Superoxide Anion Generation, Its Pathological Cellular and Molecular Roles and Pharmacological Targeting in Inflammatory Pain: Lessons from the Potassium Superoxide Model
Abstract
1. Introduction
2. ROS Biosynthesis and Physiological Function
2.1. ROS Generation Pathways
2.1.1. Mitochondrial ROS Generation
2.1.2. Cytoplasmic ROS Generation
Xanthine Oxidoreductase System (XOR)
Endoplasmic Reticulum
Iron-Dependent Reactions
Cytosolic Endogenous Antioxidant Enzymes
2.1.3. ROS Generation in the Membrane: NADPH Oxidase (NOX)
NOX1
NOX2
NOX3
NOX4
NOX5
DUOX1/2
2.2. Redox Signaling
2.2.1. ROS-Modulated Transcription Factors
Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)
NF-κB
Mitogen-Activated Proteins Kinase (MAPK)
Hypoxia-Inducible Factor 1 Alpha (HIF-1 α)
Forkhead Box O (FoxO) Factor
2.2.2. Kinases and Phosphatases Proteins
2.2.3. Epigenetic Redox Control
2.3. ROS Physiological Actions
2.3.1. Cell Proliferations
2.3.2. Cell Differentiation
2.3.3. Cell Migration
2.3.4. Cell Death
2.3.5. Immune Response
3. Molecular Mechanisms and Signaling Pathways Involved in Superoxide Anion-Induced Pain
4. Molecular Mechanisms and Signaling Pathways Involved in Potassium Superoxide (A O2•− Anion Donor)-Induced Pain
5. Therapeutic Approaches
5.1. Superoxide Anion-Induced Pain
5.2. Therapeutic Approaches Targeting Potassium Superoxide-Induced Pain
6. Clinical Prospects and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1O2 | Singlet Oxygen |
| 2-OG | 2-Oxoglutarate |
| 5-HT | Serotonin |
| 5-HT2 | 5-Hidroxitriptamina 2 |
| Acetyl-CoA | Acetylcoenzyme A |
| ADP | Adenosine Diphosphate |
| AIM2 | Absent In Melanoma 2 |
| AP-1 | Activator Protein 1 |
| APAF | Apoptotic Protease Activating Factor 1 |
| ARE | Antioxidant Response Elements |
| AKT | AKT serine/threonine kinase |
| ASC | Apoptosis-Associated Speck-Like Protein Containing a CARD |
| ASK | Apoptosis Signal-regulating Kinase |
| ATF-1 | Activating Transcription Factor 1 |
| ATP | Adenosine Triphosphate |
| BLT1 | Leukotriene B4 Receptor 1 |
| BK | Bradykinin |
| C/EBP-α/β/γ | CCAAT/Enhancer-Binding Alpha/Beta/Gamma |
| cFLIP | FLICE-like Inhibitory Protein |
| Ca2+ | Calcium Ion |
| CAT | Catalase |
| c-GAS | Cyclic GMP–AMP Synthase |
| CGD | Chronic Granulomatous Disease |
| cIAP | Cellular Inhibitor of Apoptosis Protein |
| CMKII | Ca2+/Calmodulin-Independent Protein Kinase II |
| CNS | Central Nervous System |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| COX | Cyclooxygenase |
| CPIP | Chronic Post-Ischemia Pain |
| CRPS | Complex Regional Pain Syndrome |
| c-Src | Cellular sarcoma (proto-oncogene tyrosine-protein kinase Src) |
| CYLD | Cylindromatosis |
| Cys | Cysteine |
| CUL3 | Cullin-3 |
| Cu | Copper |
| Cu2+ | Copper Ion |
| DAMP | Damage-Associated Molecular Patterns |
| dATP | Deoxyadenosine Triphosphate |
| DC | Dendritic Cells |
| DISC | Death-Inducing Signaling Complex |
| DMM | Destabilization of the Medial Meniscus |
| DNA | Deoxyribonucleic Acid |
| DRG | Dorsal Root Ganglia |
| DUOX1 | Dual oxidase 1 |
| DUOX2 | Dual oxidase 2 |
| E2F1 | E2F Transcription Factor 1 |
| EC1 | Oxidoreductase Class 1 |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| Elfo-1 | E74-Like Factor 1 |
| eIF2B | Eukaryotic Initiation Factor 2B |
| Elk1 | ETS Like-1 protein |
| EP 1-4 | E-type Prostanoid 1-4 Receptor |
| ER | Endoplasmic Reticulum |
| ERK1/2 | Extracellular Signal-Regulated Kinase 1/2 |
| ERO1 | Oxidoreductase-1 |
| ET | Endothelin |
| ET-1 | Endothelin 1 |
| ET-2 | Endothelin 2 |
| ETC | Electron Transport Chain |
| FAD | Flavin Adenine Dinucleotide |
| FADH2 | Reduced Flavin Adenine Dinucleotide |
| FADD | Fas-Associated Death Domain |
| FasL | Fas Ligand |
| FBD | FAD-Binging Domain |
| Fe | Iron |
| Fe2+ | Ferrous Ion |
| Fe3+ | Ferric ion |
| fMLF | N-Formyl-Met-Leu-Phe |
| fMLP | Formyl-Methionyl-Leucyl-Phenylalanine |
| FoXO | Forkhead Box O |
| GATA4 | GATA Binding Protein 4 |
| GATA6 | GATA Binding Protein 6 |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GCN5 | General Control Non-repressed protein 5 |
| GDP | Guanosine Diphosphate |
| GFAP | Glial Fibrillary Acidic Protein |
| GP-130 | Glycoprotein 130 |
| GPx | Glutathione Peroxidase |
| GPx-1 | Glutathione Peroxidase-1 |
| GPX4 | Glutathione Peroxidase 4 |
| GSH | Reduced Glutathione |
| GSSG | Oxidized Glutathione |
| GRB2/SOS | Growth Factor Receptor-Bound Protein 2/Guanine-Nucleotide Exchange Factor |
| GTP | Guanosine Triphosphate |
| H+ | Protons |
| H1/2 | Histamine Receptors |
| H2O | Water |
| H2O2 | Hydrogen Peroxide |
| HAT | Histone Acetylases |
| HDAC | Histone Deacetylases |
| HIF-1α | Hypoxia-Inducible Factor 1 Alpha |
| HO-1 | Heme-Oxygenase 1 |
| HOCl | Hypochlorous Acid |
| HOXA9 | Homeobox A9 |
| HOXA10 | Homeobox A10 |
| hPASMC | Human Lung Vascular Smooth Muscle Cells |
| HRAS | v-Ha-Ras |
| 5-HT2 | 5-Hidroxitriptamin 2 |
| hUVEC | Human Umbilical Vein Endothelial Cells |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| ICSBP | Interferon Consensus Sequence-Binding Protein |
| IFN | Interferon |
| IKK | IκB Kinase |
| IL | Interleukin |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| IL-17A | Interleukin-17A |
| IL-1β | Interleukin-1β |
| IL-1R | Interleukin-1 Receptor |
| IL-5R | Interleukin-5 Receptor |
| IL-17AR | Interleukin-17A Receptor |
| iPSC | Inducible Pluripotent Stem Cells |
| Iba1 | Ionized Calcium-Binding Adaptor Molecule 1 |
| IRAK1/2 | Interleukin-1 Receptor-Associated Kinase 1/2 |
| IRF1/2 | Interferon Regulatory Factor 1/2 |
| IRS | Insulin Receptor and its Substrates |
| JNK | c-Jun N-Terminal Kinases |
| Keap1 | Kelch-Like ECH-Associated Protein 1 |
| KO2 | Potassium Superoxide |
| K+ | Potassium Ion |
| KOH | Potassium Hydroxide |
| L-NAME | Nω-Nitro-L-arginine methyl ester |
| LPS | Lipopolysaccharide |
| LTB4 | Leukotriene B4 |
| MAPK | Mitogen-Activated Proteins Kinase |
| MDA | Malondialdehyde |
| Meis1 | Myeloid Ecotropic Viral Integration Site 1 |
| MEF-2B | Myocyte Enhancer Factor 2B |
| MIA | Melanoma Inhibitory Activity protein |
| MKLK | Mixed Lineage Kinase Domain-Like protein |
| MMO | Microsomal Mono-Oxidase |
| Mn | Manganese |
| MnL4 | Manganese Transporter Protein MntH-like 4 |
| MMP9 | Metalloproteinase 9 |
| MOMP | Mitochondrial Outer Membrane Permeability |
| MPO | Myeloperoxidade |
| mRNA | Messenger Ribonucleic Acid |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| mtDNA | Mitochondrial DNA |
| mtROS | Mitochondrial ROS |
| Na2+ | Sodium Ion |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| NADH | Reduced Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NBD | NADPH-Binding Domain |
| Nav | Voltage-Gated Sodium Channels |
| NET | Neutrophil Extracellular Traps |
| NF-κB | Nuclear Factor Kappa B |
| NGF | Nerve Growth Factor |
| NK | Natural Killer |
| NLRP3 | NLR-Family Pyrin Domain Containing 3 |
| NMDA | N-Methyl-D-Aspartate |
| NO● | Nitric Oxide |
| NOX | NADPH Oxidase |
| NOXO1 | NADPH Oxidase Organizer 1 |
| NOXA1 | NADPH Oxidase Activator 1 |
| NR1 | Nuclear Receptor subfamily 1 |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| O2 | Oxygen |
| O2•− | Superoxide Anion |
| OA | Osteoarthritis |
| OH● | Hydroxyl Radical |
| OH− | Hydroxide Ion |
| ONOO− | Peroxynitrite |
| OX-mtDNA | Oxidation of mtDNA |
| OXPHOS | Oxidative Phosphorylation |
| PAMP | Pathogen-Associated Molecular Patterns |
| PARP | Poly(ADP-ribose) Polymerase |
| PBN | Phenyl-N-tert-Butylnitrone |
| PBX1 | Paired Box 1 |
| pCamKII | Protein Kinase Ca2+/Calmodulin-Dependent Protein Kinase II |
| PDI | Protein Disulfide Isomerase |
| PD1 | Disulfide Isomerase Protein |
| PDGF | Plaque-Derived Growth Factor |
| PDTC | Pyrrolidine Dithiocarbonate |
| PG | Prostaglandins |
| PGE2 | Prostaglandin E2 |
| PHD | Prolyl Hydroxylase Domain |
| Pi | Inorganic Phosphate |
| PK | Protein Kinase |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| PKD | Protein Kinase D |
| PP | Protein Phosphatases |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PREPROET-1 | Preproendothelin-1 |
| PTP1B | Protein Tyrosine Phosphatase 1B |
| PUFA | Polyunsaturated fatty acid |
| RANK | Receptor Activator of Nuclear Factor κB |
| RANKL | RANK Ligand |
| RE | Endoplasmic Reticulum |
| RIP1 | Receptor-Interacting Protein 1 |
| RIPK | Receptor-Interacting Protein Kinase |
| RhoGDI | Rho GDP Dissociation Inhibitor |
| RNS | Reactive Nitrogen Species |
| RO● | Alkoryl |
| ROO● | Peroxyl Radical |
| ROS | Reactive Oxygen Species |
| RSK | Ribosomal S6 Kinase |
| RTK | Receptor Tyrosine Kinase |
| SCI | Spinal Thoracic Contusion Injury |
| SOD | Superoxide Dismutase |
| SODm | SOD Mimetic |
| SOD-NP | SOD-Loaded Porous Polymersome Nanoparticles |
| SOH | Sulfenic Acid |
| SP | Sodium Propionate |
| SPI1 | Spleen Focus-Forming Virus Proviral Integration Oncogene |
| SNT | Spinal Nerve Transection |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| TAB1/TAK1 | TAK1-Binding Protein 1/Transforming Growth Factor-β-Activated Kinase 1 |
| TCA | Tricarboxylic Acid |
| TEMPOL | 4-Hydroxy-2,2,6,6-Tetramethylpiperidine-1-Oxyl |
| Tsk4/5 | Tyrosine Kinase Substrate 4/5 |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
| TNFR | Tumor Necrosis Factor Receptor |
| TNPO | Transportin |
| TRAF | TNF Receptor-Associated Factor |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| TRADD | TNF Receptor-Associated Death Domain |
| TRAF2 | TNF Receptor-Associated Factor 2 |
| TRKA | Tropomyosin Receptor Kinase A |
| TRPA1 | Transient Receptor Potential Subfamily Ankyrine 1 |
| TRP | Transient Receptor Potential |
| TRPV1 | Transient Receptor Potential Subfamily Vanilloid 1 |
| Trx1 | Thioredoxin 1 |
| UCP | Uncoupling Proteins |
| UV | Ultraviolet |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
| VHL | Von-Hippel-Lindau |
| WT | Wide-Type |
| XDH | Xanthine Dehydrogenase |
| XO | Xanthine Oxidase |
| XOR | Xanthine Oxidoreductase |
| YY1 | Yin Yang 1 |
| Zn | Zinc |
| Zn2+ | Zinc Ion |
References
- Buetler, T.M.; Krauskopf, A.; Ruegg, U.T. Role of superoxide as a signaling molecule. News Physiol. Sci. 2004, 19, 120–123. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species (ROS), oxygen radicals and antioxidants: Where are we now, where is the field going and where should we go? Biochem. Biophys. Res. Commun. 2022, 633, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Przedborski, S.; Vila, M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: A tool to explore the pathogenesis of Parkinson’s disease. Ann. New York Acad. Sci. 2003, 991, 189–198. [Google Scholar] [CrossRef]
- Hernandes, M.S.; Café-Mendes, C.C.; Britto, L.R.G. NADPH Oxidase and the Degeneration of Dopaminergic Neurons in Parkinsonian Mice. Oxid. Med. Cell Longev. 2013, 2013, 157857. [Google Scholar] [CrossRef]
- Gamba, P.; Leonarduzzi, G.; Tamagno, E.; Guglielmotto, M.; Testa, G.; Sottero, B.; Gargiulo, S.; Biasi, F.; Mauro, A.; Viña, J.; et al. Interaction between 24-hydroxycholesterol, oxidative stress, and amyloid-β in amplifying neuronal damage in Alzheimer’s disease: Three partners in crime. Aging Cell 2011, 10, 403–417. [Google Scholar] [CrossRef]
- Park, L.; Zhou, P.; Pitstick, R.; Capone, C.; Anrather, J.; Norris, E.H.; Younkin, L.; Younkin, S.; Carlson, G.; McEwen, B.S.; et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2008, 105, 1347–1352. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, M.; Zhou, B.; Yoshii, A.; Bugg, D.; Villet, O.; Sahu, A.; Olson, G.S.; Davis, J.; Tian, R. Mitochondrial dysfunction in macrophages promotes inflammation and suppresses repair after myocardial infarction. J. Clin. Investig. 2023, 133, e159498. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- Brandes, R.P. Vascular functions of NADPH oxidases. Hypertension 2010, 56, 17–21. [Google Scholar] [CrossRef]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; de Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH Oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef]
- Meyer, J.W.; Schmitt, M.E. A central role for the endothelial NADPH oxidase in atherosclerosis. FEBS Lett. 2000, 472, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.K.; Warnholtz, A.; et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001, 88, e14–e22. [Google Scholar] [CrossRef] [PubMed]
- Balmes, J.R. Stress is in the air: Ambient reactive oxygen species and COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Henricks, P.A.J.; Nijkamp, F.P. Reactive Oxygen Species as Mediators in Asthma. Pulm. Pharmacol. Ther. 2001, 14, 409–421. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Aspects Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease. Oxid. Med. Cell Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef] [PubMed]
- Artero, N.A.; Manchope, M.F.; Carvalho, T.T.; Saraiva-Santos, T.; Bertozzi, M.M.; Carneiro, J.A.; Franciosi, A.; Dionisio, A.M.; Zaninelli, T.H.; Fattori, V.; et al. Hesperidin Methyl Chalcone Reduces the Arthritis Caused by TiO2 in Mice: Targeting Inflammation, Oxidative Stress, Cytokine Production, and Nociceptor Sensory Neuron Activation. Molecules 2023, 28, 872. [Google Scholar] [CrossRef] [PubMed]
- Laniak, O.T.; Winans, T.; Patel, A.; Park, J.; Perl, A. Redox Pathogenesis in Rheumatic Diseases. ACR Open Rheumatol. 2024, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, D.; Cao, X.; Ye, Q.; Wang, Q.; Zhang, M.; Xiao, C. The Role of Reactive Oxygen Species in the Rheumatoid Arthritis-Associated Synovial Microenvironment. Antioxidants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Shah, D.; Mahajan, N.; Sah, S.; Nath, S.K.; Paudyal, B. Oxidative stress and its biomarkers in systemic lupus erythematosus. J. Biomed. Sci. 2014, 21, 23. [Google Scholar] [CrossRef]
- Diny, N.; Radtke, D.; Choksi, Y.; Kano, G.; Tomii, T. Eosinophils in inflammatory bowel disease pathogenesis: An ROS-centric view. Front. Allergy 2025, 6, 1608202. [Google Scholar] [CrossRef]
- Wickens, A.P. Ageing and the free radical theory. Respir. Physiol. 2001, 128, 379–391. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Renaudin, X. Reactive oxygen species and DNA damage response in cancer. Int. Rev. Cell Mol. Biol. 2021, 364, 139–161. [Google Scholar] [CrossRef]
- Meitzler, J.L.; Antony, S.; Wu, Y.; Juhasz, A.; Liu, H.; Jiang, G.; Lu, J.; Roy, K.; Doroshow, J.H. NADPH Oxidases: A Perspective on Reactive Oxygen Species Production in Tumor Biology. Antioxid. Redox Signal. 2014, 20, 2873–2889. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Lourenco-Gonzalez, Y.; Fattori, V.; Domiciano, T.P.; Rossaneis, A.C.; Borghi, S.M.; Zaninelli, T.H.; Bernardy, C.C.F.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; et al. Repurposing of the nootropic drug vinpocetine as an analgesic and anti-inflammatory agent: Evidence in a mouse model of superoxide anion-triggered inflammation. Mediat. Inflamm. 2019, 2019, 6481812. [Google Scholar] [CrossRef]
- Bernardy, C.C.F.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Calixto-Campos, C.; Carvalho, T.T.; Fattori, V.; Borghi, S.M.; Casagrande, R.; Verri, W.A. Tempol, a Superoxide Dismutase Mimetic Agent, Inhibits Superoxide Anion-Induced Inflammatory Pain in Mice. Biomed. Res. Int. 2017, 2017, 9584819. [Google Scholar] [CrossRef] [PubMed]
- Silva, Á.J.C.; de Lavor, M.S.L. Nitroxidative Stress, Cell—Signaling Pathways, and Manganese Porphyrins: Therapeutic Potential in Neuropathic Pain. Int. J. Mol. Sci. 2025, 26, 2050. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of Reactive Oxygen and Nitrogen Species in Pain. Free Radic. Biol. Med. 2011, 51, 951. [Google Scholar] [CrossRef] [PubMed]
- Kallenborn-Gerhardt, W.; Schröder, K.; Schmidtko, A. NADPH Oxidases in Pain Processing. Antioxidants 2022, 11, 1162. [Google Scholar] [CrossRef]
- Carrasco, C.; Naziroglu, M.; Rodríguez, A.B.; Pariente, J.A. Neuropathic pain: Delving into the oxidative origin and the possible implication of transient receptor potential channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef]
- Jakob, H.; Leininger, S.; Lehmann, T.; Jacobi, S.; Gutewort, S. Peroxo Compounds, Inorganic; Ullmann’s Encyclopedia of Industrial Chemistry: Berlin, Germany, 2007. [Google Scholar] [CrossRef]
- Serafim, K.G.G.; Navarro, S.A.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Fattori, V.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; Casagrande, R.; Verri, W.A. Bosentan, a mixed endothelin receptor antagonist, inhibits superoxide anion-induced pain and inflammation in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 1211–1221. [Google Scholar] [CrossRef]
- Yamacita-Borin, F.Y.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Fattori, V.; Alves-Filho, J.C.; Cunha, F.Q.; Cunha, T.M.; Casagrande, R.; Verri, W.A. Superoxide anion-induced pain and inflammation depends on TNFα/TNFR1 signaling in mice. Neurosci. Lett. 2015, 605, 53–58. [Google Scholar] [CrossRef]
- Maioli, N.; Zarpelon, A.; Mizokami, S.; Calixto-Campos, C.; Guazelli, C.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Carvalho, T.T.; Manchope, M.F.; Ferraz, C.R.; et al. The superoxide anion donor, potassium superoxide, induces pain and inflammation in mice through production of reactive oxygen species and cyclooxygenase-2. Braz. J. Med. Biol. Res. 2015, 48, 321–331. [Google Scholar] [CrossRef]
- Takahashi, H.; Tran, P.O.T.; LeRoy, E.; Harmon, J.S.; Tanaka, Y.; Robertson, R.P. D-glyceraldehyde causes production of intracellular peroxide in pancreatic islets, oxidative stress, and defective beta cell function via non-mitochondrial pathways. J. Biol. Chem. 2004, 279, 37316–37323. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Shizuuchi, S.; Watanabe, H.; Hayase, F. Cytotoxicity and Oxidative Stress Induced by the Glyceraldehyde-related Maillard Reaction Products for HL-60 Cells. Biosci. Biotechnol. Biochem. 2004, 68, 333–340. [Google Scholar] [CrossRef]
- Samsuzzaman, M.; Kim, S.Y. Anti-Fibrotic Effects of DL-Glyceraldehyde in Hepatic Stellate Cells via Activation of ERK-JNK-Caspase-3 Signaling Axis. Biomol. Ther. 2023, 31, 425–433. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef]
- Hadrava Vanova, K.; Kraus, M.; Neuzil, J.; Rohlena, J. Mitochondrial complex II and reactive oxygen species in disease and therapy. Redox Report. 2020, 25, 26–32. [Google Scholar] [CrossRef]
- Antonov, A.S.; Lukashev, M.E.; Romanov, Y.A.; Tkachuk, V.A.; Repin, V.S.; Smirnov, V.N. Morphological alterations in endothelial cells from human aorta and umbilical vein induced by forskolin and phorbol 12-myristate 13-acetate: A synergistic action of adenylate cyclase and protein kinase C activators. Proc. Natl. Acad. Sci. USA 1986, 83, 9704. [Google Scholar] [CrossRef]
- Flaherty, R.L.; Owen, M.; Fagan-Murphy, A.; Intabli, H.; Healy, D.; Patel, A.; Allen, M.C.; Patel, B.A.; Flint, M.S. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017, 19, 35. [Google Scholar] [CrossRef]
- Fussell, K.C.; Udasin, R.G.; Smith, P.J.S.; Gallo, M.A.; Laskin, J.D. Catechol metabolites of endogenous estrogens induce redox cycling and generate reactive oxygen species in breast epithelial cells. Carcinogenesis 2011, 32, 1285. [Google Scholar] [CrossRef]
- Deo, S.H.; Jenkins, N.T.; Padilla, J.; Parrish, A.R.; Fadel, P.J. Norepinephrine increases NADPH oxidase-derived superoxide in human peripheral blood mononuclear cells via α-adrenergic receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The Hallmarks of Mitochondrial Dysfunction in Chronic Kidney Disease. Kidney Int. 2017, 92, 1051. [Google Scholar] [CrossRef]
- Guo, R.; Gu, J.; Zong, S.; Wu, M.; Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 2018, 41, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress. Antioxidants 2022, 11, 322. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol. Lett. 2022, 44, 1–22. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Nishino, T.; Okamoto, K.; Kawaguchi, Y.; Hori, H.; Matsumura, T.; Eger, B.T.; Pai, E.F.; Nishino, T. Mechanism of the conversion of xanthine dehydrogenase to xanthine oxidase: Identification of the two cysteine disulfide bonds and crystal structure of a non-convertible rat liver xanthine dehydrogenase mutant. J. Biol. Chem. 2005, 280, 24888–24894. [Google Scholar] [CrossRef]
- Enroth, C.; Eger, B.T.; Okamoto, K.; Nishino, T.; Nishino, T.; Pai, E.F. Crystal structures of bovinemilk xanthine dehydrogenase and xanthine oxidase: Structure-based mechanism of conversion. Proc. Natl. Acad. Sci. USA 2000, 97, 10723–10728. [Google Scholar] [CrossRef] [PubMed]
- Hille, R. Structure and function of xanthine oxidoreductase. Eur. J. Inorg. Chem. 2006, 2006, 1913–1926. [Google Scholar] [CrossRef]
- Harrison, R. Physiological roles of xanthine oxidoreductase. Drug Metab. Rev. 2004, 36, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Vorbach, C.; Harrison, R.; Capecchi, M.R. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003, 24, 512–517. [Google Scholar] [CrossRef]
- Kramer, B.; Ferrari, D.M.; Klappa, P.; Pöhlmann, N.; Söling, H.-D. Functional roles and efficiencies of the thioredoxin boxes of calcium-binding proteins 1 and 2 in protein folding. Biochem. J. 2001, 357, 83–95. [Google Scholar] [CrossRef]
- Shergalis, A.G.; Hu, S.; Bankhead, A.; Neamati, N. Role of the ERO1-PDI interaction in oxidative protein folding and disease. Pharmacol. Ther. 2020, 210, 107525. [Google Scholar] [CrossRef]
- Yoboue, E.D.; Sitia, R.; Simmen, T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 2018, 9, 331. [Google Scholar] [CrossRef]
- Liu, W.; Huan, L.; Zhang, C.; Yin, R.; Ouyang, Z.; Wei, Y. Amelioration of Acetaminophen-Induced Hepatic Oxidative Stress and Inflammation by RNAi Targeting Cyp2e1 In Vivo. Curr. Issues Mol. Biol. 2025, 47, 372. [Google Scholar] [CrossRef]
- Keyer, K.; Imlay, J.A. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 1996, 93, 13635–13640. [Google Scholar] [CrossRef]
- Halliwell, B.; Cross, C.E. Oxygen-derived species: Their relation to human disease and environmental stress. Environ. Health Perspect. 1994, 102, 5–12. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.E.; Shakarjian, M.; Kim, H.D.; Laskin, J.D.; Vetrano, A.M. Mechanisms of oxidant generation by catalase. Ann. N Y Acad. Sci. 2010, 1203, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Rajasekaran, N.S.; Connell, P.; Christians, E.S.; Yan, L.J.; Taylor, R.P.; Orosz, A.; Zhang, X.Q.; Stevenson, T.J.; Peshock, R.M.; Leopold, J.A.; et al. Human αB-Crystallin Mutation Causes Oxido-Reductive Stress and Protein Aggregation Cardiomyopathy in Mice. Cell 2007, 130, 427–439. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Bánfi, B.; Clark, R.A.; Steger, K.; Krause, K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003, 278, 3510–3513. [Google Scholar] [CrossRef]
- Suh, Y.-A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef]
- Kracun, D.; Lopes, L.R.; Cifuentes-Pagano, E.; Pagano, P.J. Nadph Oxidases: Redox Regulation of Cell Homeostasis and Disease. Physiol. Rev. 2025, 105, 1291–1428. [Google Scholar] [CrossRef]
- Manea, A.; Tanase, L.I.; Raicu, M.; Simionescu, M. JAK/STAT signaling pathway regulates Nox1 and Nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 105–112. [Google Scholar] [CrossRef]
- Kuwano, Y.; Kawahara, T.; Yamamoto, H.; Teshima-Kondo, S.; Tominaga, K.; Masuda, K.; Kishi, K.; Morita, K.; Rokutan, K. Interferon-γ activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, 433–443. [Google Scholar] [CrossRef]
- Fenyo, I.M.; Florea, I.C.; Raicu, M.; Manea, A. Tyrphostin AG490 reduces NAPDH oxidase activity and expression in the aorta of hypercholesterolemic apolipoprotein E-deficient mice. Vascul Pharmacol. 2011, 54, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Brewer, A.C.; Sparks, E.C.; Shah, A.M. Transcriptional regulation of the NADPH oxidase isoform, Nox1, in colon epithelial cells: Role of GATA-binding factor(s). Free Radic. Biol. Med. 2006, 40, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Tanase, L.I.; Raicu, M.; Simionescu, M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-κB in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2010, 396, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.O.; Katsuyama, M.; Kanda, S.; Kaneko, T.; Iwata, K.; Ibi, M.; Matsuno, K.; Kakehi, T.; Cui, W.; Sasaki, M.; et al. The AP-1 site is essential for the promoter activity of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme: Possible involvement of the ERK1/2-JunB pathway. Biochem. Biophys. Res. Commun. 2008, 374, 351–355. [Google Scholar] [CrossRef]
- Manea, A.; Manea, S.A.; Gafencu, A.V.; Raicu, M.; Simionescu, M. AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: Role of p22phox subunit. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 878–885. [Google Scholar] [CrossRef]
- Rodríguez, A.I.; Csányi, G.; Ranayhossaini, D.J.; Feck, D.M.; Blose, K.J.; Assatourian, L.; Vorp, D.A.; Pagano, P.J. MEF2B-Nox1 signaling is critical for stretch-induced phenotypic modulation of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 430–438. [Google Scholar] [CrossRef]
- Maitra, U.; Singh, N.; Gan, L.; Ringwood, L.; Li, L. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J. Biol. Chem. 2009, 284, 35403–35411. [Google Scholar] [CrossRef]
- Manea, S.A.; Todirita, A.; Raicu, M.; Manea, A. C/EBP transcription factors regulate NADPH oxidase in human aortic smooth muscle cells. J. Cell Mol. Med. 2014, 18, 1467–1477. [Google Scholar] [CrossRef]
- Katsuyama, M.; FAN, C.; Arakawa, N.; Nishinaka, T.; Miyagishi, M.; Taira, K.; Yabe-Nishimura, C. Essential role of ATF-1 in induction of NOX1, a catalytic subunit of NADPH oxidase: Involvement of mitochondrial respiratory chain. Biochem. J. 2005, 386, 255–261. [Google Scholar] [CrossRef]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.C.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48, e12951. [Google Scholar] [CrossRef] [PubMed]
- Knaus, U.G.; Heyworth, P.G.; Evans, T.; Curnutte, J.T.; Bokoch, G.M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science 1991, 254, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Abo, A.; Pick, E.; Hall, A.; Totty, N.; Teahan, C.G.; Segal, A.W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 1991, 353, 668–670. [Google Scholar] [CrossRef]
- Dang, P.M.C.; Cross, A.R.; Babior, B.M. Assembly of the neutrophil respiratory burst oxidase: A direct interaction between p67phox and cytochrome b558. Proc. Natl. Acad. Sci. USA 2001, 98, 3001–3005. [Google Scholar] [CrossRef]
- Nauseef, W.M. Myeloperoxidase in human neutrophil host defence. Cell Microbiol. 2014, 16, 1146–1155. [Google Scholar] [CrossRef]
- Gauss, K.A.; Nelson-Overton, L.K.; Siemsen, D.W.; Gao, Y.; DeLeo, F.R.; Quinn, M.T. Role of NF-κB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-α. J. Leukoc. Biol. 2007, 82, 729–741. [Google Scholar] [CrossRef]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-κB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef]
- Teissier, E.; Nohara, A.; Chinetti, G.; Paumelle, R.; Cariou, B.; Fruchart, J.-C.; Brandes, R.P.; Shah, A.; Staels, B. Peroxisome proliferator-activated receptor α induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-α activation properties. Circ. Res. 2004, 95, 1174–1182. [Google Scholar] [CrossRef]
- Diebold, I.; Petry, A.; Sabrane, K.; Djordjevic, T.; Hess, J.; Görlach, A. The HIF1 target gene NOX2 promotes angiogenesis through urotensin-II. J. Cell. Sci. 2012, 125, 956–964. [Google Scholar] [CrossRef]
- Eklund, E.A.; Jalava, A.; Kakar, R. Elf-1 and PU.1 induce expression of gp91(phox) via a promoter element mutated in a subset of chronic granulomatous disease patients. Blood 1998, 91, 2223–2234. [Google Scholar] [CrossRef]
- Eklund, E.A.; Luo, W.; Skalnik, D.G. Recruitment of CREB-binding protein by PU.1, IFN-regulatory factor-1, and the IFN consensus sequence-binding protein is necessary for IFN-γ-induced p67phox and gp91phox expression. J. Immunol. 1999, 163, 6095–6105. [Google Scholar] [CrossRef] [PubMed]
- Bei, L.; Lu, Y.F.; Eklund, E.A. HOXA9 activates transcription of the gene encoding gp91Phox during myeloid differentiation. J. Biol. Chem. 2005, 280, 12359–12370. [Google Scholar] [CrossRef] [PubMed]
- Bánfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004, 279, 46065–46072. [Google Scholar] [CrossRef] [PubMed]
- Diebold, I.; Petry, A.; Hess, J.; Görlach, A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell. 2010, 21, 2087–2096. [Google Scholar] [CrossRef]
- Zhang, L.; Sheppard, O.R.; Shah, A.M.; Brewer, A.C. Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic. Biol. Med. 2008, 45, 679–685. [Google Scholar] [CrossRef]
- Manea, A.; Manea, S.-A.; Todirita, A.; Albulescu, I.C.; Raicu, M.; Sasson, S.; Simionescu, M. High-glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARα and PPARβ/δ. Cell Tissue Res. 2015, 361, 593–604. [Google Scholar] [CrossRef]
- Hwang, J.; Kleinhenz, D.J.; Lassègue, B.; Griendling, K.K.; Dikalov, S.; Hart, C.M. Peroxisome proliferator-activated receptor-γ ligands regulate endothelial membrane superoxide production. Am. J. Physiol. Cell Physiol. 2005, 288, 899–905. [Google Scholar] [CrossRef]
- Serrander, L.; Jaquet, V.; Bedard, K.; Plastre, O.; Hartley, O.; Arnaudeau, S.; Demaurex, N.; Schlegel, W.; Krause, K. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 2007, 89, 1159–1167. [Google Scholar] [CrossRef]
- Fulton, D.J.R. Nox5 and the regulation of cellular function. Antioxid. Redox Signal 2009, 11, 2443–2452. [Google Scholar] [CrossRef]
- Bánfi, B.; Molnár, G.; Maturana, A.; Steger, K.; Hegedûs, B.; Demaurex, N.; Krause, K.-H. A Ca2+-activated NADPH Oxidase in Testis, Spleen, and Lymph Nodes. J. Biol. Chem. 2001, 276, 37594–37601. [Google Scholar] [CrossRef]
- Belaiba, R.; Djordjevic, T.; Petry, A.; Diemer, K.; Bonello, S.; Banfi, B.; Hess, J.; Pogrebniak, A.; Bickel, C.; Gorlach, A. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 2007, 42, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.A.; Savelieff, M.G.; Eid, A.A.; Feldman, E.L. Nox, Nox, Are You There? The Role of NADPH Oxidases in the Peripheral Nervous System. Antioxid. Redox Signal. 2022, 37, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Manea, S.A.; Florea, I.C.; Luca, C.M.; Raicu, M. Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic. Biol. Med. 2012, 52, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D.; Kawahara, T.; Diebold, B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007, 43, 319–331. [Google Scholar] [CrossRef]
- Wu, J.X.; Liu, R.; Song, K.; Chen, L. Structures of human dual oxidase 1 complex in low-calcium and high-calcium states. Nat. Commun. 2021, 12, 155. [Google Scholar] [CrossRef]
- Sun, J. Structures of mouse DUOX1–DUOXA1 provide mechanistic insights into enzyme activation and regulation. Nat. Struct. Mol. Biol. 2020, 27, 1086–1093. [Google Scholar] [CrossRef]
- Behring, J.B.; van der Post, S.; Mooradian, A.D.; Egan, M.J.; Zimmerman, M.I.; Clements, J.L.; Bowman, G.R.; Held, J.M. Spatial and temporal alterations in protein structure by EGF regulate cryptic cysteine oxidation. Sci. Signal. 2020, 13, eaay7315. [Google Scholar] [CrossRef]
- Sanchez, R.; Riddle, M.; Woo, J.; Momand, J. Prediction of reversibly oxidized protein cysteine thiols using protein structure properties. Protein Sci. 2008, 17, 473–481. [Google Scholar] [CrossRef]
- Go, Y.M.; Chandler, J.D.; Jones, D.P. The cysteine proteome. Free Radic. Biol. Med. 2015, 84, 227–245. [Google Scholar] [CrossRef]
- Nordzieke, D.E.; Medraño-Fernandez, I. The Plasma Membrane: A Platform for Intra- and Intercellular Redox Signaling. Antioxidants 2018, 7, 168. [Google Scholar] [CrossRef]
- Matsui, R.; Ferran, B.; Oh, A.; Croteau, D.; Shao, D.; Han, J.; Pimentel, D.R.; Bachschmid, M.M. Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation. Antioxid. Redox Signal. 2020, 32, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Keenan, E.K.; Zachman, D.K.; Hirschey, M.D. Discovering the landscape of protein modifications. Mol. Cell 2021, 81, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Saito, R.; Suzuki, T.; Hiramoto, K.; Asami, S.; Naganuma, E.; Suda, H.; Iso, T.; Yamamoto, H.; Morita, M.; Baird, L.; et al. Characterizations of Three Major Cysteine Sensors of Keap1 in Stress Response. Mol. Cell Biol. 2016, 36, 271–284. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef]
- Blank, V. Small Maf Proteins in Mammalian Gene Control: Mere Dimerization Partners or Dynamic Transcriptional Regulators? J. Mol. Biol. 2008, 376, 913–925. [Google Scholar] [CrossRef]
- Adinolfi, S.; Patinen, T.; Deen, A.J.; Pitkänen, S.; Härkönen, J.; Kansanen, E.; Küblbeck, J.; Levonen, A.-L. The KEAP1-NRF2 pathway: Targets for therapy and role in cancer. Redox Biol. 2023, 63, 102726. [Google Scholar] [CrossRef]
- Bell, K.F.; Al-Mubarak, B.; Martel, M.-A.; McKay, S.; Wheelan, N.; Hasel, P.; Márkus, N.M.; Baxter, P.; Deighton, R.F.; Serio, A.; et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat. Commun. 2015, 6, 7066. [Google Scholar] [CrossRef]
- Jiwaji, Z.; Hardingham, G.E. The consequences of neurodegenerative disease on neuron-astrocyte metabolic and redox interactions. Neurobiol. Dis. 2023, 185, 106255. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Hoffmann, A. Crosstalk via the NF-κB signaling system. Cytokine Growth Factor. Rev. 2008, 19, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB signaling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 443978. [Google Scholar] [CrossRef] [PubMed]
- Halvey, P.J.; Hansen, J.M.; Johnson, J.M.; Go, Y.M.; Samali, A.; Jones, D.P. Selective oxidative stress in cell nuclei by nuclear-targeted D-amino acid oxidase. Antioxid. Redox Signal 2007, 9, 807–816. [Google Scholar] [CrossRef]
- Reynaert, N.L.; Van Der Vliet, A.; Guala, A.S.; McGovern, T.; Hristova, M.; Pantano, C.; Heintz, N.H.; Heim, J.; Ho, Y.-S.; Matthews, D.E.; et al. Dynamic redox control of NF-κB through glutaredoxin-regulated S-glutathionylation of inhibitory κB kinase β. Proc. Natl. Acad. Sci. USA 2006, 103, 13086–13091. [Google Scholar] [CrossRef]
- Kumar, S.; Vinayak, M. NADPH oxidase1 inhibition leads to regression of central sensitization during formalin induced acute nociception via attenuation of ERK1/2-NFκB signaling and glial activation. Neurochem. Int. 2020, 134, 104652. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Lutsenko, S.V.; Terentiev, A.A. Reactive oxygen and nitrogen species–induced protein modifications: Implication in carcinogenesis and anticancer therapy. Cancer Res. 2018, 78, 6040–6047. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Pacquelet, S.; Johnson, J.L.; Ellis, B.A.; Brzezinska, A.A.; Lane, W.S.; Munafo, D.B.; Catz, S.D. Cross-talk between IRAK-4 and the NADPH oxidase. Biochem. J. 2007, 403, 451–461. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, D.; Zhang, H.; Yao, H.; Wang, Y. Hypoxia-Inducible Factor-1, A Potential Target to Treat Acute Lung Injury. Oxid. Med. Cell Longev. 2020, 2020, 8871476. [Google Scholar] [CrossRef]
- Priya Dharshini, L.C.; Vishnupriya, S.; Sakthivel, K.M.; Rasmi, R.R. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms. Cell Signal. 2020, 72, 109670. [Google Scholar] [CrossRef]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive oxygen species formation in the brain at different oxygen levels: The role of hypoxia inducible factors. Front. Cell Dev. Biol. 2018, 6, 409700. [Google Scholar] [CrossRef] [PubMed]
- Soh, R.; Hardy, A.; zur Nieden, N.I. The FOXO signaling axis displays conjoined functions in redox homeostasis and stemness. Free Radic. Biol. Med. 2021, 169, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Dansen, T.B.; Smits, L.M.M.; van Triest, M.H.; de Keizer, P.L.J.; van Leenen, D.; Koerkamp, M.G.; Szypowska, A.; Meppelink, A.; Brenkman, A.B.; Yodoi, J.; et al. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat. Chem. Biol. 2009, 5, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.F. Mechanisms for redox-regulation of protein kinase C. Front. Pharmacol. 2015, 6, 150500. [Google Scholar] [CrossRef] [PubMed]
- Roe, N.D.; Ren, J. Oxidative activation of Ca2+/calmodulin-activated kinase II mediates ER stress-induced cardiac dysfunction and apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, 828–839. [Google Scholar] [CrossRef]
- Womersley, J.S.; Uys, J.D. S-Glutathionylation and Redox Protein Signaling in Drug Addiction. Prog. Mol. Biol. Transl. Sci. 2015, 137, 87. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin Oxidase-Induced ROS Generation Modulates PKC Biphasic Effect of Resveratrol on Endothelial Cell Survival. Biomolecules 2019, 9, 209. [Google Scholar] [CrossRef]
- Talior, I.; Tennenbaum, T.; Kuroki, T.; Eldar-Finkelman, H. PKC-δ-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: Role for NADPH oxidase. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 405–411. [Google Scholar] [CrossRef]
- Gozal, E.; Metz, C.J.; Dematteis, M.; Sachleben, L.R.; Schurr, A.; Rane, M.J. PKA activity exacerbates hypoxia-induced ROS formation and hypoxic injury in PC-12 cells. Toxicol. Lett. 2017, 279, 107–114. [Google Scholar] [CrossRef]
- Heppner, D.E.; Dustin, C.M.; Liao, C.; Hristova, M.; Veith, C.; Little, A.C.; Ahlers, B.A.; White, S.L.; Deng, B.; Lam, Y.-W.; et al. Direct cysteine sulfenylation drives activation of the Src kinase. Nat. Commun. 2018, 9, 4522. [Google Scholar] [CrossRef]
- Kemble, D.J.; Sun, G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc. Natl. Acad. Sci. USA 2009, 106, 5070–5075. [Google Scholar] [CrossRef]
- Sundaresan, M.; Yu, Z.-X.; Ferrans, V.J.; Irani, K.; Finkel, T. Requirement for Generation of H2O2 for Platelet-Derived Growth Factor Signal Transduction. Science 1995, 270, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Mondol, A.S.; Tonks, N.K.; Kamata, T. Nox4 redox regulation of PTP1B contributes to the proliferation and migration of glioblastoma cells by modulating tyrosine phosphorylation of coronin-1C. Free Radic. Biol. Med. 2014, 67, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Feron, O. Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 7–15. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Cyr, A.R.; Domann, F.E. The redox basis of epigenetic modifications: From mechanisms to functional consequences. Antioxid. Redox Signal 2011, 15, 551–589. [Google Scholar] [CrossRef]
- Afanas’ev, I. New Nucleophilic Mechanisms of Ros-Dependent Epigenetic Modifications: Comparison of Aging and Cancer. Aging Dis. 2014, 5, 52–62. [Google Scholar] [CrossRef]
- Kietzmann, T.; Petry, A.; Shvetsova, A.; Gerhold, J.M.; Görlach, A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1533–1554. [Google Scholar] [CrossRef]
- Ranjan, P.; Anathy, V.; Burch, P.M.; Weirather, K.; Lambeth, J.D.; Heintz, N.H. Redox-dependent expression of cyclin D1 and cell proliferation by Nox1 in mouse lung epithelial cells. Antioxid. Redox Signal 2006, 8, 1447–1460. [Google Scholar] [CrossRef]
- Hood, K.Y.; Montezano, A.C.; Harvey, A.P.; Nilsen, M.; Maclean, M.R.; Touyz, R.M. Nicotinamide adenine dinucleotide phosphate oxidase-mediated redox signaling and vascular remodeling by 16α-hydroxyestrone in human pulmonary artery cells. Hypertension 2016, 68, 796–808. [Google Scholar] [CrossRef]
- Olashaw, N.E.; Kowalik, T.F.; Huang, E.S.; Pledger, W.J. Induction of NF-κB-like activity by platelet-derived growth factor in mouse fibroblasts. Mol. Biol. Cell 1992, 3, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, L.; Chen, S.; Zhao, G.; Fu, C.; Xu, B.; Tan, X.; Xiang, Y.; Chen, G. Carbon monoxide inhibits T cell proliferation by suppressing reactive oxygen species signaling. Antioxid. Redox Signal 2020, 32, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Soneja, A.; Drews, M.; Malinski, T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005, 57, 108–119. [Google Scholar] [PubMed]
- Loo, A.E.K.; Ho, R.; Halliwell, B. Mechanism of hydrogen peroxide-induced keratinocyte migration in a scratch-wound model. Free Radic. Biol. Med. 2011, 51, 884–892. [Google Scholar] [CrossRef]
- Shi, M.M.; Godleski, J.J.; Paulauskis, J.D. Regulation of macrophage inflammatory protein-1α mRNA by oxidative stress. J. Biol. Chem. 1996, 271, 5878–5883. [Google Scholar] [CrossRef]
- Murrell, G.A.C.; Francis, M.J.O.; Bromley, L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem. J. 1990, 265, 659–665. [Google Scholar] [CrossRef]
- Yang, J.Q.; Buettner, G.R.; Domann, F.E.; Li, Q.; Engelhardt, J.F.; Weydert, C.D.; Oberley, L.W. v-Ha-ras mitogenic signaling through superoxide and derived reactive oxygen species. Mol. Carcinog. 2002, 33, 206–218. [Google Scholar] [CrossRef]
- Pervaiz, S.; Clément, M.V. A Permissive Apoptotic Environment: Function of a Decrease in Intracellular Superoxide Anion and Cytosolic Acidification. Biochem. Biophys. Res. Commun. 2002, 290, 1145–1150. [Google Scholar] [CrossRef]
- Xu, Q.; Choksi, S.; Qu, J.; Jang, J.; Choe, M.; Banfi, B.; Engelhardt, J.F.; Liu, Z.-G. NADPH oxidases are essential for macrophage differentiation. J. Biol. Chem. 2016, 291, 20030–20041. [Google Scholar] [CrossRef]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef]
- Kang, X.; Wei, X.; Wang, X.; Jiang, L.; Niu, C.; Zhang, J.; Chen, S.; Meng, D. Nox2 contributes to the arterial endothelial specification of mouse induced pluripotent stem cells by upregulating Notch signaling. Sci. Rep. 2016, 6, 33737. [Google Scholar] [CrossRef]
- Nayernia, Z.; Colaianna, M.; Robledinos-Antón, N.; Gutzwiller, E.; Sloan-Béna, F.; Stathaki, E.; Hibaoui, Y.; Cuadrado, A.; Hescheler, J.; Stasia, M.-J.; et al. Decreased neural precursor cell pool in NADPH oxidase 2-deficiency: From mouse brain to neural differentiation of patient derived iPSC. Redox Biol. 2017, 13, 82–93. [Google Scholar] [CrossRef]
- Ibi, M.; Katsuyama, M.; Fan, C.; Iwata, K.; Nishinaka, T.; Yokoyama, T.; Yabe-Nishimura, C. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free Radic. Biol. Med. 2006, 40, 1785–1795. [Google Scholar] [CrossRef]
- Okamoto, T.; Taguchi, M.; Osaki, T.; Fukumoto, S.; Fujita, T. Phosphate enhances reactive oxygen species production and suppresses osteoblastic differentiation. J. Bone Miner. Metab. 2014, 32, 393–399. [Google Scholar] [CrossRef]
- Han, J.; Park, D.; Park, J.Y.; Han, S. Inhibition of NADPH Oxidases Prevents the Development of Osteoarthritis. Antioxidants 2022, 11, 2346. [Google Scholar] [CrossRef]
- Schröder, K. NADPH oxidases in bone homeostasis and osteoporosis. Free Radic. Biol. Med. 2019, 132, 67–72. [Google Scholar] [CrossRef]
- Zimmerman, M.C.; Takapoo, M.; Jagadeesha, D.K.; Stanic, B.; Banfi, B.; Bhalla, R.C.; Miller, F.J. Activation of NADPH oxidase 1 increases intracellular calcium and migration of smooth muscle cells. Hypertension 2011, 58, 446–453. [Google Scholar] [CrossRef]
- Cholan, P.M.; Cartland, S.P.; Kavurma, M.M. NADPH Oxidases, Angiogenesis, and Peripheral Artery Disease. Antioxidants 2017, 6, 56. [Google Scholar] [CrossRef]
- Garrido-Urbani, S.; Jemelin, S.; Deffert, C.; Carnesecchi, S.; Basset, O.; Szyndralewiez, C.; Heitz, F.; Page, P.; Montet, X.; Michalik, L.; et al. Targeting Vascular NADPH Oxidase 1 Blocks Tumor Angiogenesis through a PPARα Mediated Mechanism. PLoS ONE 2011, 6, e14665. [Google Scholar] [CrossRef]
- Fang, J.; Sheng, R.; Qin, Z.H. NADPH Oxidases in the Central Nervous System: Regional and Cellular Localization and the Possible Link to Brain Diseases. Antioxid. Redox Signal 2021, 35, 951–973. [Google Scholar] [CrossRef] [PubMed]
- Rouyère, C.; Serrano, T.; Frémont, S.; Echard, A. Oxidation and reduction of actin: Origin, impact in vitro and functional consequences in vivo. Eur. J. Cell Biol. 2022, 101, 151249. [Google Scholar] [CrossRef] [PubMed]
- Balta, E.; Kramer, J.; Samstag, Y. Redox Regulation of the Actin Cytoskeleton in Cell Migration and Adhesion: On the Way to a Spatiotemporal View. Front. Cell Dev. Biol. 2021, 8, 618261. [Google Scholar] [CrossRef]
- Kang, M.-I.; Kobayashi, A.; Wakabayashi, N.; Kim, S.G.; Yamamoto, M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA 2004, 101, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Roebuck, K.A.; Finnegan, A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 1999, 66, 876–888. [Google Scholar] [CrossRef]
- Lo, S.K.; Janakidevi, K.; Lai, L.; Malik, A.B. Hydrogen peroxide-induced increase in endothelial adhesiveness is dependent on ICAM-1 activation. Am. J. Physiol. Lung. Cell. Mol. Physiol. 1993, 264, L406–L412. [Google Scholar] [CrossRef]
- Ledebur, H.C.; Parks, T.P. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells: Essential roles of a variant NF-κB site and p65 homodimers. J. Biol. Chem. 1995, 270, 933–943. [Google Scholar] [CrossRef]
- Fulda, S. Regulation of necroptosis signaling and cell death by reactive oxygen species. Biol. Chem. 2016, 397, 657–660. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Pérez, S.; Toledano, M.B.; Sastre, J. Mitochondrial Reactive Oxygen Species and Lytic Programmed Cell Death in Acute Inflammation. Antioxid. Redox Signal 2023, 39, 708. [Google Scholar] [CrossRef]
- Wang, Y.; Kanneganti, T.D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021, 19, 4641. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-K.; Chang, W.-T.; Lin, I.-L.; Chen, Y.-F.; Padalwar, N.B.; Cheng, K.-C.; Teng, Y.-N.; Wang, C.-H.; Chiu, C.-C. The Role of Necroptosis in ROS-Mediated Cancer Therapies and Its Promising Applications. Cancers 2020, 12, 2185. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Pandian, N.; Kanneganti, T.-D. PANoptosis: A unique inflammatory cell death modality. J. Immunol. 2022, 209, 1625. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Dianzani, M.U. Lipid peroxidation: Control of cell proliferation, cell differentiation and cell death. Mol. Asp. Med. 2008, 29, 1–8. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, J. Necroptosis in health and diseases. Semin. Cell Dev. Biol. 2014, 35, 14–23. [Google Scholar] [CrossRef]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4, e128834. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Se-Kwon, K. Cell Survival and Apoptosis Signaling as Therapeutic Target for Cancer: Marine Bioactive Compounds. Int. J. Mol. Sci. 2013, 14, 2334. [Google Scholar] [CrossRef]
- Feltham, R.; Silke, J. The small molecule that packs a punch: Ubiquitin-mediated regulation of RIPK1/FADD/caspase-8 complexes. Cell Death Differ. 2017, 24, 1196. [Google Scholar] [CrossRef]
- Van Raam, B.J.; Salvesen, G.S. Proliferative versus Apoptotic Functions of Caspase-8 Hetero or Homo: The Caspase-8 Dimer Controls Cell Fate. Biochim. Biophys. Acta 2011, 1824, 113. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.S.; Zhao, S.; Yang, Z.; Zhong, C.-Q.; Chen, X.; Cai, Q.; Yang, Z.-H.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8, 14329. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61. [Google Scholar] [CrossRef]
- Boada-Romero, E.; Martinez, J.; Heckmann, B.L.; Green, D.R. Mechanisms and physiology of the clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 2020, 21, 398. [Google Scholar] [CrossRef]
- Koren, E.; Fuchs, Y. Modes of Regulated Cell Death in Cancer. Cancer Discov. 2021, 11, 245–265. [Google Scholar] [CrossRef]
- Opferman, J.T.; Korsmeyer, S.J. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 2003, 4, 410–415. [Google Scholar] [CrossRef]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 548553. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Zhang, L.; Guo, L.; Wang, X.; Pan, Z.; Jiang, X.; Wu, F.; He, G. Mechanisms of PANoptosis and relevant small-molecule compounds for fighting diseases. Cell Death Dis. 2023, 14, 851. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Bao, Q.; Shi, Y. Apoptosome: A platform for the activation of initiator caspases. Cell Death Differ. 2007, 14, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Anadón, A.; Wang, X.; et al. Cancer Metabolism: The Role of ROS in DNA Damage and Induction of Apoptosis in Cancer Cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef]
- Jing, W.; Liu, C.; Su, C.; Liu, L.; Chen, P.; Li, X.; Zhang, X.; Yuan, B.; Wang, H.; Du, X. Role of reactive oxygen species and mitochondrial damage in rheumatoid arthritis and targeted drugs. Front. Immunol. 2023, 14, 1107670. [Google Scholar] [CrossRef]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.R.; Amin, J.K.; Xiao, L.; Miller, T.; Viereck, J.; Oliver-Krasinski, J.; Baliga, R.; Wang, J.; Siwik, D.A.; Singh, K.; et al. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ. Res. 2001, 89, 453–460. [Google Scholar] [CrossRef]
- Clément, M.V.; Stamenkovic, I. Superoxide anion is a natural inhibitor of FAS-mediated cell death. EMBO J. 1996, 15, 216. [Google Scholar] [CrossRef]
- Tiwari, R.; Mondal, Y.; Bharadwaj, K.; Mahajan, M.; Mondal, S.; Sarkar, A. Reactive Oxygen Species (ROS) and Their Profound Influence on Regulating Diverse Aspects of Cancer: A Concise Review. Drug Dev. Res. 2025, 86, e70107. [Google Scholar] [CrossRef]
- Magnani, A.; Brosselin, P.; Beauté, J.; de Vergnes, N.; Mouy, R.; Debré, M.; Suarez, F.; Hermine, O.; Lortholary, O.; Blanche, S.; et al. Inflammatory manifestations in a single-center cohort of patients with chronic granulomatous disease. J. Allergy Clin. Immunol. 2014, 134, 655–662.e8. [Google Scholar] [CrossRef]
- Song, E.K.; Jaishankar, G.B.; Saleh, H.; Jithpratuck, W.; Sahni, R.; Krishnaswamy, G. Chronic granulomatous disease: A review of the infectious and inflammatory complications. Clin. Mol. Allergy 2011, 9, 10. [Google Scholar] [CrossRef]
- Chen, Y.H.; Xu, X.; Sheng, M.J.; Zheng, Z.; Gu, Q. Effects of asymmetric dimethylarginine on bovine retinal capillary endothelial cell proliferation, reactive oxygen species production, permeability, intercellular adhesion molecule-1, and occludin expression. Mol. Vis. 2011, 17, 332. [Google Scholar]
- Henderson, L.M.; Chappell, J.B. NADPH oxidase of neutrophils. Biochim. Biophys. Acta (BBA)—Bioenerg. 1996, 1273, 87–107. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, K.; Deng, L.; Chen, Y.; Nice, E.C.; Huang, C. Redox Regulation of Inflammation: Old Elements, a New Story. Med. Res. Rev. 2015, 35, 306–340. [Google Scholar] [CrossRef]
- Fu, H.; Bylund, J.; Karlsson, A.; Pellmé, S.; Dahlgren, C. The mechanism for activation of the neutrophil NADPH-oxidase by the peptides formyl-Met-Leu-Phe and Trp-Lys-Tyr-Met-Val-Met differs from that for interleukin-8. Immunology 2004, 112, 201–210. [Google Scholar] [CrossRef]
- Rothfork, J.M.; Timmins, G.S.; Harris, M.N.; Chen, X.; Lusis, A.J.; Otto, M.; Cheung, A.L.; Gresham, H.D. Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: New role for the NADPH oxidase in host defense. Proc. Natl. Acad. Sci. USA 2004, 101, 13867–13872. [Google Scholar] [CrossRef]
- Schoen, J.; Euler, M.; Schauer, C.; Schett, G.; Herrmann, M.; Knopf, J.; Yaykasli, K.O. Neutrophils’ Extracellular Trap Mechanisms: From Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 12855. [Google Scholar] [CrossRef]
- Douda, D.N.; Yip, L.; Khan, M.A.; Grasemann, H.; Palaniyar, N. Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood 2014, 123, 597–600. [Google Scholar] [CrossRef]
- Fonseca, Z.; Díaz-Godínez, C.; Mora, N.; Alemán, O.R.; Uribe-Querol, E.; Carrero, J.C.; Rosales, C. Entamoeba histolytica induce signaling via Raf/MEK/ERK for neutrophil extracellular trap (NET) formation. Front. Cell Infect. Microbiol. 2018, 8, 378104. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2010, 7, 75–77. [Google Scholar] [CrossRef]
- Dingjan, I.; Verboogen, D.R.; Paardekooper, L.M.; Revelo, N.H.; Sittig, S.P.; Visser, L.J.; von Mollard, G.F.; Henriet, S.S.; Figdor, C.G.; ter Beest, M.; et al. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 2016, 6, 22064. [Google Scholar] [CrossRef]
- Savina, A.; Jancic, C.; Hugues, S.; Guermonprez, P.; Vargas, P.; Moura, I.C.; Lennon-Duménil, A.-M.; Seabra, M.C.; Raposo, G.; Amigorena, S. NOX2 Controls Phagosomal pH to Regulate Antigen Processing during Crosspresentation by Dendritic Cells. Cell 2006, 126, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, M.; Garg, N.J. P47phox−/− Mice Are Compromised in Expansion and Activation of CD8+ T Cells and Susceptible to Trypanosoma cruzi Infection. PLoS Pathog. 2014, 10, e1004516. [Google Scholar] [CrossRef] [PubMed]
- A Lang, P.; Xu, H.C.; Grusdat, M.; McIlwain, D.R.; A Pandyra, A.; Harris, I.S.; Shaabani, N.; Honke, N.; Maney, S.K.; Lang, E.; et al. Reactive oxygen species delay control of lymphocytic choriomeningitis virus. Cell Death Differ. 2013, 20, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Bassoy, E.Y.; Walch, M.; Martinvalet, D. Reactive Oxygen Species: Do They Play. a Role in Adaptive Immunity? Front. Immunol. 2021, 12, 755856. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef]
- Rada, B.; Leto, T.L. Characterization of hydrogen peroxide production by Duox in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett. 2010, 584, 917–922. [Google Scholar] [CrossRef]
- Rada, B.; Leto, T. Oxidative Innate Immune Defenses by Nox/Duox Family NADPH Oxidases. Contrib. Microbiol. 2008, 15, 164–187. [Google Scholar] [CrossRef]
- El Hassani, R.A.; Benfares, N.; Caillou, B.; Talbot, M.; Sabourin, J.-C.; Belotte, V.; Morand, S.; Gnidehou, S.; Agnandji, D.; Ohayon, R.; et al. Dual oxidase2 is expressed all along the digestive tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G933–G942. [Google Scholar] [CrossRef]
- Nunes, P.R.; Romao-Veiga, M.; Peracoli, M.T.S.; Peracoli, J.C.; Sandrim, V.C. Potential role of uric acid to activate NLRP3 inflammasome triggering endothelial dysfunction in preeclampsia. Clin. Immunol. Commun. 2022, 2, 69–75. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Nishino, T.; Okamoto, K.; Matsumura, T.; Eger, B.T.; Pai, E.F.; Nishino, T. Unique amino acids cluster for switching from the dehydrogenase to oxidase form of xanthine oxidoreductase. Proc. Natl. Acad. Sci. USA 2003, 100, 8170–8175. [Google Scholar] [CrossRef]
- Cantu-Medellin, N.; Kelley, E.E. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol. 2013, 1, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Komaki, Y.; Sugiura, H.; Koarai, A.; Tomaki, M.; Ogawa, H.; Akita, T.; Hattori, T.; Ichinose, M. Cytokine-mediated xanthine oxidase upregulation in chronic obstructive pulmonary disease’s airways. Pulm. Pharmacol. Ther. 2005, 18, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Little, J.W.; Doyle, T.; Salvemini, D. Reactive nitroxidative species and nociceptive processing: Determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids 2012, 42, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Porreca, F.; Cuzzocrea, S.; Galen, K.; Lightfoot, R.; Masini, E.; Muscoli, C.; Mollace, V.; Ndengele, M.; Ischiropoulos, H.; et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004, 309, 869–878. [Google Scholar] [CrossRef]
- Ogata, N.; Yamamoto, H.; Kugiyama, K.; Yasue, H.; Miyamoto, E. Involvement of protein kinase C in superoxide anion-induced activation of nuclear factor-κB in human endothelial cells. Cardiovasc. Res. 2000, 45, 513–521. [Google Scholar] [CrossRef]
- Janes, K.; Neumann, W.L.; Salvemini, D. Anti-superoxide and anti-peroxynitrite strategies in pain suppression. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 815–821. [Google Scholar] [CrossRef]
- Humphries, K.M.; Pennypacker, J.K.; Taylor, S.S. Redox regulation of cAMP-dependent protein kinase signaling: Kinase versus phosphatase inactivation. J. Biol. Chem. 2007, 282, 22072–22079. [Google Scholar] [CrossRef]
- Hongpaisan, J.; Winters, C.A.; Andrews, S.B. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J. Neurosci. 2004, 24, 10878–10887. [Google Scholar] [CrossRef]
- Kim, H.Y.; Wang, J.; Lu, Y.; Chung, J.M.; Chung, K. Superoxide signaling in pain is independent of nitric oxide signaling. Neuroreport 2009, 20, 1424–1428. [Google Scholar] [CrossRef]
- Lee, B.H.; Kang, J.; Kim, H.Y.; Gwak, Y.S. The roles of superoxide on at-level spinal cord injury pain in rats. Int. J. Mol. Sci. 2021, 22, 2672. [Google Scholar] [CrossRef] [PubMed]
- Naziroğlu, M.; Braidy, N. Thermo-sensitive TRP channels: Novel targets for treating chemotherapy-induced peripheral pain. Front. Physiol. 2017, 8, 1040. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M.; Radu, B.M.; Cucu, D. Editorial: Transient receptor potential (TRP) ion channels in non-excitable cells. Front. Physiol. 2023, 14, 1213332. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J. Update on the neurophysiology of pain transmission and modulation: Focus on the NMDA-receptor. J. Pain. Symptom Manag. 2000, 19, 2–6. [Google Scholar] [CrossRef]
- Brenner, G.J.; Ji, R.R.; Shaffer, S.; Woolf, C.J. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur. J. Neurosci. 2004, 20, 375–384. [Google Scholar] [CrossRef]
- Ndengele, M.M.; Cuzzocrea, S.; Esposito, E.; Mazzon, E.; Di Paola, R.; Matuschak, G.M.; Salvemini, D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. FASEB J. 2008, 22, 3154–3164. [Google Scholar] [CrossRef]
- Lasa, M.; Mahtani, K.R.; Finch, A.; Brewer, G.; Saklatvala, J.; Clark, A.R. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol. Cell. Biol. 2000, 20, 4265–4274. [Google Scholar] [CrossRef]
- Borghi, S.M.; Carvalho, T.T.; Bertozzi, M.M.; Bernardy, C.C.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Calixto-Campos, C.; Fattori, V.; Alves-Filho, J.C.; Cunha, T.M.; et al. Role of the interleukin-33 (IL-33)/suppressor of tumorigenicity 2 (ST2) signaling in superoxide anion-triggered inflammation and pain behavior in mice. Chem. Biol. Interact. 2025, 413, 111476. [Google Scholar] [CrossRef]
- Fattori, V.; Serafim, K.G.G.; Zarpelon, A.C.; Borghi, S.M.; Pinho-Ribeiro, F.A.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; Casagrande, R.; Verri, W.A. Differential regulation of oxidative stress and cytokine production by endothelin ETA and ETB receptors in superoxide anion-induced inflammation and pain in mice. J. Drug Target. 2017, 25, 264–274. [Google Scholar] [CrossRef]
- Fattori, V.; Pinho-Ribeiro, F.A.; Borghi, S.M.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; Casagrande, R.; Verri, W.A. Curcumin inhibits superoxide anion-induced pain-like behavior and leukocyte recruitment by increasing Nrf2 expression and reducing NF-κB activation. Inflamm. Res. 2015, 64, 993–1003. [Google Scholar] [CrossRef]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A. Naringenin inhibits superoxide anion-induced inflammatory pain: Role of oxidative stress, cytokines, Nrf-2 and the no-cGMP-PKG-KATP channel signaling pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Fattori, V.; Zarpelon, A.C.; Borghi, S.M.; Staurengo-Ferrari, L.; Carvalho, T.T.; Alves-Filho, J.C.; Cunha, F.Q.; Cunha, T.M.; Casagrande, R.; et al. Pyrrolidine dithiocarbamate inhibits superoxide anion-induced pain and inflammation in the paw skin and spinal cord by targeting NF-κB and oxidative stress. Inflammopharmacology 2016, 24, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Zarpelon, A.C.; Rodrigues, F.C.; Lopes, A.H.; Souza, G.R.; Carvalho, T.T.; Pinto, L.G.; Xu, D.; Ferreira, S.H.; Alves-Filho, J.C.; McInnes, I.B.; et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J. 2016, 30, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.M.; Lemos, H.P.; Grespan, R.; Napimoga, M.H.; Dal-Secco, D.; Freitas, A.; Cunha, T.M.; Verri Jr, W.A.; Souza-Junior, D.A.; Jamur, M.C.; et al. A crucial role for TNF-α in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5, RESEARCH PAPER. Br. J. Pharmacol. 2009, 158, 779–789. [Google Scholar] [CrossRef]
- Cunha, F.Q.; Poole, S.; Lorenzetti, B.B.; Ferreira, S.H. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992, 107, 660–664. [Google Scholar] [CrossRef]
- Jin, X.; Gereau, R.W. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-α. J. Neurosci. 2006, 26, 246–255. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164. [Google Scholar] [CrossRef]
- Olukman, M.; Önal, A.; Celenk, F.G.; Uyanıkgil, Y.; Cavuşoğlu, T.; Düzenli, N.; Ülker, S. Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats. Neural Regen. Res. 2018, 13, 1657–1664. [Google Scholar] [CrossRef]
- Ryu, T.H.; Jung, K.Y.; Ha, M.J.; Kwak, K.H.; Lim, D.G.; Hong, J.G. Superoxide and Nitric Oxide Involvement in Enhancing of N-methyl-D-aspartate Receptor-Mediated Central Sensitization in the Chronic Post-ischemia Pain Model. Korean J. Pain. 2010, 23, 1–10. [Google Scholar] [CrossRef]
- Kwak, K.H.; Han, C.G.; Lee, S.H.; Jeon, Y.; Park, S.S.; Kim, S.O.; Baek, W.Y.; Hong, J.G.; Lim, D.G. Reactive oxygen species in rats with chronic post-ischemia pain. Acta Anaesthesiol. Scand. 2009, 53, 648–656. [Google Scholar] [CrossRef]
- Kim, D.; You, B.; Jo, E.K.; Han, S.K.; Simon, M.I.; Lee, S.J. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14851–14856. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chung, J.M.; Chung, K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: The effect of mitochondrial electron transport complex inhibitors. Neurosci. Lett. 2008, 447, 87–91. [Google Scholar] [CrossRef]
- Khattab, M.M. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: A key role for superoxide anion. Eur. J. Pharmacol. 2006, 548, 167–173. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Bani, D.; Bencini, A.; Brandi, M.L.; Calosi, L.; Cantore, M.; Carossino, A.M.; Ghelardini, C.; Valtancoli, B.; Failli, P. Therapeutic effects of the superoxide dismutase mimetic compound MnII MeO2A on experimental articular pain in rats. Mediat. Inflamm. 2013, 2013, 905360. [Google Scholar] [CrossRef]
- Gui, T.; Luo, L.; Chhay, B.; Zhong, L.; Wei, Y.; Yao, L.; Yu, W.; Li, J.; Nelson, C.L.; Tsourkas, A.; et al. Superoxide dismutase-loaded porous polymersomes as highly efficient antioxidant nanoparticles targeting synovium for osteoarthritis therapy. Biomaterials 2022, 283, 121437. [Google Scholar] [CrossRef] [PubMed]
- Little, J.W.; Cuzzocrea, S.; Bryant, L.; Esposito, E.; Doyle, T.; Rausaria, S.; Neumann, W.L.; Salvemini, D. Spinal mitochondrial-derived peroxynitrite enhances neuroimmune activation during morphine hyperalgesia and antinociceptive tolerance. Pain 2013, 154, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Bryant, L.; Muscoli, C.; Cuzzocrea, S.; Esposito, E.; Chen, Z.; Salvemini, D. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci. Lett. 2010, 483, 85–89. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Liu, X.; Wang, L.; Ha, J.; Gao, Z.; He, X.; Wu, Z.; Chen, A.; Jewell, L.L.; et al. Treatment of Cerebral Ischemia Through NMDA Receptors: Metabotropic Signaling and Future Directions. Front. Pharmacol. 2022, 13, 831181. [Google Scholar] [CrossRef]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; Clair, D.S.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2012, 1822, 794–814. [Google Scholar] [CrossRef]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. The anti-inflammatory and antioxidant effects of sodium propionate. Int. J. Mol. Sci. 2020, 21, 3026. [Google Scholar] [CrossRef]
- Actelion Pharmaceuticals Ltd. Actelion receives FDA Approval of Tracleer (Bosentan) for Use in Pediatric Patients with Pulmonary Arterial Hypertension [Internet]; Johnson & Johnson: Titusville, NJ, USA, 2017; Available online: https://www.jnj.com/media-center/press-releases/actelion-receives-fda-approval-of-tracleer-bosentan-for-use-in-pediatric-patients-with-pulmonary-arterial-hypertension (accessed on 6 October 2025).
- Channick, R.N.; Simonneau, G.; Sitbon, O.; Robbins, I.M.; Frost, A.; Tapson, V.F.; Badesch, D.B.; Roux, S.; Rainisio, M.; Bodin, F.; et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: A randomized placebo-controlled study. N. Engl. J. Med. 2001, 344, 896–903. [Google Scholar] [CrossRef]
- Cingolani, H.E.; Villa-Abrille, M.C.; Cornelli, M.; Nolly, M.B.; Ennis, I.L.; Garciarena, C.D.; Suburo, A.M.; Aiello, E.A. Bosentan attenuates monocrotaline-induced pulmonary arterial hypertension through inhibition of oxidative stress and restoration of eNOS/NO pathway. Eur. J. Pharmacol. 2011, 650, 323–331. [Google Scholar] [CrossRef]
- de Man, F.S.; Tu, L.; Handoko, M.L.; Rain, S.; Ruiter, G.; François, C.; Schalij, I.; Dorfmüller, P.; Simonneau, G.; Fadel, E.; et al. Dysregulated renin–angiotensin–aldosterone system contributes to pulmonary arterial hypertension pathogenesis. Nat. Metab. 2020, 2, 803–812. [Google Scholar] [CrossRef]

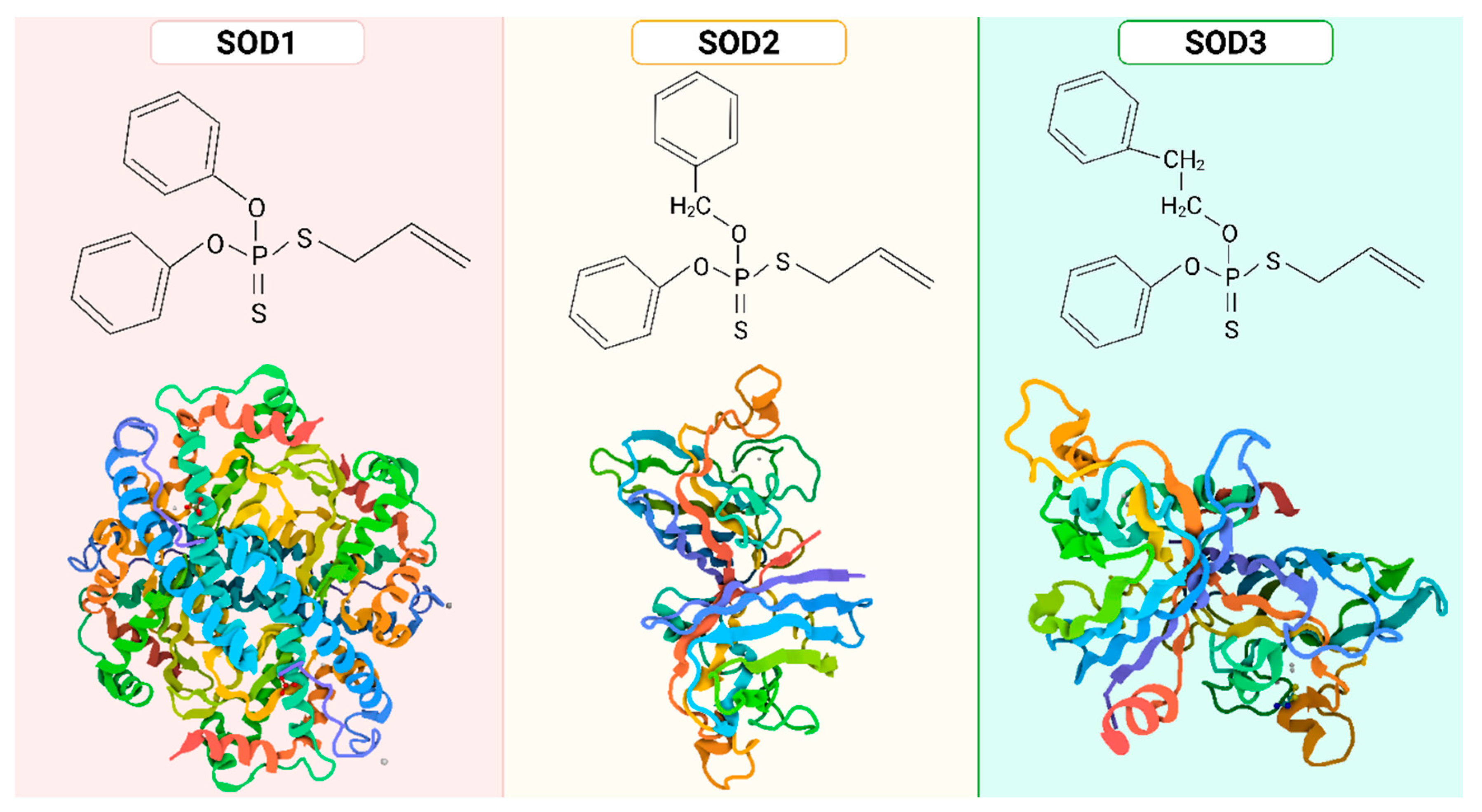
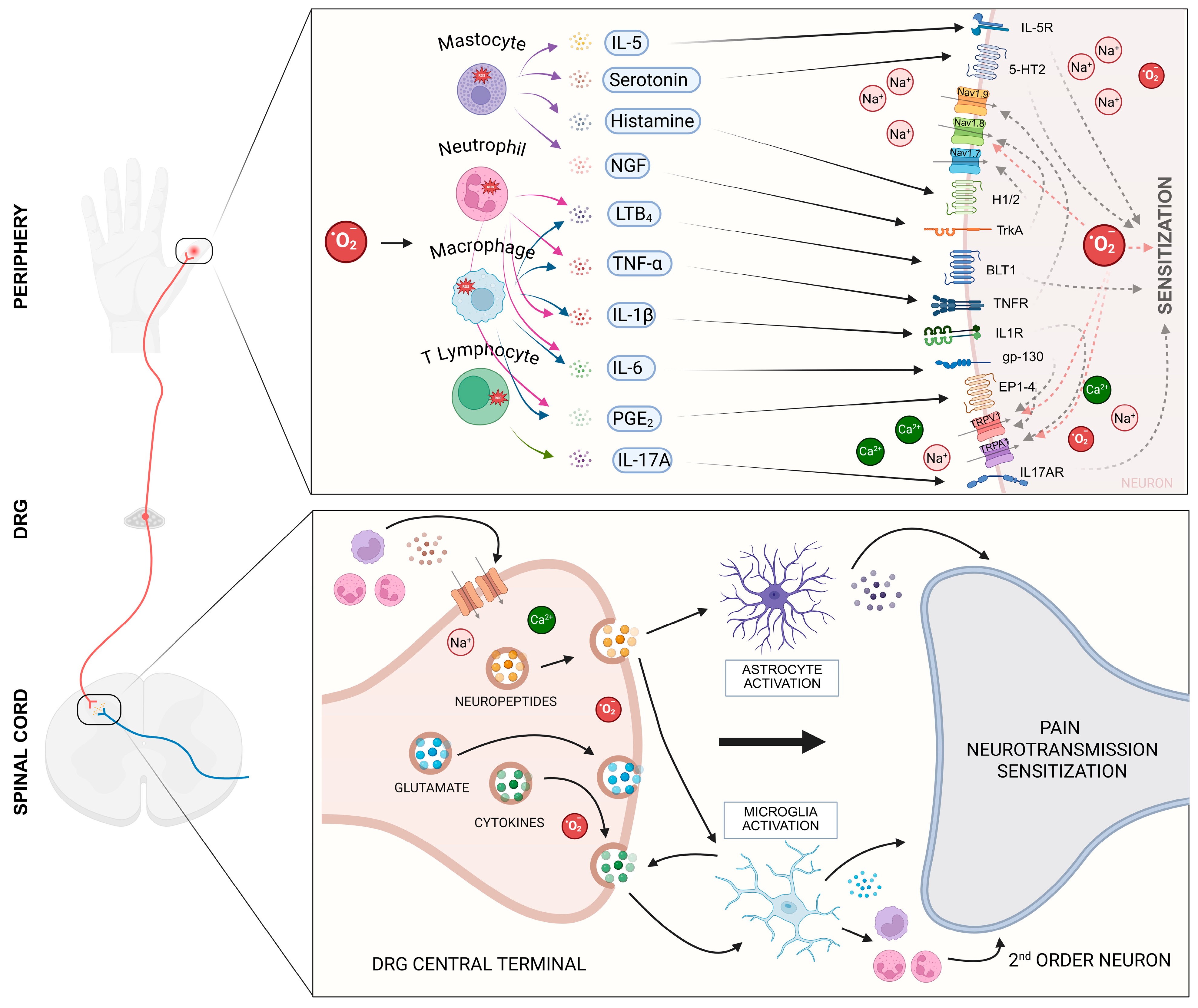
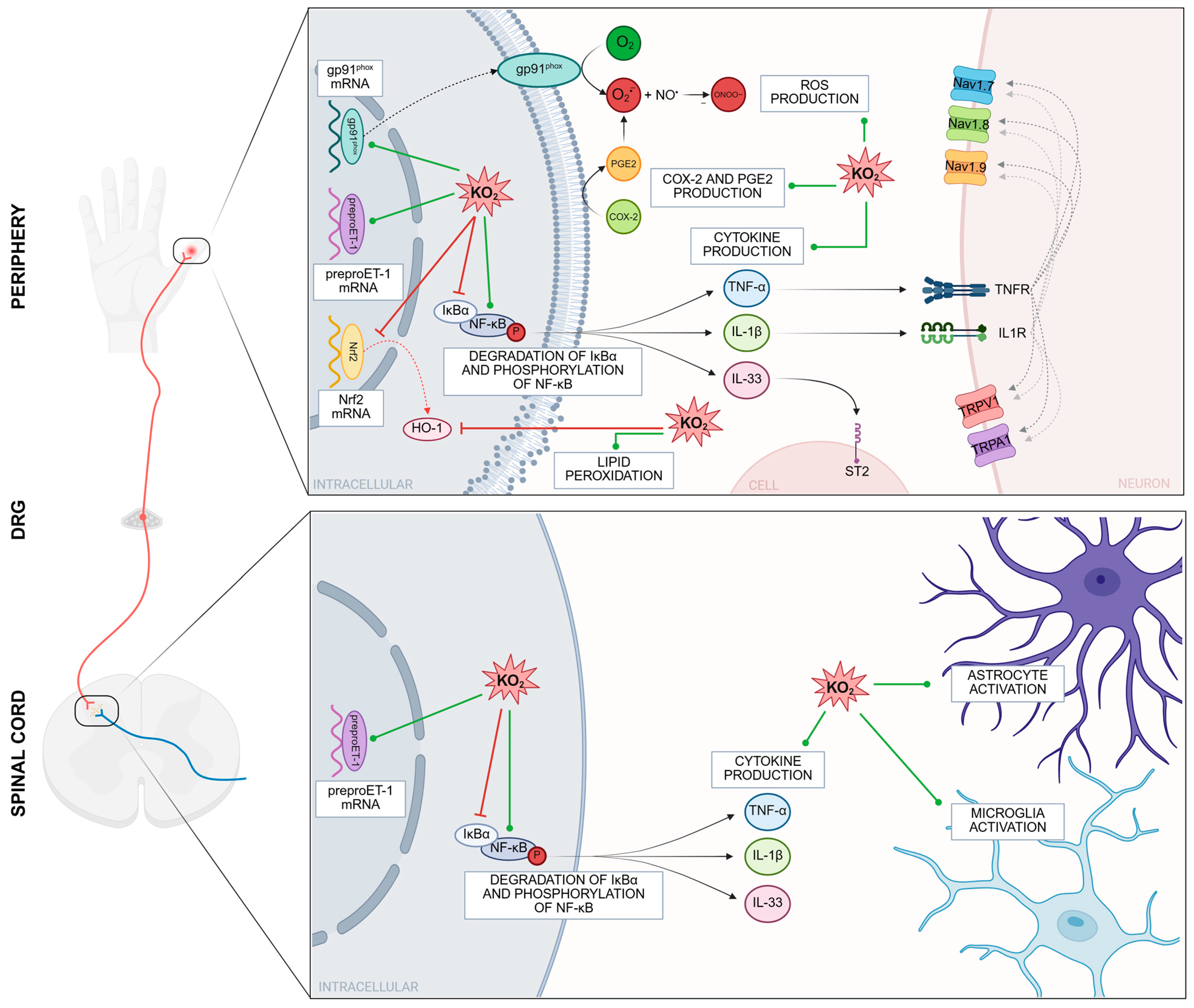
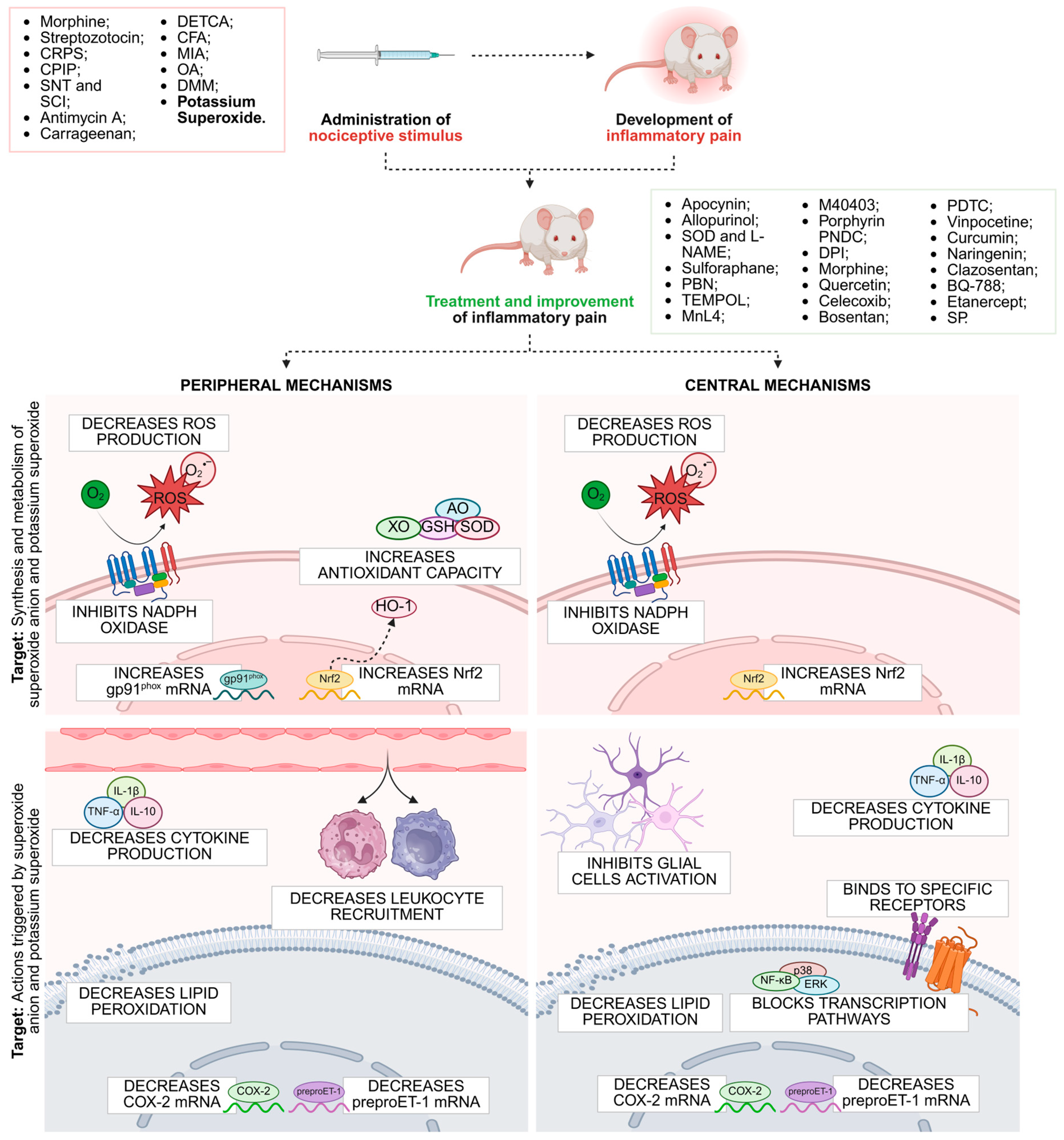
| Treatment | Experimental Model | Route of Administration and Dose | Model | Analgesic Effects | Mechanisms of Action | Reference |
|---|---|---|---|---|---|---|
| Apocynin | Rats | Intratecal 0.1–0.6 nmol/day | Morphine tolerance | Inhibited thermal hyperalgesia | Blocking NADPH oxidase activation | [268] |
| Apocynin | Rats | Intraperitoneal 100 mg/kg | Streptozotocin-induced diabetes | Ameliorated hyperglycemia-induced hyperalgesia and prevented sciatic nerve damage | NADPH oxidase inhibition | [269] |
| Allopurinol, SOD L-NAME | Rats | Intraperitoneal 40 mg/kg 4000 U/kg 10 mg/kg | CRPS | Mechanical allodynia reduction | Activation of NMDA receptor phosphorylation in the spinal dorsal horn | [270] |
| Allopurinol, SOD L-NAME | Rats | Intraperitoneal 40 mg/kg | CIPIP | Alleviation in mechanical and cold allodynia | XO inhibition | [271] |
| Sulforaphane | Mice (C57BL/6) | Intrathecal 10 mg/kg or 50 mg/kg Intraperitoneal 50 μmol/kg or 50 mg/kg | SNT | Allodynia and inflammatory hyperalgesia reduction | Inducing nuclear translocation of Nrf-2 with subsequent HO-1 expression in myeloid cells, including the microglia | [272] |
| PBN TEMPOL | Mice (C57BL/6) | Intraperitoneal 100 mg/kg 300 mg/kg | Antimycin A | Mechanical hyperalgesia reduction | Transient antinociception | [273] |
| TEMPOL | Rats | 60 mg/kg | Carrageenan | Inhibition of edema and hyperalgesia | SOD mimetics | [274] |
| TEMPOL | Rats | 15, 30, and 60 mg/kg | Carrageenan DETCA | Attenuates paw edema and thermal hyperalgesia | SOD mimetics | [274] |
| MnL4 | Rats | Intraperitoneal or subcutaneously 15 mg kg−1 | Carrageenan, CFA, and MIA | Decrease mechanical hypersensitivity and prevent the hind limb weight bearing alterations | SOD mimetic inhibition of PARP activation | [275] |
| M40403 | Rats | 1–10 mg/kg | Carrageenan | inhibition of edema and hyperalgesia | Antinociceptive effect | [246] |
| SOD-loaded nanoparticles | Mice (C57BL/6) | Intra-articular 500 U/mL | OA DMM | Allodynia reduction | Reduced ROS production and the synthesis of catabolic proteases | [276] |
| TEMPOL | Rats | Intrathecal 1 mg/kg | SCI | Mechanical sensitivity decrease | SOD mimetics | [252] |
| Porphyrin PNDCs | Rats | Intrathecal | Morphine | Attenuates morphine hyperalgesia and antinociceptive tolerance | Reduces PN-mediated mitochondrial nitroxidative stress in the spinal cord | [277] |
| Apocynin and DPI | Rats | Intrathecal 100 mg/kg 1 mg/kg | Morphine | Blocked spinal NADPH oxidase activation | NADPH oxidase inhibition | [278] |
| Treatment | Experimental Model | Route of Administration and Dose | Analgesic Effects | Mechanisms of Action | Reference |
|---|---|---|---|---|---|
| Morphine | Mice (Swiss) | Intraplantar 12 µg/paw | Reduction in mechanical and thermal hyperalgesia, and inhibition of overt pain behaviors | - | [41] |
| Quercetin | Mice (Swiss) | Intraperitoneal 100 mg/kg | Inhibited mechanical and thermal hyperalgesia, paw edema, and overt pain behaviors | Inhibited the recruitment of total leukocytes, mononuclear and polymorphonuclear leukocytes into the peritoneal cavity. Reduced oxidative stress in plantar tissue | [41] |
| Celecoxib | Mice (Swiss) | Intraperitoneal 30 mg/kg | Inhibited mechanical and thermal hyperalgesia, and overt pain behaviors | Inhibited COX-2 mRNA expression in plantar tissue | [41] |
| Bosentan | Mice (Swiss) | Oral 100 mg/kg | Inhibited mechanical and thermal hyperalgesia, overt pain behavior, and paw edema | Inhibited MPO activity in plantar tissue, leukocyte recruitment into the peritoneal cavity, and pro-inflammatory cytokine production. Increased anti-inflammatory cytokine production and antioxidant capacity. Induced prepro-ET-1 mRNA expression | [39] |
| TEMPOL | Mice (Balb/c) | Intraperitoneal 100 mg/kg | Inhibited mechanical and thermal hyperalgesia, and paw edema | Inhibited the mRNA expression of pro-inflammatory cytokines, COX-2 and prepro-ET-1. Improved the depletion of antioxidant capacity, and inhibited oxidative stress and glial cell activation | [33] |
| Pyrrolidine Dithiocarbamate | Mice (Swiss) | Subcutaneous 100 mg/kg | Inhibits mechanical and thermal hyperalgesia, and paw edema | Inhibits activity of MPO and NAG in plantar tissue, the recruitment of leukocytes into the peritoneal cavity, the degradation of IkBa, the activation of NF-kB, the production of pro-inflammatory cytokines and oxidative stress. | [263] |
| Intrathecal 300 µg/animal | Inhibits activity of MPO in plantar tissue | ||||
| Vinpocetine | Mice (Swiss and LysM-eGFP) | Oral 30 mg/kg | Inhibited mechanical and thermal hyperalgesia, overt pain behavior, and paw edema | Restored local antioxidant capacity, increased Nrf2 and HO-1 expression. Reduced leukocyte recruitment in plantar tissue and peritoneal cavity, oxidative stress, pro-inflammatory cytokine production, ET-1 and COX-2 expression, and NF-κB activation | [32] |
| Curcumin | Mice (Swiss) | Subcutaneous 10 mg/kg | Inhibited mechanical and thermal hyperalgesia, and overt pain behaviors | Inhibited leukocyte recruitment, MPO activity, pro-inflammatory cytokine production, NF-kB activation, and oxidative stress. Increased Nrf2 and HO-1 mRNA expression | [261] |
| Naringenin | Mice (Swiss) | Oral 50 mg/kg | Inhibited mechanical and thermal hyperalgesia, and overt pain behaviors | Inhibited MPO activity, oxidative stress, pro-inflammatory cytokine production, and gp91phox, COX-2, and preproET-1 mRNA expression. Increased antioxidant capacity and Nrf2 and HO-1 mRNA expression. Activated NO-cGMP-PKG-ATP (KATP)-sensitive potassium channels | [262] |
| Clazosentan | Mice (Swiss) | Intraplantar 30 nmol/animal | Inhibited mechanical and thermal hyperalgesia, overt pain behaviors, and edema | Inhibited MPO activity, oxidative stress and pro-inflammatory cytokine production | [260] |
| Intraperitoneal 30 nm/animal | Inhibited overt pain behavior | Inhibited the recruitment of total leukocytes | |||
| BQ-788 | Mice (Swiss) | Intraplantar 30 nmol/animal | Inhibited mechanical and thermal hyperalgesia, overt pain behaviors, and edema | Inhibited MPO activity and oxidative stress | [260] |
| Intraperitoneal 30 nm/animal | Inhibited overt pain behavior | Inhibited the recruitment of total leukocytes | |||
| Etanercept | Mice (C57BL/6) | Intraperitoneal 10 mg/kg | Inhibited mechanical hyperalgesia and overt pain behaviors | Inhibited MPO activity | [40] |
| Sodium Propionate | Rats (Sprague-Dawley) | Intraplantar 30 and 100 mg/kg | Inhibited paw edema, nociceptive stimulation and overt pain behaviors | Reduced the recruitment of inflammatory cells, tissue morphological changes, and MPO activity. Increased the expression of SOD2, HO-1, and GSH. | [281] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchini, B.H.S.; Martelossi-Cebinelli, G.; Carneiro, J.A.; Rasquel-Oliveira, F.S.; Casagrande, R.; Verri, W.A. Superoxide Anion Generation, Its Pathological Cellular and Molecular Roles and Pharmacological Targeting in Inflammatory Pain: Lessons from the Potassium Superoxide Model. Future Pharmacol. 2025, 5, 60. https://doi.org/10.3390/futurepharmacol5040060
Bianchini BHS, Martelossi-Cebinelli G, Carneiro JA, Rasquel-Oliveira FS, Casagrande R, Verri WA. Superoxide Anion Generation, Its Pathological Cellular and Molecular Roles and Pharmacological Targeting in Inflammatory Pain: Lessons from the Potassium Superoxide Model. Future Pharmacology. 2025; 5(4):60. https://doi.org/10.3390/futurepharmacol5040060
Chicago/Turabian StyleBianchini, Beatriz Hoffmann Sales, Geovana Martelossi-Cebinelli, Jessica Aparecida Carneiro, Fernanda Soares Rasquel-Oliveira, Rubia Casagrande, and Waldiceu A. Verri. 2025. "Superoxide Anion Generation, Its Pathological Cellular and Molecular Roles and Pharmacological Targeting in Inflammatory Pain: Lessons from the Potassium Superoxide Model" Future Pharmacology 5, no. 4: 60. https://doi.org/10.3390/futurepharmacol5040060
APA StyleBianchini, B. H. S., Martelossi-Cebinelli, G., Carneiro, J. A., Rasquel-Oliveira, F. S., Casagrande, R., & Verri, W. A. (2025). Superoxide Anion Generation, Its Pathological Cellular and Molecular Roles and Pharmacological Targeting in Inflammatory Pain: Lessons from the Potassium Superoxide Model. Future Pharmacology, 5(4), 60. https://doi.org/10.3390/futurepharmacol5040060








