Altered Antimicrobial Activity and Selectivity of Dihydro-Protoberberines over Their Corresponding Protoberberines
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
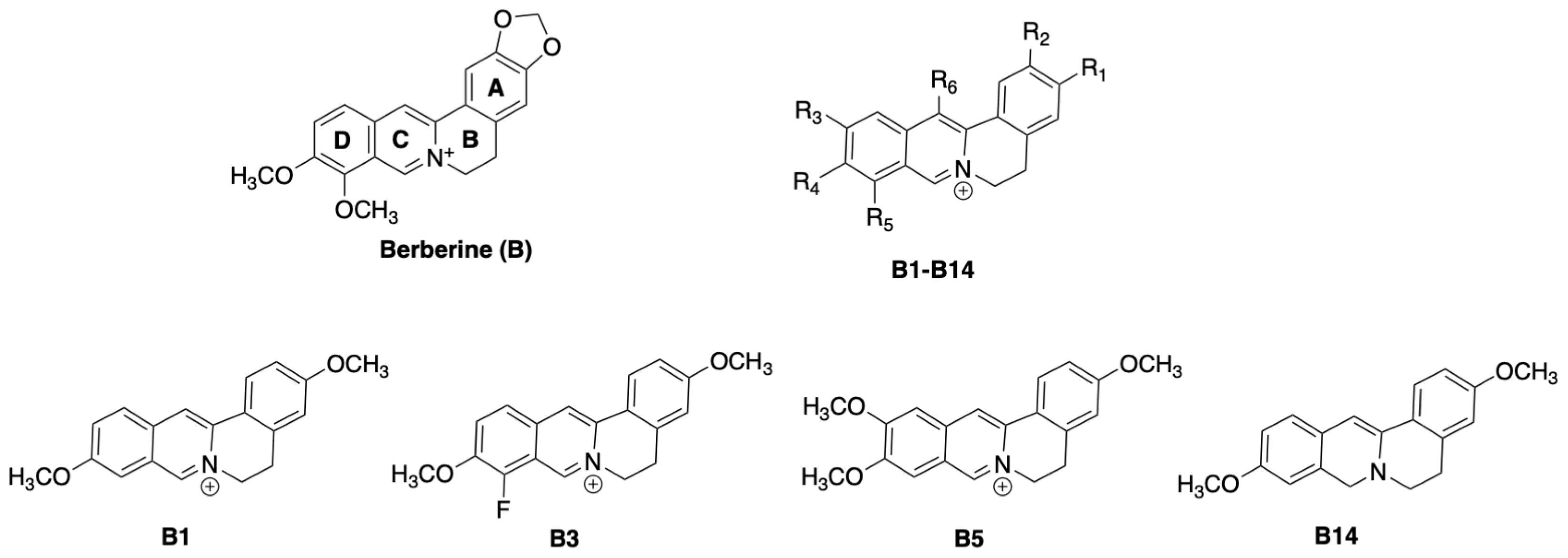
2.2. Synthesis of Protoberberines (General Method A)
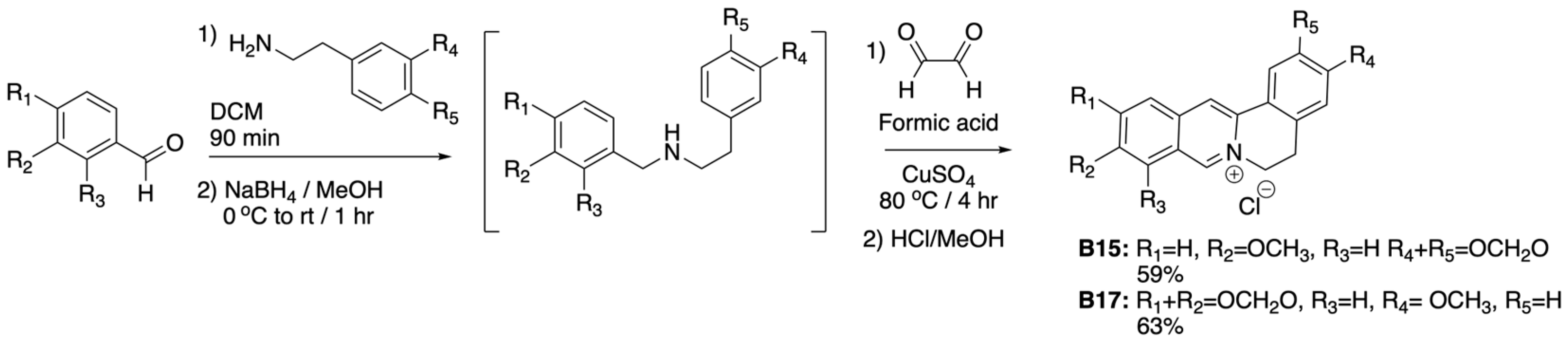
2.3. Synthesis of Dihydro-Protoberberines (General Method B)
2.4. Antimicrobial (Kirby–Bauer) Disc Diffusion Assay
2.5. Cell Culture
2.6. Variant Treatment and Viability Assay of Colon Cancer Cells
2.7. Statistical Analysis
3. Results
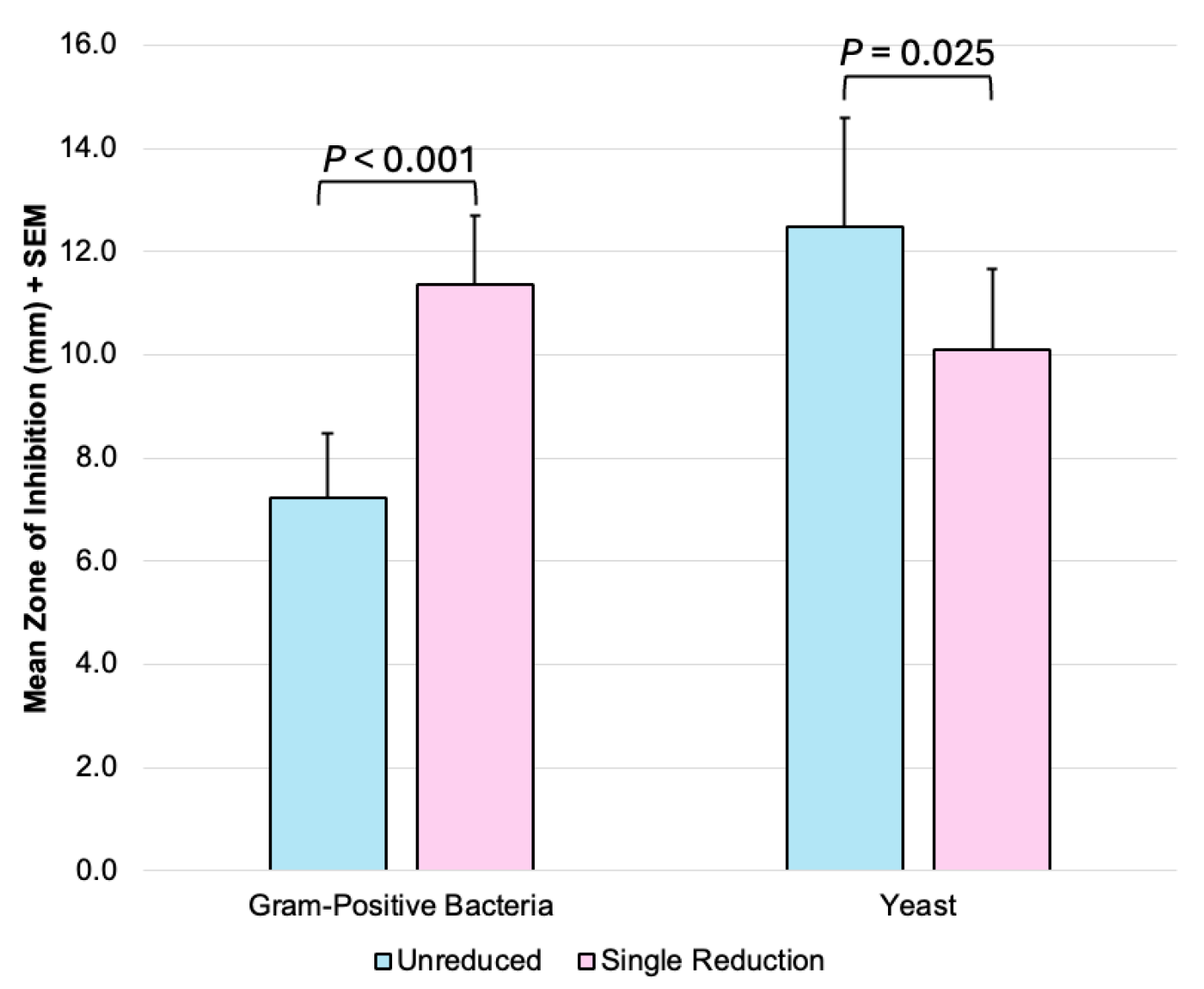
3.1. Antimicrobial Effects
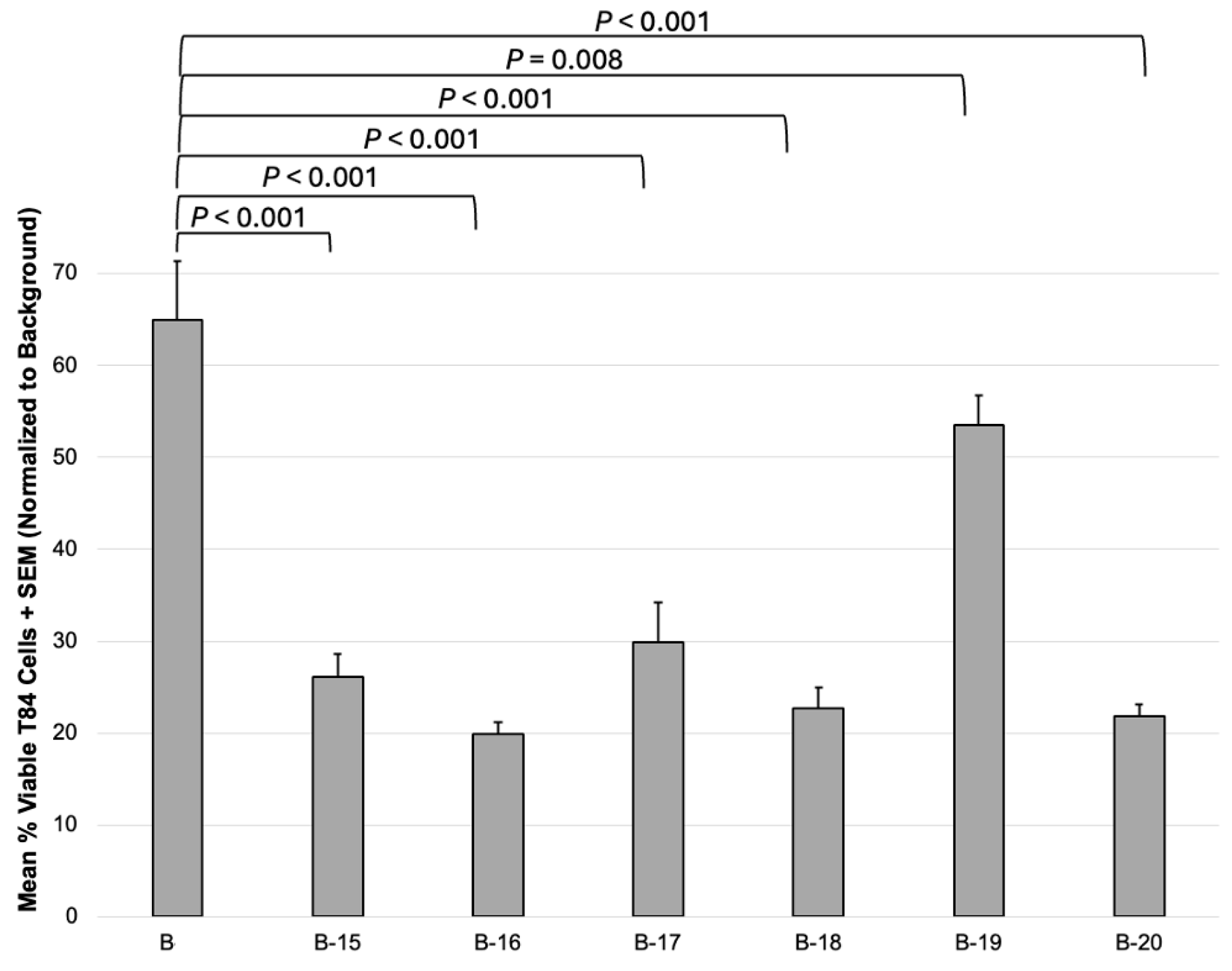
3.2. Anticancer Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTT | 3-(4,5-di methyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| FtsZ | Filamenting temperature-sensitive mutant Z |
| NMR | Nuclear magnetic resonance |
| DMSO | Dimethyl sulfoxide |
| ISS | International space station |
| UPLC | Ultra performance liquid chromatography |
| QDa | Quadrupole Dalton |
| DCM | Dichloromethane |
| ADMEM | Advanced Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| SEM | Standard error of the mean |
References
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2014. [Google Scholar]
- Nordmann, P.; Naas, T.; Fortineau, N.; Poirel, L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr. Opin. Microbiol. 2007, 10, 436–440. [Google Scholar] [CrossRef]
- Checinska Sielaff, A.; Urbaniak, C.; Mohan, G.B.M.; Stepanov, V.G.; Tran, Q.; Wood, J.M.; Minich, J.; McDonald, D.; Mayer, T.; Knight, R.; et al. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome 2019, 7, 50. [Google Scholar] [CrossRef]
- Brahmachari, G.; Gorai, D.; Roy, R. Argemone mexicana: Chemical and pharmacological aspects. Rev. Bras. Farmacogn. 2013, 23, 559–575. [Google Scholar] [CrossRef]
- Moore, M.; Kamp, M. Los Remedios: Traditional Herbal Remedies of the Southwest; Museum of New Mexico Press: Santa Fe, NM, USA, 2008. [Google Scholar]
- Orozco-Nunnelly, D.A.; Pruet, J.; Rios-Ibarra, C.P.; Bocangel Gamarra, E.L.; Lefeber, T.; Najdeska, T. Characterizing the cytotoxic effects and several antimicrobial phytocompounds of Argemone mexicana. PLoS ONE 2021, 16, e0249704. [Google Scholar] [CrossRef]
- Villegas, J.; Ball, B.C.; Shouse, K.M.; VanArragon, C.W.; Wasserman, A.N.; Bhakta, H.E.; Oliver, A.G.; Orozco-Nunnelly, D.A.; Pruet, J.M. Synthesis and biological evaluation of Argemone mexicana-inspired antimicrobials. Beilstein J. Org. Chem. 2023, 19, 1511–1524. [Google Scholar] [CrossRef]
- Schmeller, T.; Latz-Brüning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- Čerňáková, M.; Košťálová, D. Antimicrobial activity of berberine—A constituent of Mahonia aquifolium. Folia Microbiol. 2002, 47, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Grycová, L.; Dostál, J.; Marek, R. Quaternary protoberberine alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Sun, N.; Chan, F.-Y.; Lu, Y.-J.; Neves, M.A.C.; Lui, H.-K.; Wang, Y.; Chow, K.-Y.; Chan, K.-F.; Yan, S.-C.; Leung, Y.-C.; et al. Rational Design of Berberine-Based FtsZ Inhibitors with Broad-Spectrum Antibacterial Activity. PLoS ONE 2014, 9, e97514. [Google Scholar] [CrossRef]
- Boberek, J.M.; Stach, J.; Good, L. Genetic Evidence for Inhibition of Bacterial Division Protein FtsZ by Berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef]
- Kosalec, I.; Jembrek, M.J.; Vlainić, J. The Spectrum of Berberine Antibacterial and Antifungal Activities. In Promising Antimicrobials from Natural Products; Rai, M., Kosalec, I., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Ma, Y.; Liu, Y.; Xue, Y.; Li, Y.; Gao, X.; Wang, Y.; Chu, M. Advance in identified targets of berberine. Front. Pharmacol. 2025, 16, 1500511. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Song, D.-Q.; Li, Y.-H.; Kong, W.-J.; Wang, Y.-X.; Gao, L.-M.; Liu, S.-Y.; Cao, R.-Q.; Jiang, J.-D. Synthesis and structure–Activity relationships of berberine analogues as a novel class of low-density-lipoprotein receptor up-regulators. Bioorg. Med. Chem. Lett. 2008, 18, 4675–4677. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jiang, X.; Xiong, J.; Lan, J.; Tian, Y.; Zhong, L.; Wang, X.; Xu, N.; Cao, H.; Zhang, W.; et al. Structure–Activity Relationship Study Enables the Discovery of a Novel Berberine Analogue as the RXRα Activator to Inhibit Colon Cancer. J. Med. Chem. 2020, 63, 5841–5855. [Google Scholar] [CrossRef]
- Salam, A.; Al-Amin, Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional methods and future trends in antimicrobial susceptibility testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Dharmsathaphorn, K.; McRoberts, J.A.; Mandel, K.G.; Tisdale, L.D.; Masui, H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am. J. Physiol.-Gastrointest. Liver Physiol. 1984, 246, G204–G208. [Google Scholar] [CrossRef]
- Bouyer, P.G.; Tang, X.; Weber, C.R.; Shen, L.; Turner, J.R.; Matthews, J.B. Capsaicin induces NKCC1 internalization and inhibits chloride secretion in colonic epithelial cells independently of TRPV1. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G142–G156. [Google Scholar] [CrossRef] [PubMed]
- Kettmann, V.; Košt’álová, D.; Höltje, H.-D. Human topoisomerase I poisoning: Docking protoberberines into a structure-based binding site model. J. Comput.-Aided Mol. Des. 2004, 18, 785–796. [Google Scholar] [CrossRef]
- Li, T.-K.; Bathory, E.; LaVoie, E.J.; Srinivasan, A.R.; Olson, W.K.; Sauers, R.R.; Liu, L.F.; Pilch, D.S. Human Topoisomerase I Poisoning by Protoberberines: Potential Roles for Both Drug–DNA and Drug–Enzyme Interactions. Biochemistry 2000, 39, 7107–7116. [Google Scholar] [CrossRef]
- Haque, S.; Mathkor, D.M.; Bhat, S.A.; Musayev, A.; Khitoyva, L.; Ramniwas, S.; Phillips, E.; Swamy, N.; Kumar, S.; Yerer, M.B.; et al. A Comprehensive Review Highlighting the Prospects of Phytonutrient Berberine as an Anticancer Agent. J. Biochem. Mol. Toxicol. 2024, 39, e70073. [Google Scholar] [CrossRef] [PubMed]

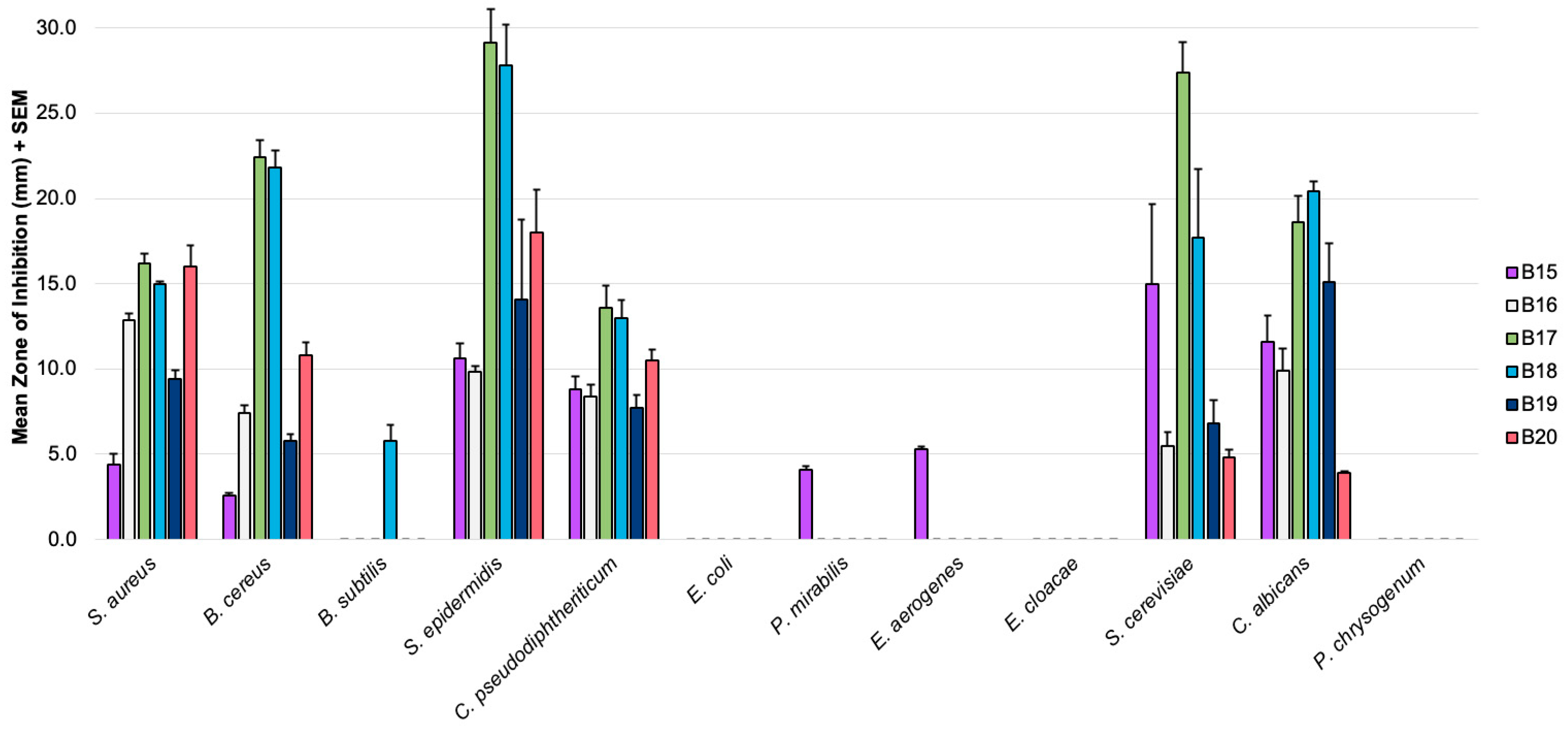
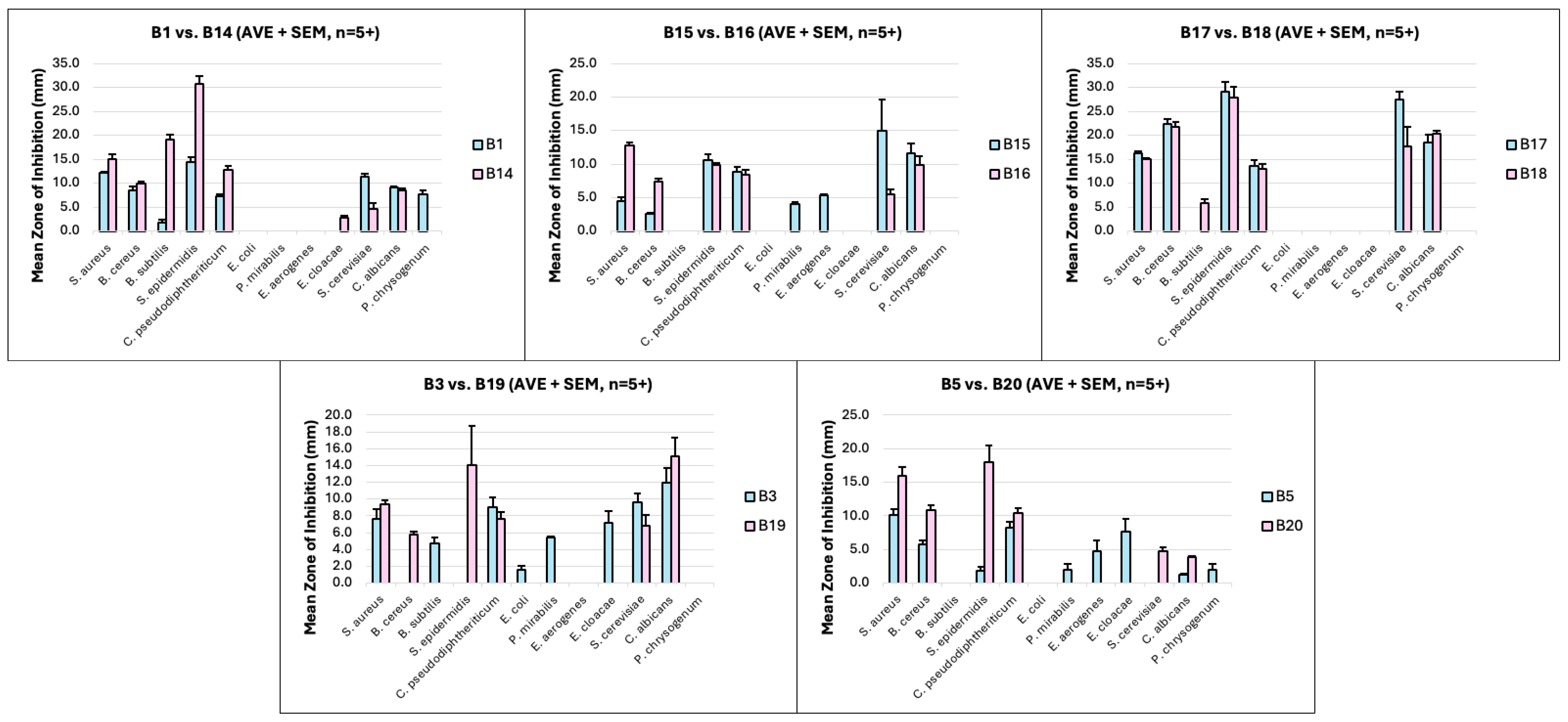
| Microbe | Mean Zones of Inhibition (mm) 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protoberberines 2 | Dihydro-Protoberberines 2 | |||||||||
| B1 [8] (n = 5) | B15 (n = 6) | B17 (n = 5) | B3 [8] (n = 5) | B5 [8] (n = 5) | B14 [8] (n = 5) | B16 (n = 6) | B18 (n = 5) | B19 (n = 5) | B20 (n = 5) | |
| S. aureus | 12.2 | 4.4 | 16.2 | 7.6 | 10.2 | 15.1 | 12.8 | 15.0 | 9.4 | 16.0 |
| B. cereus | 8.5 | 2.6 | 22.4 | - | 5.8 | 10.0 | 7.4 | 21.8 | 5.8 | 10.8 |
| B. subtilis | - | - | - | 4.7 | - | 19.2 | - | 5.8 | - | - |
| S. epidermidis | 14.4 | 10.6 | 29.1 | - | 1.8 | 30.8 | 9.9 | 27.8 | 14.1 | 18.0 |
| C. pseudodiphtheriticum | 7.4 | 8.8 | 13.6 | 9.0 | 8.2 | 12.8 | 8.4 | 13.0 | 7.7 | 10.5 |
| E. coli | - | - | - | 2.7 | - | - | - | - | - | - |
| P. mirabilis | - | 4.1 | - | 5.4 | 2.0 | - | - | - | - | - |
| E. aerogenes | - | 5.3 | - | - | 4.8 | - | - | - | - | - |
| E. cloacae | - | - | - | 7.2 | 7.6 | 2.9 | - | - | - | - |
| S. cerevisiae | 11.4 | 15.0 | 27.4 | 9.6 | - | 4.6 | 5.5 | 17.7 | 6.8 | 4.8 |
| C. albicans | 9.1 | 11.6 | 18.6 | 12.0 | 1.3 | 8.6 | 9.9 | 20.4 | 15.1 | 3.9 |
| P. chrysogenum | 7.8 | - | - | - | 2.0 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostos-Hernandez, J.; Bhakta, H.; VanArragon, C.; Sirhan, L.; Orozco-Nunnelly, D.; Pruet, J. Altered Antimicrobial Activity and Selectivity of Dihydro-Protoberberines over Their Corresponding Protoberberines. Future Pharmacol. 2025, 5, 53. https://doi.org/10.3390/futurepharmacol5030053
Ostos-Hernandez J, Bhakta H, VanArragon C, Sirhan L, Orozco-Nunnelly D, Pruet J. Altered Antimicrobial Activity and Selectivity of Dihydro-Protoberberines over Their Corresponding Protoberberines. Future Pharmacology. 2025; 5(3):53. https://doi.org/10.3390/futurepharmacol5030053
Chicago/Turabian StyleOstos-Hernandez, Juan, Hannah Bhakta, Caleb VanArragon, Lanna Sirhan, Danielle Orozco-Nunnelly, and Jeffrey Pruet. 2025. "Altered Antimicrobial Activity and Selectivity of Dihydro-Protoberberines over Their Corresponding Protoberberines" Future Pharmacology 5, no. 3: 53. https://doi.org/10.3390/futurepharmacol5030053
APA StyleOstos-Hernandez, J., Bhakta, H., VanArragon, C., Sirhan, L., Orozco-Nunnelly, D., & Pruet, J. (2025). Altered Antimicrobial Activity and Selectivity of Dihydro-Protoberberines over Their Corresponding Protoberberines. Future Pharmacology, 5(3), 53. https://doi.org/10.3390/futurepharmacol5030053







