Cannabidiol Modulates the Effects of Levetiracetam on Seizure Parameters and Behavioral Outcomes in Pentylenetetrazol-Kindled Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
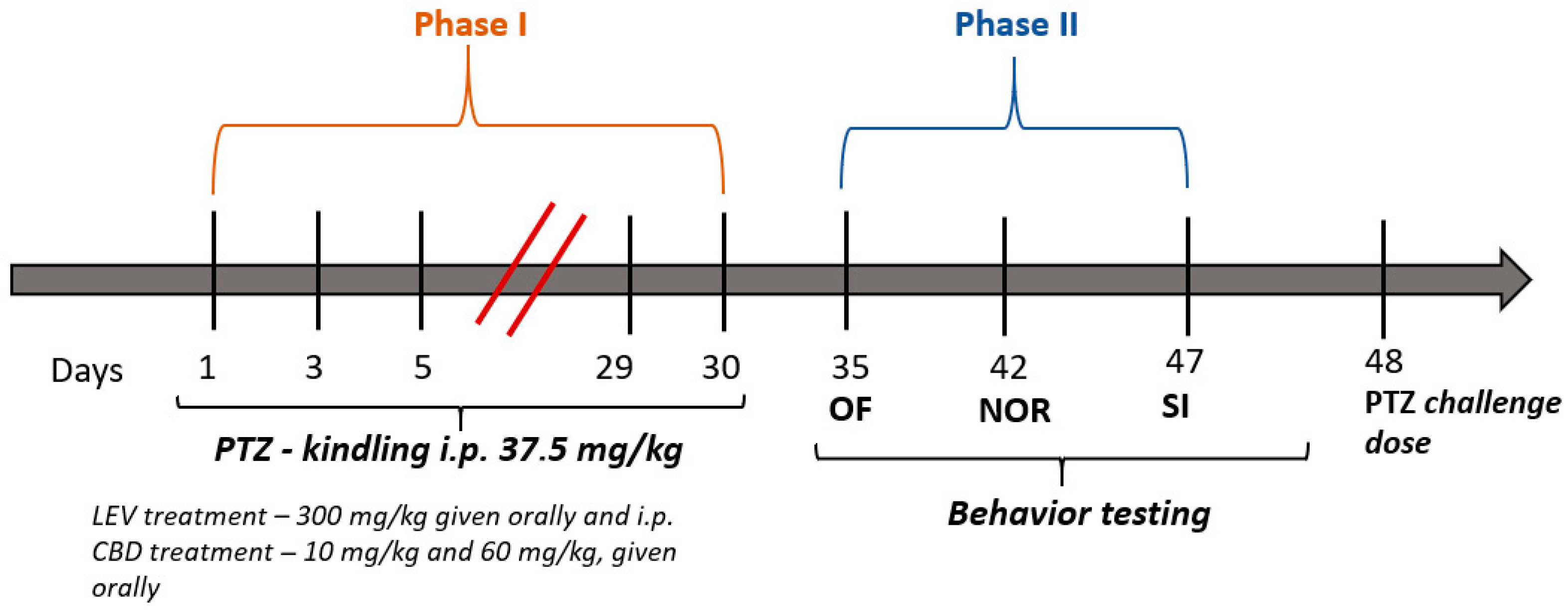
2.2. Pentylenetetrazol Kindling Model of Epilepsy
2.3. Preparation and Administration of Active Substances
2.4. Behavioral Tests
2.4.1. Open–Field Test
2.4.2. Novel Object Recognition Test
2.4.3. Social Interaction Test
2.5. Statistical Analysis
3. Results
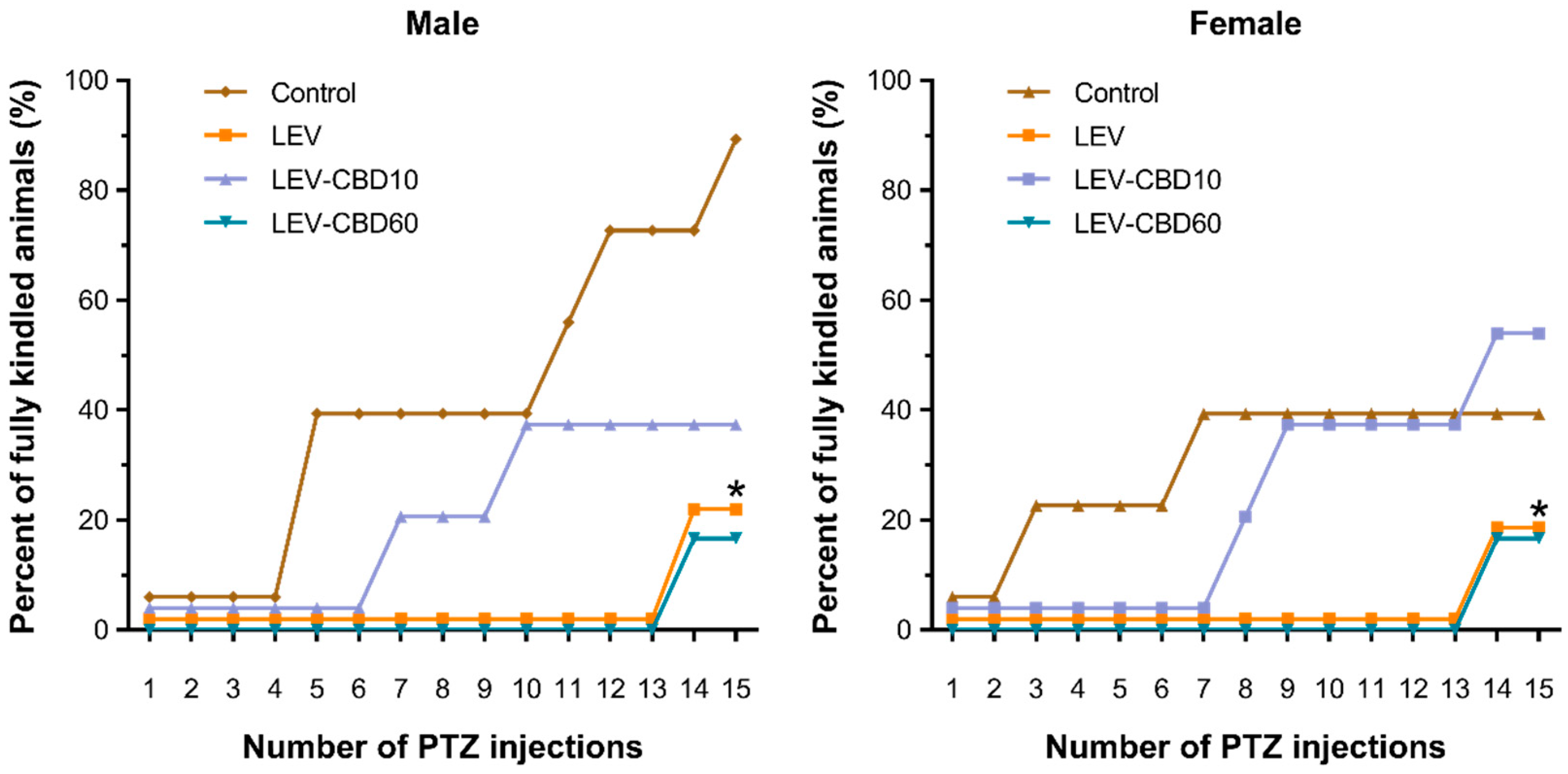
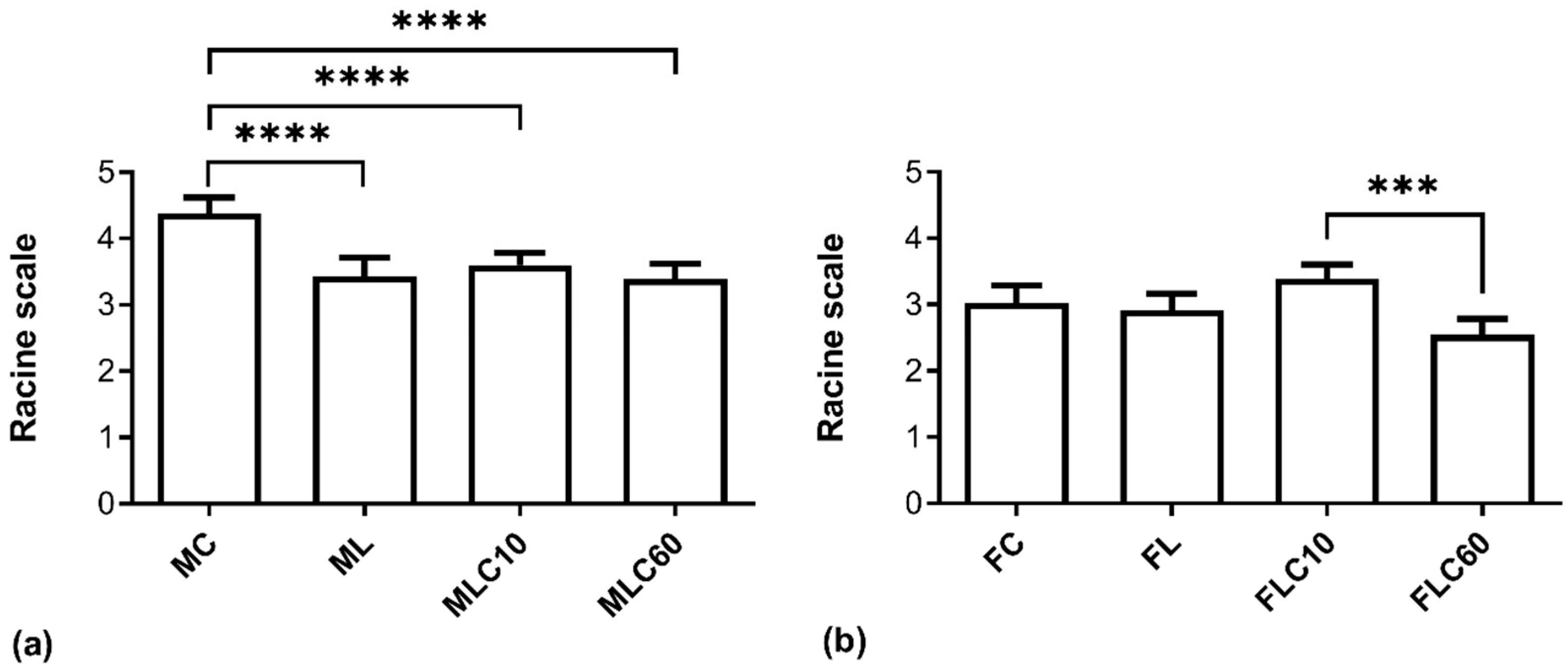
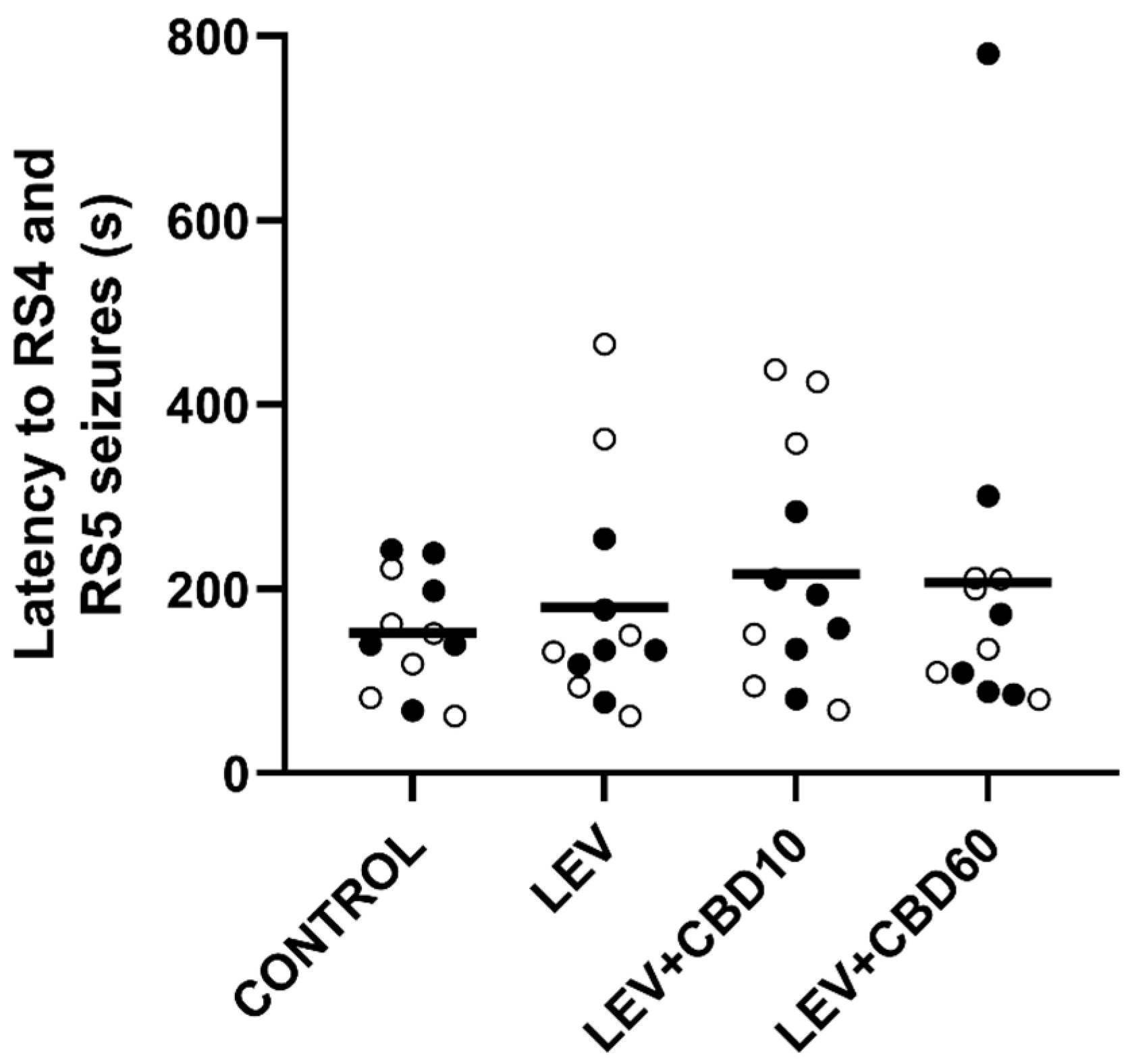
3.1. Antiseizure and Anti-Kindling Effects
3.2. Behavioral Effects
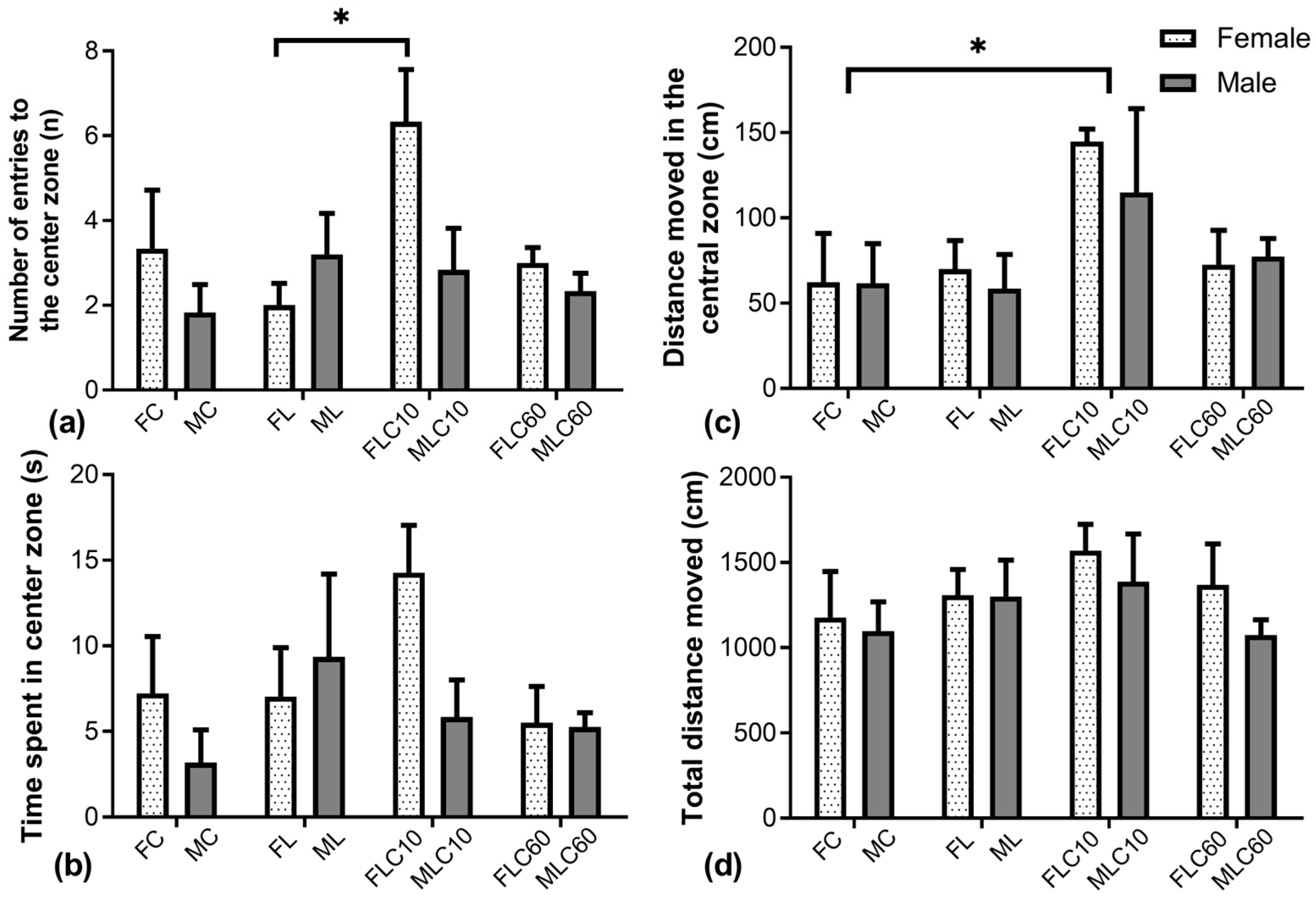
3.2.1. Open Field Test
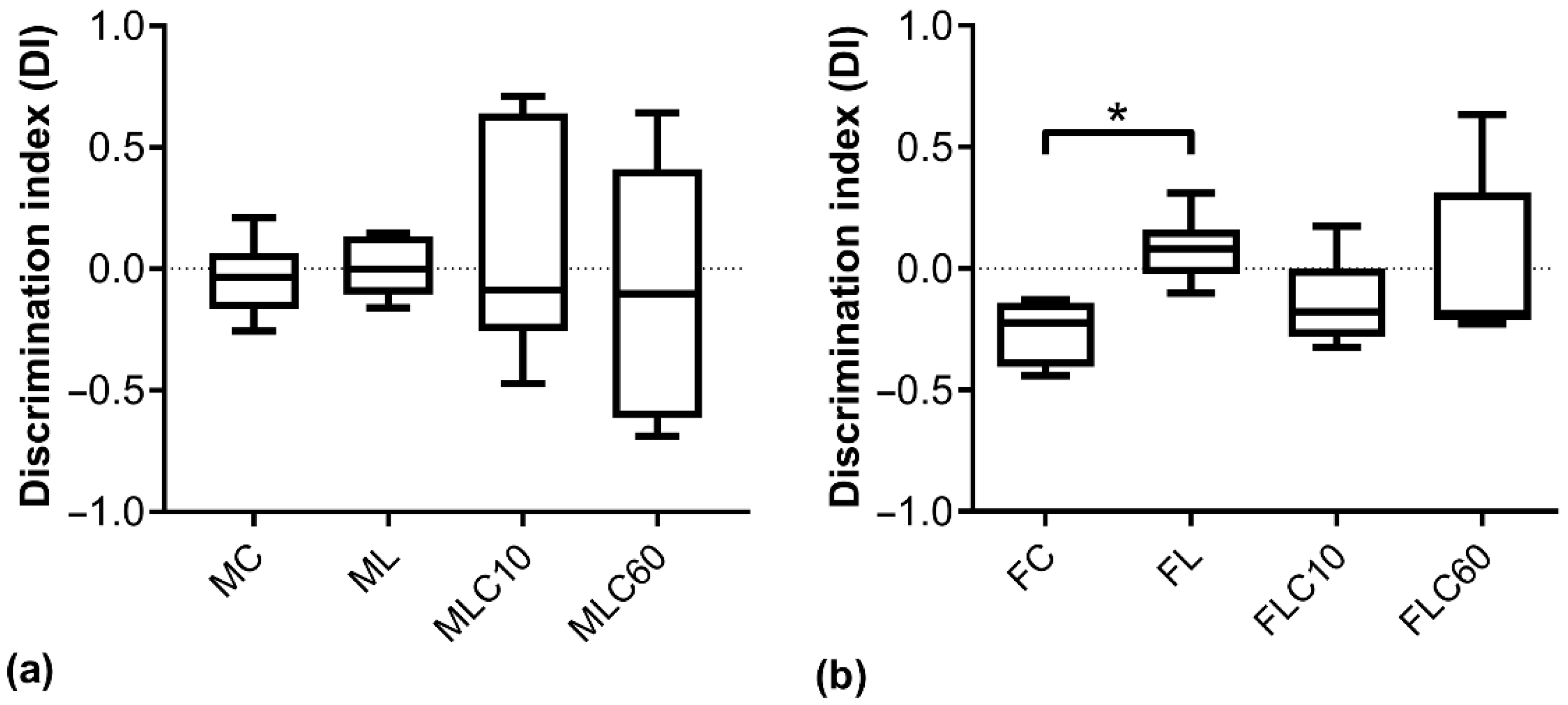
3.2.2. Novel Object Recognition Test
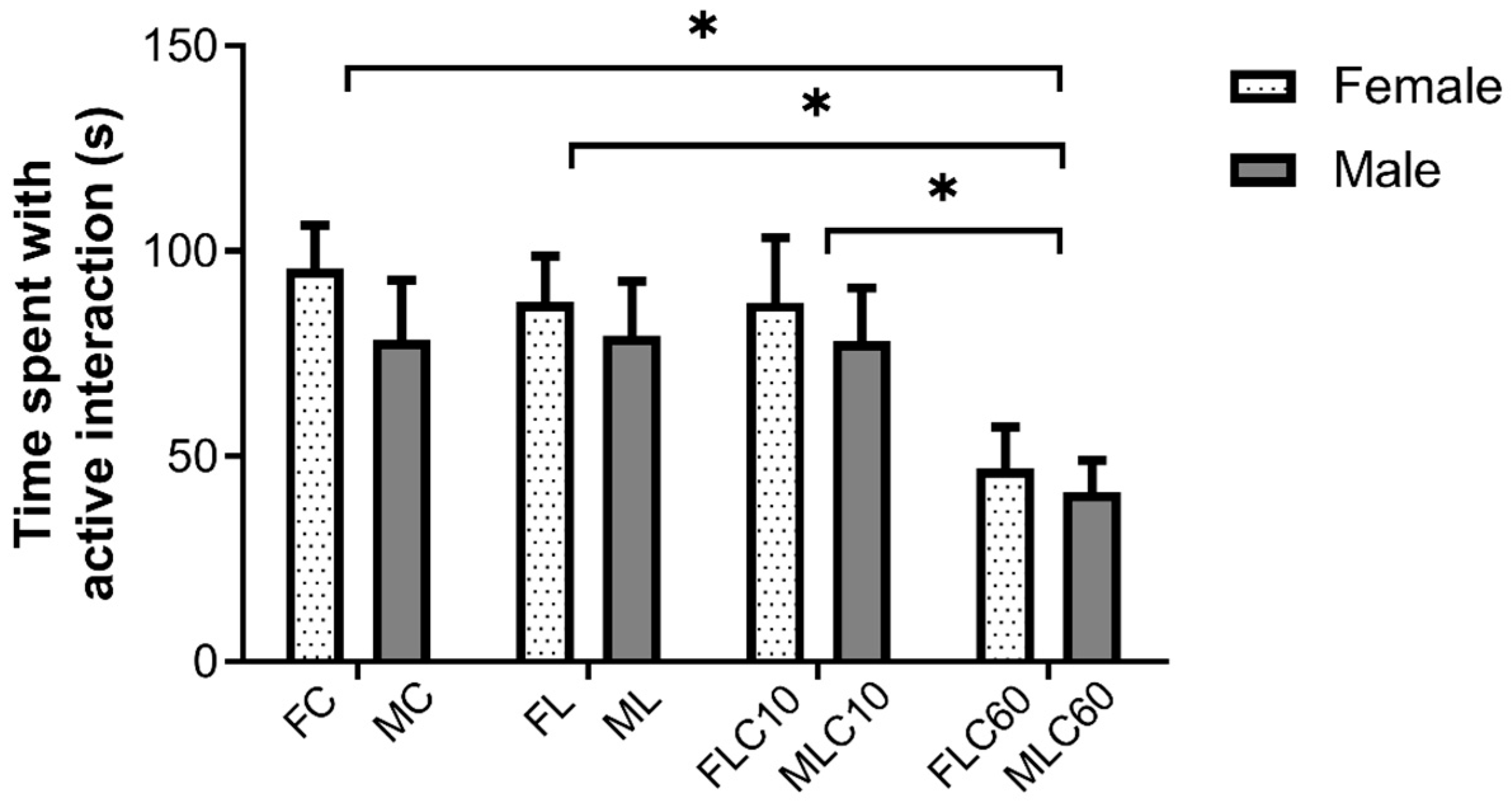
3.2.3. Social Interaction Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASM | Antiseizure medication |
| CBD | Cannabidiol |
| DI | Discrimination index |
| DRS | Duration of seizures |
| FC | Female controls |
| FDR | False discovery rate |
| FL | Female levetiracetam-treated |
| FLC | Female levetiracetam and cannabidiol treated |
| LAT | Latency to first seizures |
| LEV | Levetiracetam |
| MC | Male control |
| ML | Male levetiracetam-treated |
| MLC | Male levetiracetam and cannabidiol treated |
| MWM | Morris water maze |
| NOR | Novel object recognition |
| OF | Open field |
| PTZ | Pentylenetetrazol |
| RSMax | Maximum seizure severity according to Racine scale |
| SI | Social interaction |
References
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018, 59, 2179–2193. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Khan, T.; Devaraju, K.S.; Singh, P.; Vaibhav, K.; Gaur, P. Pharmacological and Therapeutic Approaches in the Treatment of Epilepsy. Biomedicines 2021, 9, 470. [Google Scholar] [CrossRef]
- Kaufman, K.R. Antiepileptic drugs in the treatment of psychiatric disorders. Epilepsy Behav. 2011, 21, 1–11. [Google Scholar] [CrossRef]
- García-Morales, I.; de la Peña Mayor, P.; Kanner, A.M. Psychiatric comorbidities in epilepsy: Identification and treatment. Neurologist 2008, 14, S15–S25. [Google Scholar] [CrossRef]
- Elger, C.E.; Johnston, S.A.; Hoppe, C. Diagnosing and treating depression in epilepsy. Seizure 2017, 44, 184–193. [Google Scholar] [CrossRef]
- Elger, C.E.; Schmidt, D. Modern management of epilepsy: A practical approach. Epilepsy Behav. 2008, 12, 501–539. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J.; Mintzer, S.; Pack, A.M.; Gidal, B.E.; Vecht, C.J.; Schmidt, D. Enzyme induction with antiepileptic drugs: Cause for concern? Epilepsia 2013, 54, 11–27. [Google Scholar] [CrossRef]
- Wahab, A. Difficulties in Treatment and Management of Epilepsy and Challenges in New Drug Development. Pharmaceuticals 2010, 3, 2090–2110. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, I.J.; Cárdenas-Rodríguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macías, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 475. [Google Scholar] [CrossRef] [PubMed]
- de Toledo, M.; de la Fuente, E.; Ramos, C.; Ferreiros-Martinez, R.; Muro, I.; Vieira Campos, A.; de Toledo, M.P.; Lagares, A.; Sobrado, M.; Ovejero-Benito, M.C. Extensive pharmacokinetic variability of Levetiracetam. ¿Are doctors aware? Epilepsy Res. 2022, 187, 107029. [Google Scholar] [CrossRef]
- Abou-Khalil, B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr. Dis. Treat. 2008, 4, 507–523. [Google Scholar] [CrossRef]
- Wright, C.; Downing, J.; Mungall, D.; Khan, O.; Williams, A.; Fonkem, E.; Garrett, D.; Aceves, J.; Kirmani, B. Clinical Pharmacology and Pharmacokinetics of Levetiracetam. Front. Neurol. 2013, 4, 192. [Google Scholar] [CrossRef]
- Tao, K.; Chen, H.; Chen, Y.; Gu, Y.; Wang, X. Levetiracetam induces severe psychiatric symptoms in people with epilepsy. Seizure Eur. J. Epilepsy 2024, 116, 147–150. [Google Scholar] [CrossRef]
- Czégény, Z.; Nagy, G.; Babinszki, B.; Bajtel, Á.; Sebestyén, Z.; Kiss, T.; Csupor-Löffler, B.; Tóth, B.; Csupor, D. CBD, a precursor of THC in e-cigarettes. Sci. Rep. 2021, 11, 8951. [Google Scholar] [CrossRef]
- Silvestro, S.; Mammana, S.; Cavalli, E.; Bramanti, P.; Mazzon, E. Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules 2019, 24, 1459. [Google Scholar] [CrossRef] [PubMed]
- Sanmartin, P.E.; Detyniecki, K. Cannabidiol for Epilepsy: New Hope on the Horizon? Clin. Ther. 2018, 40, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Roberta Cilio, M.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Consultant, I.; Di Marzo, V.; Jutras-Aswad, D.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Mao, K.; You, C.; Lei, D.; Zhang, H. High dosage of cannabidiol (CBD) alleviates pentylenetetrazole-induced epilepsy in rats by exerting an anticonvulsive effect. Int. J. Clin. Exp. Med. 2015, 8, 8820–8827. [Google Scholar]
- Gáll, Z.; Kelemen, K.; Tolokán, A.; Zolcseak, I.; Sável, I.; Bod, R.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Anticonvulsant Action and Long-Term Effects of Chronic Cannabidiol Treatment in the Rat Pentylenetetrazole-Kindling Model of Epilepsy. Biomedicines 2022, 10, 1811. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; García-Fuster, M.J. Dose-Dependent Antidepressant-Like Effects of Cannabidiol in Aged Rats. Front. Pharmacol. 2022, 13, 891842. [Google Scholar] [CrossRef]
- Socała, K.; Wyska, E.; Szafarz, M.; Nieoczym, D.; Wlaź, P. Acute effect of cannabidiol on the activity of various novel antiepileptic drugs in the maximal electroshock- and 6 Hz-induced seizures in mice: Pharmacodynamic and pharmacokinetic studies. Neuropharmacology 2019, 158, 107733. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kim, J.S.; Abejuela, V.A.; Lamano, J.B.; Klein, N.J.; Christian, C.A. Disrupted female estrous cyclicity in the intrahippocampal kainic acid mouse model of temporal lobe epilepsy. Epilepsia Open 2017, 2, 39–47. [Google Scholar] [CrossRef]
- Svalheim, S.; Taubøll, E.; Surdova, K.; Ormel, L.; Dahl, E.; Aleksandersen, M.; McNeilly, A.; Gjerstad, L.; Ropstad, E. Long-term levetiracetam treatment affects reproductive endocrine function in female Wistar rats. Seizure 2008, 17, 203–209. [Google Scholar] [CrossRef]
- Dhir, A. Pentylenetetrazol (PTZ) Kindling Model of Epilepsy. In Current Protocols in Neuroscience; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 9–37. ISBN 9780471142300. [Google Scholar]
- Hoeller, A.; de Carvalho, C.; Franco, P.; Formolo, D.; Imthon, A.; dos Santos, H.; Eidt, I.; Souza, G.; Constantino, L.; Ferreira, C.; et al. Behavioral and Neurochemical Consequences of Pentylenetetrazol-Induced Kindling in Young and Middle-Aged Rats. Pharmaceuticals 2017, 10, 75. [Google Scholar] [CrossRef]
- Samokhina, E.; Samokhin, A. Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int. J. Neurosci. 2018, 128, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, M.; Shojaei, A.; Palizvan, M.R.; Javan, M.; Mirnajafi-Zadeh, J. Comparison between standard protocol and a novel window protocol for induction of pentylenetetrazol kindled seizures in the rat. Epilepsy Res. 2013, 106, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lüttjohann, A.; Fabene, P.F.; van Luijtelaar, G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol. Behav. 2009, 98, 579–586. [Google Scholar] [CrossRef]
- Gáll, Z.; Kelemen, K.; Mihály, I.; Salamon, P.; Miklóssy, I.; Zsigmond, B.; Kolcsár, M. Role of Lacosamide in Preventing Pentylenetetrazole Kindling-Induced Alterations in the Expression of the Gamma-2 Subunit of the GABAA Receptor in Rats. Curr. Mol. Pharmacol. 2020, 13, 251–260. [Google Scholar] [CrossRef]
- Becker, A.; Braun, H.; Schröder, H.; Grecksch, G.; Höllt, V. Effects of enadoline on the development of pentylenetetrazol kindling, learning performance, and hippocampal morphology. Brain Res. 1999, 823, 191–197. [Google Scholar] [CrossRef]
- Sarangi, S.C.; Pattnaik, S.S.; Katyal, J.; Kaleekal, T.; Dinda, A.K. An interaction study of Ocimum sanctum L. and levetiracetam in pentylenetetrazole kindling model of epilepsy. J. Ethnopharmacol. 2020, 249, 112389. [Google Scholar] [CrossRef] [PubMed]
- Gáll, Z.; Farkas, S.; Albert, Á.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Effects of Chronic Cannabidiol Treatment in the Rat Chronic Unpredictable Mild Stress Model of Depression. Biomolecules 2020, 10, 801. [Google Scholar] [CrossRef]
- Strolin Benedetti, M.; Coupez, R.; Whomsley, R.; Nicolas, J.M.; Collart, P.; Baltes, E. Comparative pharmacokinetics and metabolism of levetiracetam, a new anti-epileptic agent, in mouse, rat, rabbit and dog. Xenobiotica 2004, 34, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.D.; Saletti, P.G.; Lisgaras, C.P.; Casillas-Espinosa, P.M.; Liu, W.; Li, Q.; Jones, N.C.; Shultz, S.; Ali, I.; Brady, R.; et al. Levetiracetam Pharmacokinetics and Brain Uptake in a Lateral Fluid Percussion Injury Rat Model. J. Pharmacol. Exp. Ther. 2023, 386, 259–265. [Google Scholar] [CrossRef]
- Löscher, W.; Hönack, D.; Rundfeldt, C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 1998, 284, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, H.; Matagne, A.; Gobert, J.; Wülfert, E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur. J. Pharmacol. 1998, 353, 191–206. [Google Scholar] [CrossRef]
- Matagne, A.; Margineanu, D.; Kenda, B.; Michel, P.; Klitgaard, H. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br. J. Pharmacol. 2008, 154, 1662–1671. [Google Scholar] [CrossRef]
- Rehman, Z.; Farooq, T.; Javaid, S.; Ashraf, W.; Fawad Rasool, M.; Samad, N.; Tariq, M.; Muhammad Muneeb Anjum, S.; Sivandzade, F.; Alotaibi, F.; et al. Combination of levetiracetam with sodium selenite prevents pentylenetetrazole-induced kindling and behavioral comorbidities in rats. Saudi Pharm. J. 2022, 30, 494–507. [Google Scholar] [CrossRef]
- Christian-Hinman, C.A. The Promise and Practicality of Addressing Sex as a Biological Variable and the Ovarian Cycle in Preclinical Epilepsy Research. Epilepsy Curr. 2024, 24, 274–279. [Google Scholar] [CrossRef]
- Kelly, M.J.; Rønnekleiv, O.K. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol. Cell. Endocrinol. 2009, 308, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kapur, J.; Joshi, S. Progesterone modulates neuronal excitability bidirectionally. Neurosci. Lett. 2021, 744, 135619. [Google Scholar] [CrossRef]
- Velíšková, J.; DeSantis, K.A. Sex and hormonal influences on seizures and epilepsy. Horm. Behav. 2013, 63, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Pollo, M.L.M.; Gimenes, C.; Covolan, L. Male rats are more vulnerable to pentylenetetrazole-kindling model but females have more spatial memory-related deficits. Epilepsy Behav. 2022, 129, 108632. [Google Scholar] [CrossRef]
- Silote, G.P.; Gatto, M.C.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S.R.L. Strain-, Sex-, and Time-Dependent Antidepressant-like Effects of Cannabidiol. Pharmaceuticals 2021, 14, 1269. [Google Scholar] [CrossRef]
- Lucchi, C.; Marcucci, M.; Aledresi, K.A.M.S.; Costa, A.-M.; Cannazza, G.; Biagini, G. Subthreshold Cannabidiol Potentiates Levetiracetam in the Kainic Acid Model of Temporal Lobe Epilepsy: A Pilot Study. Pharmaceuticals 2024, 17, 1187. [Google Scholar] [CrossRef]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Grayson, L.; Szaflarski, J.P. Final analysis of potential drug–drug interactions between highly purified cannabidiol and anti-seizure medications in an open-label expanded access program. Epilepsia Open 2023, 8, 1405–1412. [Google Scholar] [CrossRef]
- Gilmartin, C.G.S.; Dowd, Z.; Parker, A.P.J.; Harijan, P. Interaction of cannabidiol with other antiseizure medications: A narrative review. Seizure 2021, 86, 189–196. [Google Scholar] [CrossRef]
- Costa, A.-M.; Lucchi, C.; Malkoç, A.; Rustichelli, C.; Biagini, G. Relationship between Delta Rhythm, Seizure Occurrence and Allopregnanolone Hippocampal Levels in Epileptic Rats Exposed to the Rebound Effect. Pharmaceuticals 2021, 14, 127. [Google Scholar] [CrossRef]
- Lynch, B.A.; Lambeng, N.; Nocka, K.; Kensel-Hammes, P.; Bajjalieh, S.M.; Matagne, A.; Fuks, B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl. Acad. Sci. USA 2004, 101, 9861–9866. [Google Scholar] [CrossRef] [PubMed]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef]
- Cramer, J. A systematic review of the behavioral effects of levetiracetam in adults with epilepsy, cognitive disorders, or an anxiety disorder during clinical trials. Epilepsy Behav. 2003, 4, 124–132. [Google Scholar] [CrossRef]
- Brandt, C.; Glien, M.; Gastens, A.M.; Fedrowitz, M.; Bethmann, K.; Volk, H.A.; Potschka, H.; Löscher, W. Prophylactic treatment with levetiracetam after status epilepticus: Lack of effect on epileptogenesis, neuronal damage, and behavioral alterations in rats. Neuropharmacology 2007, 53, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Zimcikova, E.; Simko, J.; Karesova, I.; Kremlacek, J.; Malakova, J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35–40. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, F.; Zhao, Y.; He, M.; Luo, Y.; Cheng, Y.; Luo, J.; Li, Z.; Yang, J. The regulative effects of levetiracetam on adult hippocampal neurogenesis in mice via Wnt/β-catenin signaling. Neurochem. Int. 2020, 133, 104643. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.G.; Chaves Filho, A.J.M.; Souza Oliveira, J.V.; de Souza, D.A.A.; Lopes, I.S.; de Carvalho, M.A.J.; de Lima, K.A.; Florenço Sousa, F.C.; Mendes Vasconcelos, S.M.; Macedo, D.; et al. Prevention of pentylenetetrazole-induced kindling and behavioral comorbidities in mice by levetiracetam combined with the GLP-1 agonist liraglutide: Involvement of brain antioxidant and BDNF upregulating properties. Biomed. Pharmacother. 2019, 109, 429–439. [Google Scholar] [CrossRef]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaça, M.V.; Aguiar, D.C.; Díaz-Alonso, J.; Ortega-Gutiérrez, S.; Vázquez-Villa, H.; Moreira, F.A.; Guzmán, M.; et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef]
- Kolcsar, M.; Gáll, Z.; Dogaru, M.T. Dose dependent effects of serotonergic agents on anxiety. Acta Physiol. Hung. 2014, 101, 479–487. [Google Scholar] [CrossRef]
- Austrich-Olivares, A.; García-Gutiérrez, M.S.; Illescas, L.; Gasparyan, A.; Manzanares, J. Cannabinoid CB1 Receptor Involvement in the Actions of CBD on Anxiety and Coping Behaviors in Mice. Pharmaceuticals 2022, 15, 473. [Google Scholar] [CrossRef] [PubMed]
- Gomer, B.; Wagner, K.; Frings, L.; Saar, J.; Carius, A.; Härle, M.; Steinhoff, B.J.; Schulze-Bonhage, A. The influence of antiepileptic drugs on cognition: A comparison of levetiracetam with topiramate. Epilepsy Behav. 2007, 10, 486–494. [Google Scholar] [CrossRef]
- Lamberty, Y.; Margineanu, D.G.; Klitgaard, H. Absence of Negative Impact of Levetiracetam on Cognitive Function and Memory in Normal and Amygdala-Kindled Rats. Epilepsy Behav. 2000, 1, 333–342. [Google Scholar] [CrossRef]
- Zwierzyńska, E.; Pietrzak, B. The differential effect of levetiracetam on memory and anxiety in rats. Epilepsy Behav. 2022, 136, 108917. [Google Scholar] [CrossRef]
- Zwierzyńska, E.; Klimczak, M.; Nasiadek, M.; Stragierowicz, J.; Pietrzak, B. Impact of levetiracetam and ethanol on memory, selected neurotransmitter levels, oxidative stress parameters, and essential elements in rats. Pharmacol. Rep. 2024, 76, 1363–1376. [Google Scholar] [CrossRef]
- Malone, D.T.; Jongejan, D.; Taylor, D.A. Cannabidiol reverses the reduction in social interaction produced by low dose Δ9-tetrahydrocannabinol in rats. Pharmacol. Biochem. Behav. 2009, 93, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zlatanova-Tenisheva, H.; Georgieva-Kotetarova, M.; Vilmosh, N.; Kandilarov, I.; Delev, D.; Dermendzhiev, T.; Kostadinov, I.D. Exploring the Anxiolytic, Antidepressant, and Immunomodulatory Effects of Cannabidiol in Acute Stress Rat Models. Appl. Biosci. 2025, 4, 4. [Google Scholar] [CrossRef]
- Lucindo, M.S.S.; Albuquerque, A.L.S.; Pereira, K.A.; Salgado, K.D.C.B.; Oliveira, L.A.M.; Engel, D.F.; Nogueira, K.O.P.C. Chronic cannabidiol administration modulates depressive and cognitive alterations induced by social isolation in male mice. Behav. Brain Res. 2025, 480, 115408. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Pathapati, N.; Schultz, S.T.; Nunn, M.C.; Pierce, B.L.; Sanchez, Y.R.; Murrell, M.D.; Ginsburg, B.C.; Onaivi, E.S.; Gould, G.G. Acute cannabidiol treatment enhances social interaction in adult male mice. Adv. Drug Alcohol Res. 2023, 3, 11163. [Google Scholar] [CrossRef] [PubMed]
- Cavichioli, A.M.; Santos-Silva, T.; Grace, A.A.; Guimarães, F.S.; Gomes, F.V. Levetiracetam Attenuates Adolescent Stress-induced Behavioral and Electrophysiological Changes Associated with Schizophrenia in Adult Rats. Schizophr. Bull. 2023, 49, 68–77. [Google Scholar] [CrossRef]
- Almeida, V.; Levin, R.; Peres, F.F.; Niigaki, S.T.; Calzavara, M.B.; Zuardi, A.W.; Hallak, J.E.; Crippa, J.A.; Abílio, V.C. Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 41, 30–35. [Google Scholar] [CrossRef]

| Treatment Group | On PTZ Days | On Non-PTZ Days |
|---|---|---|
| FC and MC | i.p.—sodium chloride 0.9% solution for infusion | p.o.—placebo tablet |
| FL and ML | i.p.—300 mg/kg body weight LEV solution for infusion | p.o.—300 mg/kg body weight LEV tablet |
| FLC10 and MLC10 | i.p.—300 mg/kg body weight LEV solution for infusion + p.o. 10 mg/kg body weight CBD pellet | p.o.—300 mg/kg body weight LEV tablet + 10 mg/kg body weight CBD pellet |
| FLC60 and MLC60 | i.p.—300 mg/kg body weight LEV solution for infusion + p.o. 60 mg/kg body weight CBD pellet | p.o.—300 mg/kg body weight LEV tablet + 60 mg/kg body weight CBD pellet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, E.; Miklós, N.; Frandeș, S.-D.; Kolcsar, M.; Gáll, Z. Cannabidiol Modulates the Effects of Levetiracetam on Seizure Parameters and Behavioral Outcomes in Pentylenetetrazol-Kindled Rats. Future Pharmacol. 2025, 5, 62. https://doi.org/10.3390/futurepharmacol5040062
Simon E, Miklós N, Frandeș S-D, Kolcsar M, Gáll Z. Cannabidiol Modulates the Effects of Levetiracetam on Seizure Parameters and Behavioral Outcomes in Pentylenetetrazol-Kindled Rats. Future Pharmacology. 2025; 5(4):62. https://doi.org/10.3390/futurepharmacol5040062
Chicago/Turabian StyleSimon, Emília, Noémi Miklós, Sorana-Denisa Frandeș, Melinda Kolcsar, and Zsolt Gáll. 2025. "Cannabidiol Modulates the Effects of Levetiracetam on Seizure Parameters and Behavioral Outcomes in Pentylenetetrazol-Kindled Rats" Future Pharmacology 5, no. 4: 62. https://doi.org/10.3390/futurepharmacol5040062
APA StyleSimon, E., Miklós, N., Frandeș, S.-D., Kolcsar, M., & Gáll, Z. (2025). Cannabidiol Modulates the Effects of Levetiracetam on Seizure Parameters and Behavioral Outcomes in Pentylenetetrazol-Kindled Rats. Future Pharmacology, 5(4), 62. https://doi.org/10.3390/futurepharmacol5040062










