Effect of Calcium on the Characteristics of Action Potential Under Different Electrical Stimuli
Abstract
1. Introduction
2. Neuron Model
2.1. HH Model
2.2. HH + Ca Model
3. Action Potential Simulation Based on HH and HH + Ca Models
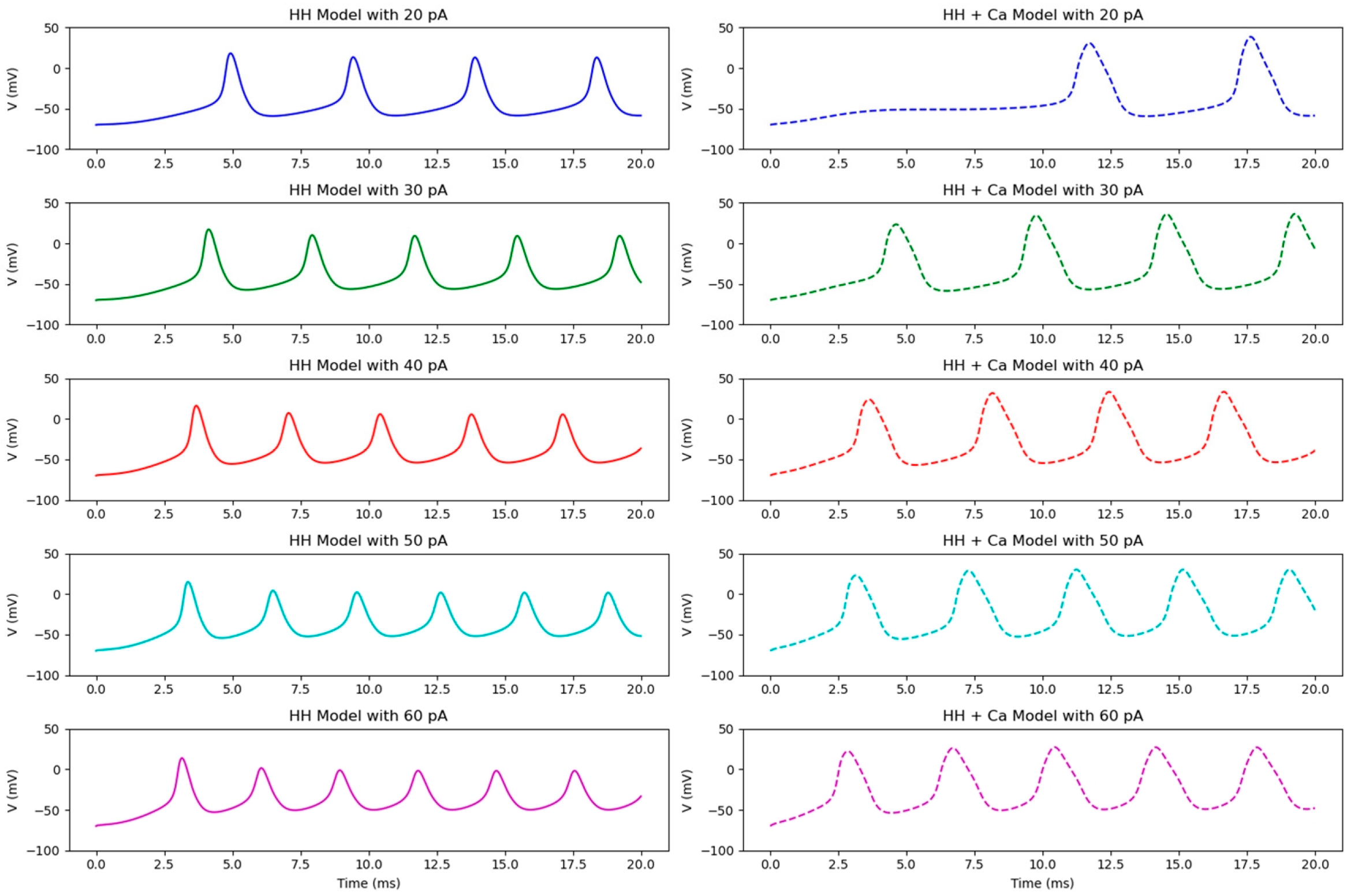
3.1. Characteristics of Action Potential Release Under Direct Current Stimulation
3.1.1. Characteristics of Action Potential Release Under Weak Current Stimulation
3.1.2. Characteristics of Action Potential Release Under Moderate to Strong Current Stimulation
3.1.3. Characteristics of Action Potential Release Under Strong Current Stimulation
3.2. Characteristics of Action Potential Release Under Step Current Stimulation
3.3. Characteristics of Action Potential Release Under Square Wave Current Stimulation
3.4. Characteristics of Action Potential Release Under Sine Current Stimulation
4. Discussions
5. Conclusions
- (1)
- Under a direct current stimulation, the frequency of the action potential release increases with the current intensity, and the delay time of the first action potential is shortened. However, when the current stimulation exceeds a certain threshold, , the peak amplitude of the action potential gradually diminishes. The delay time of the first action potential in the HH + Ca model is longer than that in the HH model, while the peak amplitude after a stable release is greater in the HH + Ca model. The firing frequency of the HH model is higher than that of the HH + Ca model. Under a strong current stimulation, the HH + Ca model demonstrates a greater capacity to sustain the action potential release compared to the HH model.
- (2)
- When a step current is applied, the results are approximately equivalent to those observed under the constant direct current stimulation. The step change in the current induces the depolarization of the membrane potential, thereby triggering the action potential release. As the current intensity increases, the frequency of the action potential release in both models also rises. The HH model exhibits greater sensitivity to current stimulations, as smaller currents can elicit action potentials, whereas the HH + Ca model requires higher current intensities to initiate action potentials. The HH + Ca model may exhibit subthreshold oscillations under weak current stimulations below the threshold, with the oscillation amplitude increasing alongside the current intensity.
- (3)
- When a square wave current is applied, an increase in the current intensity leads to an increase in the frequency of the action potential release in both models, accompanied by a reduction in the delay time. Increasing the frequency of the square wave current diminishes the number of peak action potentials per cluster. At a current intensity of 10 pA, high-frequency action potentials are completely suppressed in the HH + Ca model, a phenomenon not observed in the HH model. In the HH model, action potentials can still be generated when the square wave current is zero, whereas this does not occur in the HH + Ca model.
- (4)
- When a sine current is introduced, the frequency of the action potential release in both models increases with the rising current intensity at the same sine current frequency. As the frequency of the sine current escalates, the number of spikes in the action potential clusters decreases. At high current intensities, both models display lower action potential amplitudes near the peak of the sine wave. The continuous depolarizing current provided by the calcium ion current partially offsets the hyperpolarizing effect of the potassium ion current. Additionally, the slow response of the calcium-activated potassium ion channels in the HH + Ca model, along with the rapid activation and inactivation of the A-type potassium ion currents, mitigates the potassium ion outflow and prevents the excessive hyperpolarization of the membrane potential. Collectively, these factors result in a lesser degree of action potential depression in the HH + Ca model compared to the HH model.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Hall, W.C.; LaMantia, A.S.; McNamara, J.O.; White, L.E. Neuroscience, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2008; pp. 432–434. [Google Scholar]
- Than, M.; Szabo, B. Analysis of the Function of GABAB Receptors on Inhibitory Afferent Neurons of Purkinje Cells in the Cerebellar Cortex of the Rat. Eur. J. Neurosci. 2002, 15, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, Y.; Schaefer, A.T.; Wagner, M.J.; Senn, W.; Larkum, M.E. Bistability of Cerebellar Purkinje Cells Modulated by Sensory Stimulation. Nat. Neurosci. 2005, 8, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, K.; Kawabata Galbraith, K.; Kengaku, M. Dendritic Self-Avoidance and Morphological Development of Cerebellar Purkinje Cells. Cerebellum 2018, 17, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, R.C.A.; Burgis, V.; Holmgren, E.; McBride, W.J. A Comparison of the Inhibitory Effects of Taurine and GABA on Identified Purkinje Cells and Other Neurons in the Cerebellar Cortex of the Rat. Brain Res. 1978, 145, 117–126. [Google Scholar] [CrossRef]
- Purkinje Cell | Granule Cells, Cerebellum & Neurons | Britannica. Available online: https://www.britannica.com (accessed on 16 January 2024).
- Paul, M.S.; Limaiem, F. Histology, Purkinje Cells. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551704/ (accessed on 16 January 2024). [PubMed]
- Fry, C.H.; Jabr, R.I. The Action Potential and Nervous Conduction. Surgery 2010, 28, 49–54. [Google Scholar]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D. (Eds.) Voltage-Gated Ion Channels. In Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Raghavan, M.; Fee, D.; Barkhaus, P.E. Generation and Propagation of the Action Potential. In Handbook of Clinical Neurology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 3–22. [Google Scholar]
- Ren, X. The Effect of Calcium Ion Oscillations on Neuronal and Network Discharge Activity. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2022. [Google Scholar] [CrossRef]
- Li, R.T.; Wei, H.F.; Qin, Y.J.; Xiong, L.Q.; Shen, B.R.; Wang, Y.Y.; Huang, D.M.; Huang, J.; He, S.; Wu, G.Y. Osthole Reduces Compound Action Potential Amplitude in Frog Sciatic Nerve by Blocking Calcium Channels. Liaoning J. Tradit. Chin. Med. 2024, 51, 134–136+222–224. [Google Scholar] [CrossRef]
- Pattillo, J.; Yazejian, B.; DiGregorio, D.; Vergara, J.; Grinnell, A.; Meriney, S. Contribution of Presynaptic Calcium-Activated Potassium Currents to Transmitter Release Regulation in Cultured Xenopus Nerve–Muscle Synapses. Neuroscience 2001, 102, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Helton, T.D.; Xu, W.; Lipscombe, D. Neuronal L-Type Calcium Channels Open Quickly and Are Inhibited Slowly. J. Neurosci. 2005, 25, 10247–10251. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Veldkamp, M.W.; Abbate, F.; Antoons, G.; Bouman, L.N.; Ravesloot, J.H.; van Ginneken, A.C.G. Two Types of Action Potential Configuration in Single Cardiac Purkinje Cells of Sheep. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, H1299–H1310. [Google Scholar] [CrossRef] [PubMed]
- Connors, B.W.; Gutnick, M.J. Intrinsic Firing Patterns of Diverse Neocortical Neurons. Trends Neurosci. 1990, 13, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.R.; Christensen, S.R.; Stuart, G.J.; Häusser, M. Membrane Potential Bistability Is Controlled by the Hyperpolarization-Activated Current IH in Rat Cerebellar Purkinje Neurons in Vitro. J. Physiol. 2002, 539, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, M.; Qu, X.; Zhou, S. Exploring Neuronal Action Potential Firing Characteristics Using the Izhikevich Model. Ind. Technol. Innov. 2022, 9, 100–107. [Google Scholar]
- Liu, J. Simulation of Firing Characteristics of Several Different Types of Neuronal Action Potentials. Ph.D. Thesis, Harbin Normal University, Harbin, China, 2024. [Google Scholar] [CrossRef]
- Baranauskas, G.; Martina, M. Sodium Currents Activate Without a Hodgkin-and-Huxley-Type Delay in Central Mammalian Neurons. J. Neurosci. 2006, 26, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Huxley, A.F. A Quantitative Description of Membrane Current and Its Application to Conduction and Excitation in Nerve. Bull. Math. Biol. 1990, 52, 25–71. [Google Scholar] [CrossRef] [PubMed]
- Kameneva, T.; Meffin, H.; Burkitt, A.N.; Grayden, D.B. Bistability in Hodgkin-Huxley-Type Equations. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 4728–4731. [Google Scholar]
- Kameneva, T.; Meffin, H.; Burkitt, A.N. Modelling Intrinsic Electrophysiological Properties of ON and OFF Retinal Ganglion Cells. J. Comput. Neurosci. 2011, 31, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Fohlmeister, J.F.; Coleman, P.A.; Miller, R.F. Modeling the Repetitive Firing of Retinal Ganglion Cells. Brain Res. 1990, 510, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M. Simulation of Retinal Ganglion Cell Action Potentials Based on HH and FCM Models. Ph.D. Thesis, Harbin Normal University, Harbin, China, 2024. [Google Scholar] [CrossRef]
- Stevens, C.F. Neurophysiology: A Primer; John Wiley and Sons: New York, NY, USA, 1966; ISBN 9780471824367. [Google Scholar]
- Chen, Y.R. Research Progress on Cell Membrane Oscillation and Its Regulation of Motor Neuron Excitability. Sports Sci. Technol. Lit. Bull. 2023, 31, 249–251. [Google Scholar]
- Amitai, Y. Membrane Potential Oscillations Underlying Firing Patterns in Neocortical Neurons. Neuroscience 1994, 63, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.K.; Türker, K.S. Deciphering the Contribution of Intrinsic and Synaptic Currents to the Effects of Transient Synaptic Inputs on Human Motor Unit Discharge. Clin. Neurophysiol. 2010, 121, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dai, Y.; Zhou, J.; Ge, R.; Hua, Y.; Powers, R.K.; Binder, M.D. The Effects of Membrane Potential Oscillations on the Excitability of Rat Hypoglossal Motoneurons. Front. Physiol. 2022, 13, 955566. [Google Scholar] [CrossRef] [PubMed]






















| channel | ||
| channel | ||
| channel | ||
(HH model) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, X.; Yao, W. Effect of Calcium on the Characteristics of Action Potential Under Different Electrical Stimuli. AppliedMath 2024, 4, 1358-1381. https://doi.org/10.3390/appliedmath4040072
Qiao X, Yao W. Effect of Calcium on the Characteristics of Action Potential Under Different Electrical Stimuli. AppliedMath. 2024; 4(4):1358-1381. https://doi.org/10.3390/appliedmath4040072
Chicago/Turabian StyleQiao, Xuan, and Wei Yao. 2024. "Effect of Calcium on the Characteristics of Action Potential Under Different Electrical Stimuli" AppliedMath 4, no. 4: 1358-1381. https://doi.org/10.3390/appliedmath4040072
APA StyleQiao, X., & Yao, W. (2024). Effect of Calcium on the Characteristics of Action Potential Under Different Electrical Stimuli. AppliedMath, 4(4), 1358-1381. https://doi.org/10.3390/appliedmath4040072







