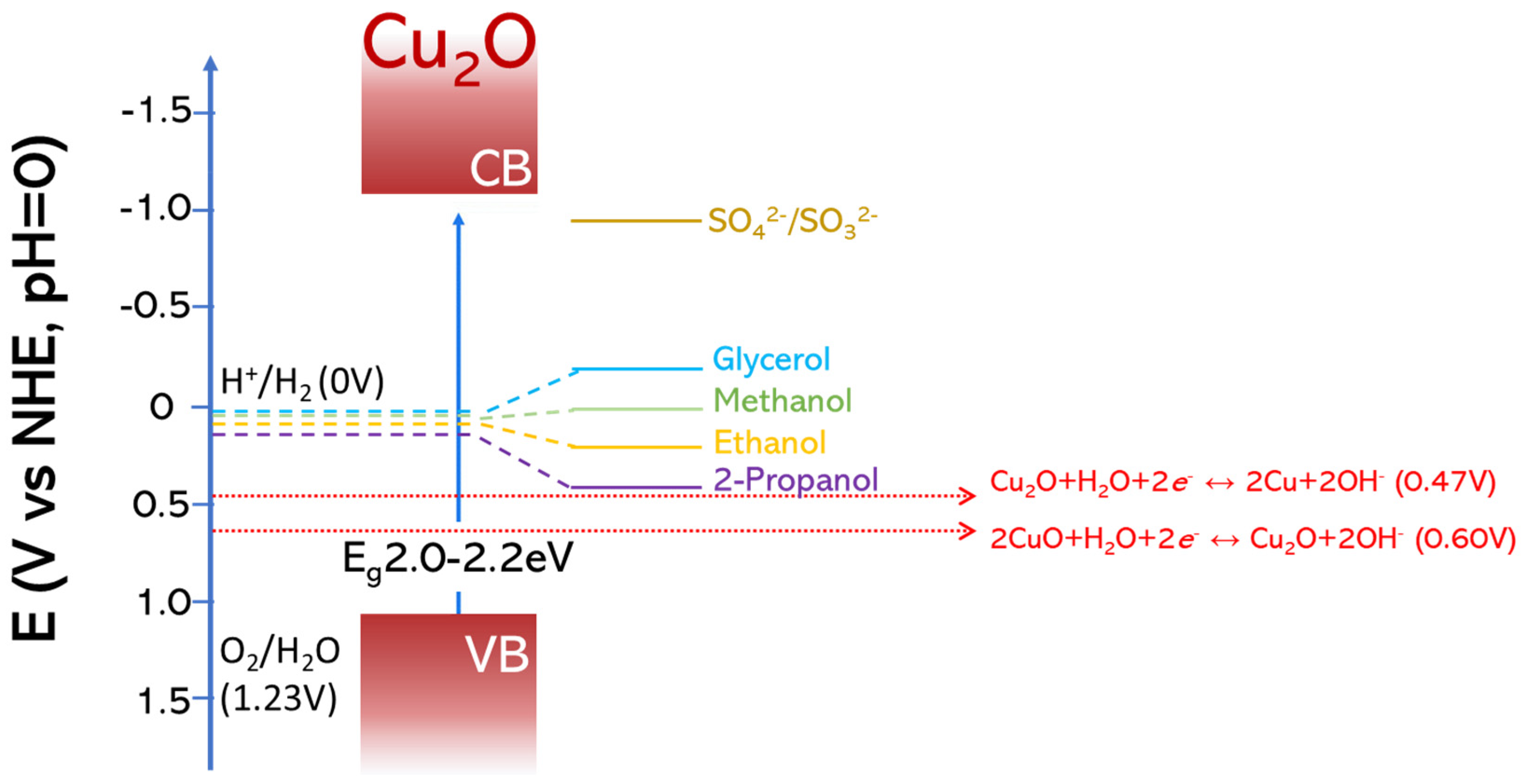
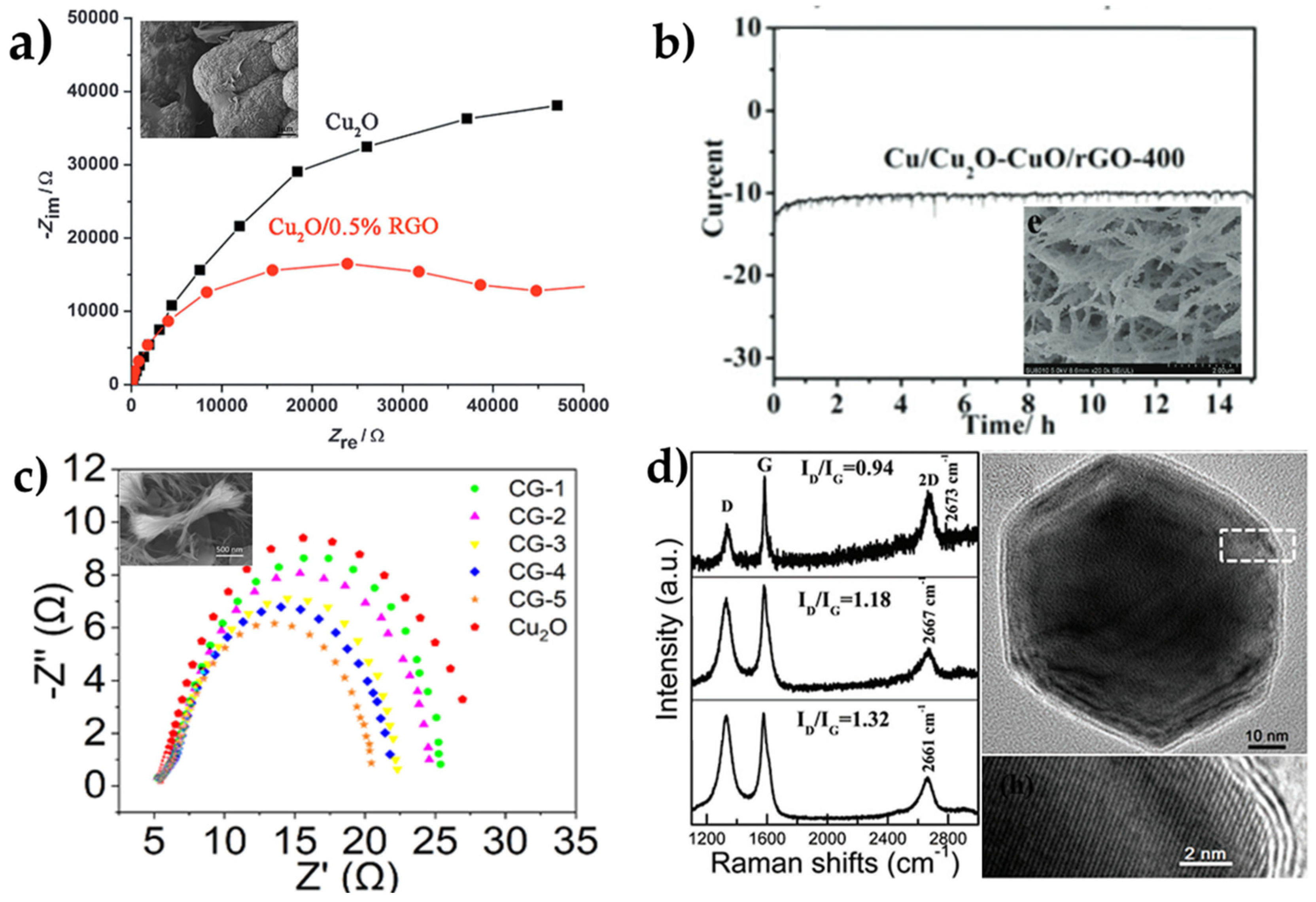


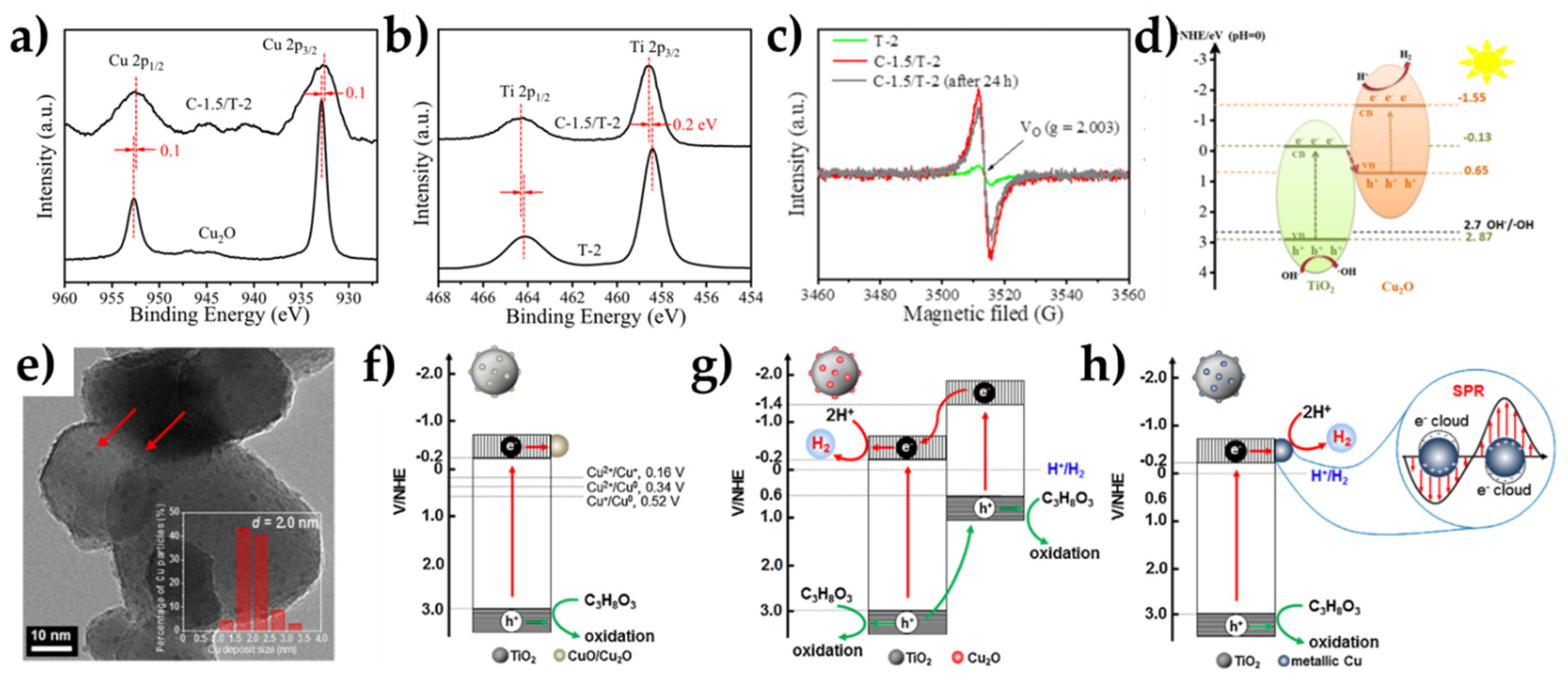
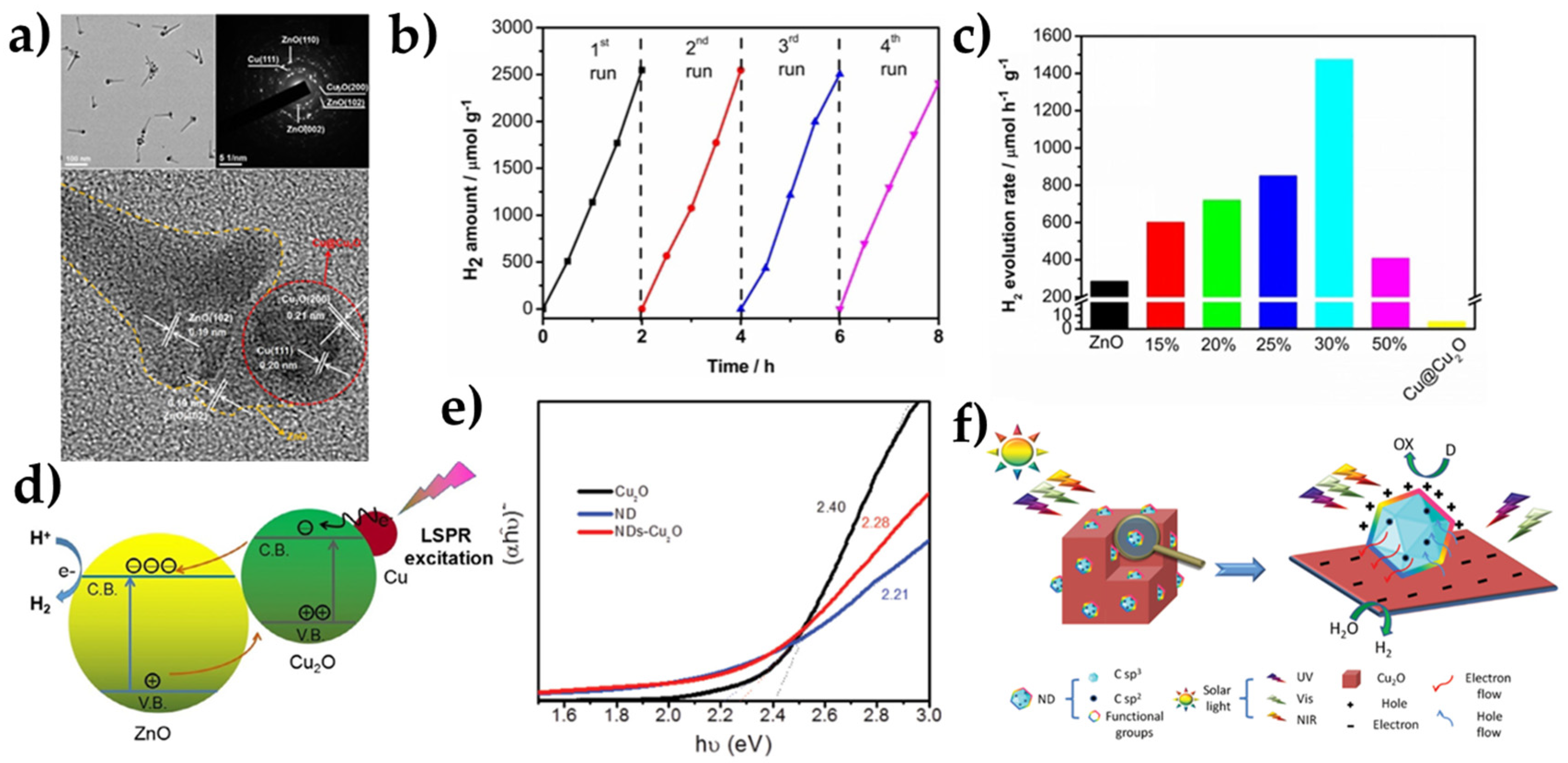
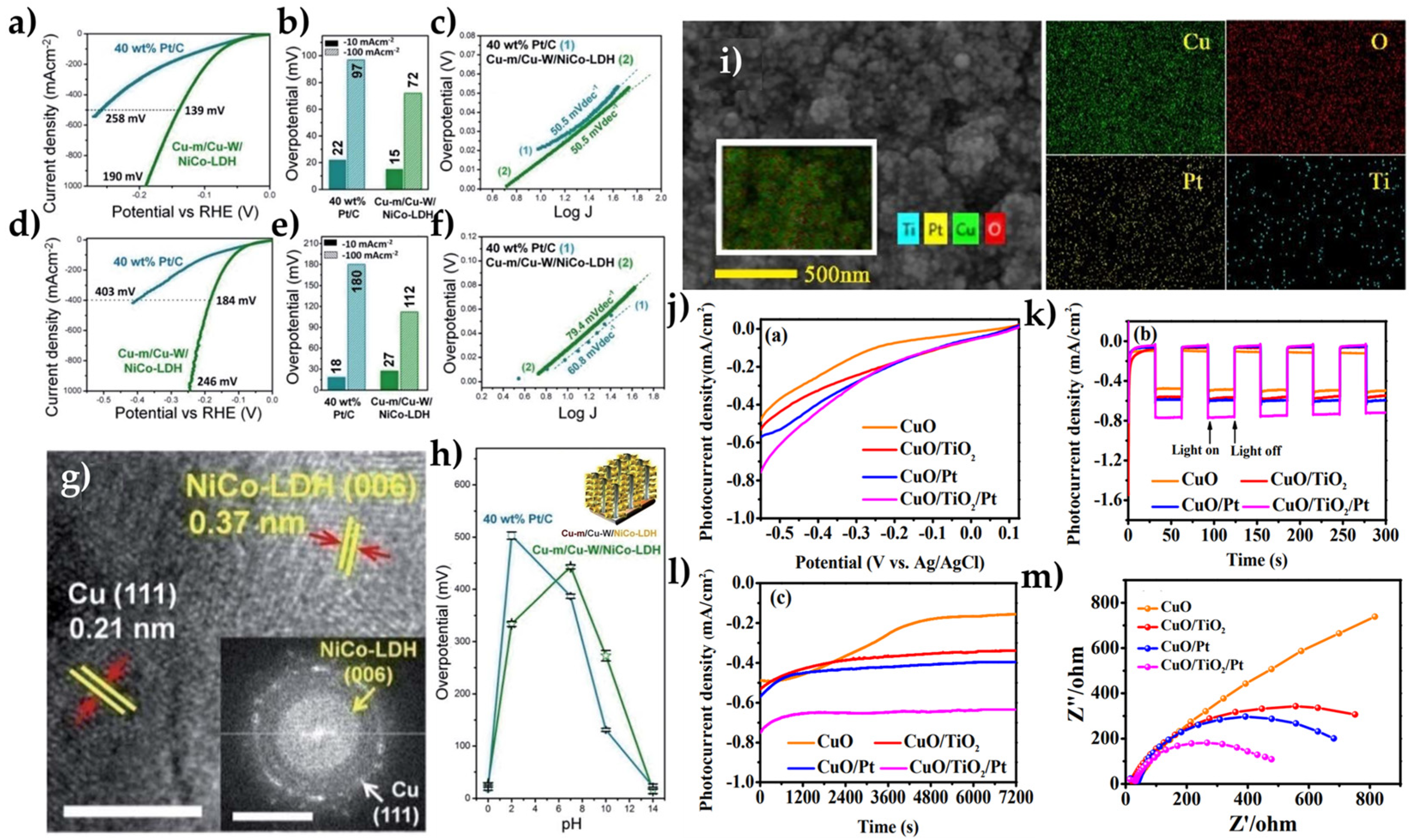
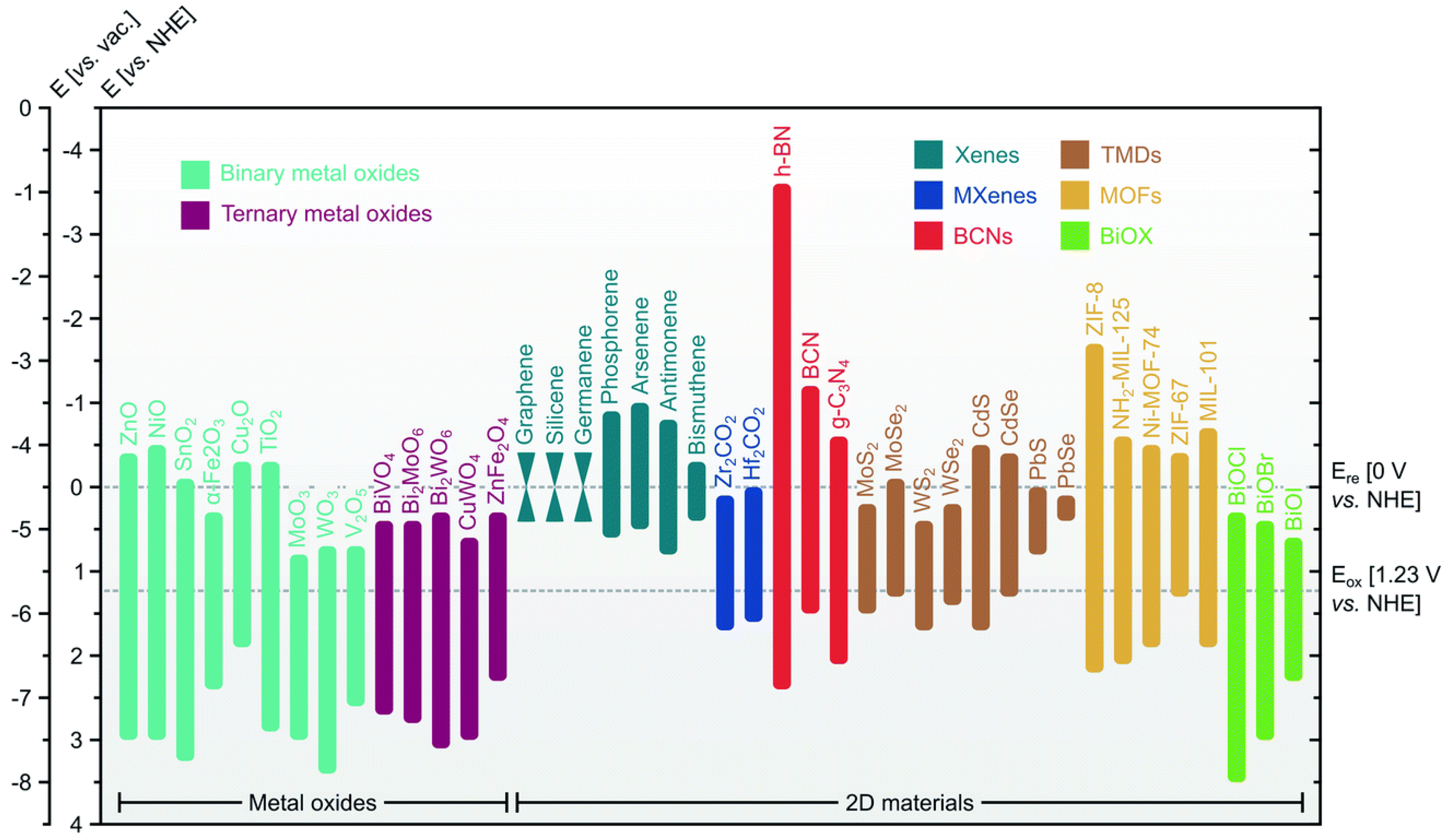
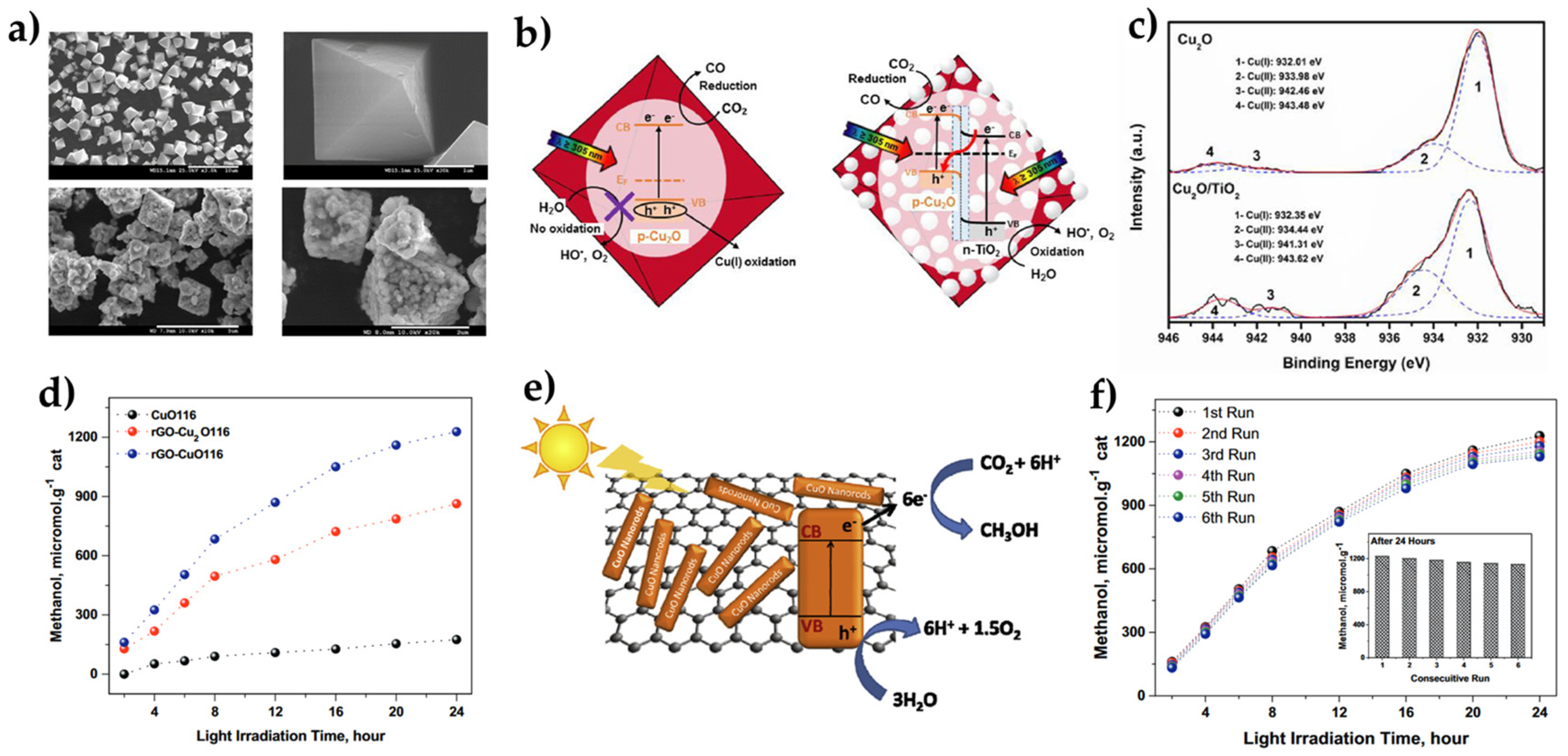
| Overall Reaction (Conditions) | Reaction Pathway | Equations |
|---|---|---|
| Acidic Media | 1.i | |
| 1.ii | ||
| 1.iii | ||
| Alkaline Media | 2.i | |
| 2.ii | ||
| 2.iii |
| Electrocatalytic Hydrogen Production | |||||
|---|---|---|---|---|---|
| Catalyst | Electrolyte or pH | Cdl (mF/cm2) | η(mV) @ −10 mA/cm2 | Tafel Slope (mV dec−1) | Ref. |
| Cu/Cu2O-CuO/rGO-400 | 1 M KOH | 130 | 105 | 124 | [44] |
| PS-Cu | 0.5 M H2SO4 | 8.77 | 182 | 99.16 | [27] |
| 1 M PBS | 10.01 | 261 | 143.58 | ||
| 1 M KOH | 13.56 | 121 | 136.54 | ||
| Cu-foam | 0.5 M H2SO4 | 4.86 | 411 | 192.22 | |
| 1 M PBS | 5.60 | 429 | 211.95 | ||
| 1 M KOH | 5.91 | 473 | 230.27 | ||
| Cu-NPs | 0.5 M H2SO4 | 6.87 | 530 | 132.17 | |
| 1 M PBS | 6.47 | 707 | 285.99 | ||
| 1 M KOH | 9.72 | 454 | 148.86 | ||
| Pt-foil | 0.5 M H2SO4 | 78 | 79.09 | ||
| 1 M PBS | 186 | 194.05 | |||
| 1 M KOH | 178 | 167.84 | |||
| NiCu@C-1 | pH = 0 | 48 | 94.5 | [33] | |
| pH = 7 | 164 | 94.6 | |||
| pH = 14 | 74 | 74 | |||
| Graphene coated Cu | 0.5 M H2SO4 | 252 | 67 | [77] | |
| Cu@NC NT/CF-500 | 1 M KOH | 101 | 123 | 61 | [78] |
| Cu53Ru47 | 1 M KOH | 59 | 15 | 30 | [28] |
| 1 M PbS | 59 | 41 | 35 | ||
| Cu-m/Cu-W/NiCo-LDH | 0.5 M H2SO4 | 15 | 79.4 | [79] | |
| 1 M KOH | 19.8 | 27 | 50.5 | ||
| Photocatalytic CO2 Reduction | ||||
|---|---|---|---|---|
| Catalyst | Hole Scavenger/ Reaction Conditions | Irradiation Source | Main Products | Ref. |
| (110) Cu2O | Saturated H2O in CO2 | 300 W Xe-Lamp | CH3OH: 1.2 mol g−1 h−1 | [90] |
| Dodeca-Cu2O/rGO | Saturated H2O in CO2 | 300 W Xe-Lamp (λ ≤ 420 nm) | CH3OH: 355.3 μmol g−1 h−1 | [91] |
| Pt-Cu2O/TiO2 | Saturated H2O in CO2 (71kPa) | 500 W Xe-arc Lamp (300 nm < λ < 400 nm) (20.5 mW cm−2) | CH4: 1.42 μmol g−1 h−1 CO: 0.05 μmol g−1 h−1 | [92] |
| Octa-Cu2O/TiO2 | Water vapor—1atm CO2 (g) | 1 kW Hg (Xe) arc lamp (λ ≥ 305 nm) | CO: 2.11 μmol g−1 h−1 | [67] |
| 1% Cu/TiO2 (H2) | Mixed gas CO2/H2O | 150 W solar simulator (90 mW cm−2, 200 ≤ λ ≤ 1000 nm) | CH4: 25 μmol g−1 h−1 CO: 4.4 μmol g−1 h−1 | [93] |
| 2%CuO-19%ZnO/TiO2 | Saturated H2O in CO2, 0.2 M NaOH | 18 W Hg-Lamp (λ = 25 4nm) | CH4: 184 μmol g−1 (after 24 h) | [94] |
| ZnO-Cu2O | Saturated H2O in CO2, 0.2 M Na2CO3 | 300 W Xe-Lamp | CH4: 1080 μmol g1 h−1 | [95] |
| 5wt% CuO/NaTaO3 | CO2, Isopropanol | 250 W Hg-Lamp (365 nm) | CH3OH: 1302.22 μmol g−1 h−1 | [96] |
| Cu2O | Saturated H2O in CO2, NaOH, Na2SO3 | 500 W Xe-arc Lamp (400 nm < λ < 700nm) | CH3OH: 104 μmol g−1 | [97] |
| Cu2O-SiC | CH3OH: 191 μmol g−1 | |||
| BiVO4/C-coated Cu2O | Saturated H2O in CO2 | 300 W Xe-Lamp (100 mW cm−2) (λ > 420 nm) | CO: ~3 μmol g−1 h−1 | [98] |
| 3%Cu/TiO2 | Saturated H2O in CO2, 1 M NaOH | UV-Lamp (254 nm, 5.4 mW cm−2) Visible: 500 W Xe-arc Lamp | HCOOH: > 4500 μmol g−1 h−1 (Visible) // CH3OH: ~300 μmol g−1 h−1 (Visible) HCOOH: >2000 μmol g−1 h−1 (UV)// CH3OH: >75 μmol g−1 h−1 (UV) | [99] |
| 3% Cu/g-C3N4 | HCOOH: >3500 μmol g−1 h−1 (Visible)//CH3OH: ~350 μmol g−1 h−1 (Visible) HCOOH: >3750 μmol g−1 h−1 (UV)// CH3OH: >200 μmol g−1 h−1 (UV) | |||
| (g-C3N4)/(3%Cu/TiO2) (30:70) | HCOOH: 5069 μmol g−1 (Visible)// CH3OH: 2574 μmol g−1 (Visible) HCOOH: 6709 μmol g−1 (UV)// CH3OH: 614 μmol g−1 (UV) | |||
| rGO-CuO | DMF, Saturated H2O in CO2 | 20 W LED (85 W m−2) | CH3OH: 1228 μmol g−1 | [100] |
| rGO-Cu2O | CH3OH: 862 μmol g−1 | |||
| c-Cu2O_gCN | ~1 bar CO2 with moisture | 8 W LED Lamp | CO: 0.002 μmol g1 h−1 | [101] |
| g-C3N4/CuO@MIL-125(Ti) | H2O 0.3%CO2 (1 MPa) | 300 W Xe-Lamp (326.1 W m−2) (λ = 420 nm) | CO: 180.1 μmol g−1 CH3OH: 997.2 μmol g−1 C2H5OH: 531.5 μmol g−1 CH3CHO: 1505.7 μmol g−1 | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zindrou, A.; Belles, L.; Deligiannakis, Y. Cu-Based Materials as Photocatalysts for Solar Light Artificial Photosynthesis: Aspects of Engineering Performance, Stability, Selectivity. Solar 2023, 3, 87-112. https://doi.org/10.3390/solar3010008
Zindrou A, Belles L, Deligiannakis Y. Cu-Based Materials as Photocatalysts for Solar Light Artificial Photosynthesis: Aspects of Engineering Performance, Stability, Selectivity. Solar. 2023; 3(1):87-112. https://doi.org/10.3390/solar3010008
Chicago/Turabian StyleZindrou, Areti, Loukas Belles, and Yiannis Deligiannakis. 2023. "Cu-Based Materials as Photocatalysts for Solar Light Artificial Photosynthesis: Aspects of Engineering Performance, Stability, Selectivity" Solar 3, no. 1: 87-112. https://doi.org/10.3390/solar3010008
APA StyleZindrou, A., Belles, L., & Deligiannakis, Y. (2023). Cu-Based Materials as Photocatalysts for Solar Light Artificial Photosynthesis: Aspects of Engineering Performance, Stability, Selectivity. Solar, 3(1), 87-112. https://doi.org/10.3390/solar3010008