A Single-Group, Open-Label Study on the Systemic Bioavailability, Safety, and Local Tolerability of a New L-Thyroxine/Escin Gel Formulation in Healthy Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Investigational Medicinal Product (IMP)
2.3. Objectives and Endpoints
2.4. Participants and Biological Sample Management
2.5. Safety Assessment
2.6. Statistical and Analytical Plan
2.7. Study Plan
3. Results
3.1. Study Population
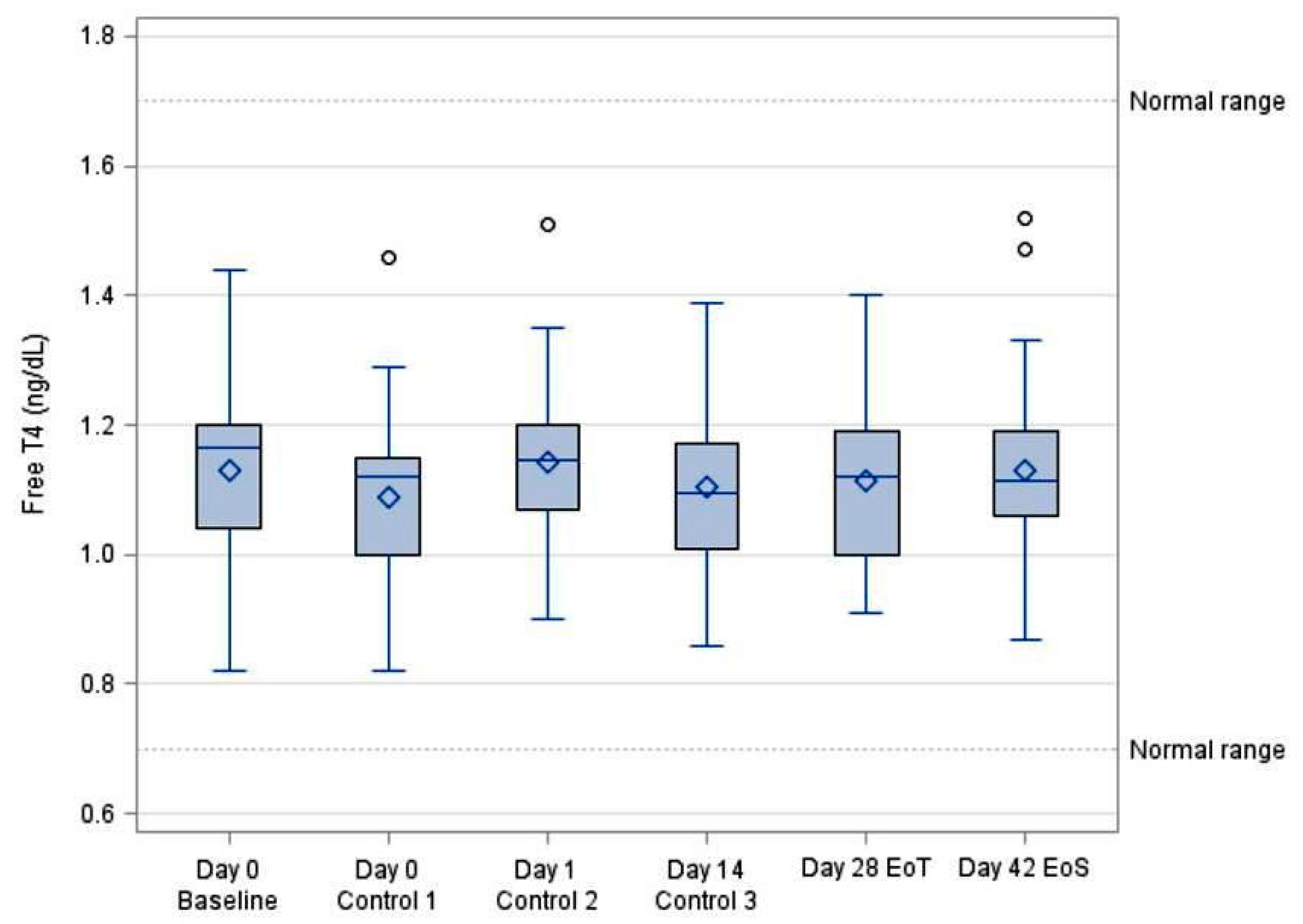
3.2. Systemic Bioavailability of FT4
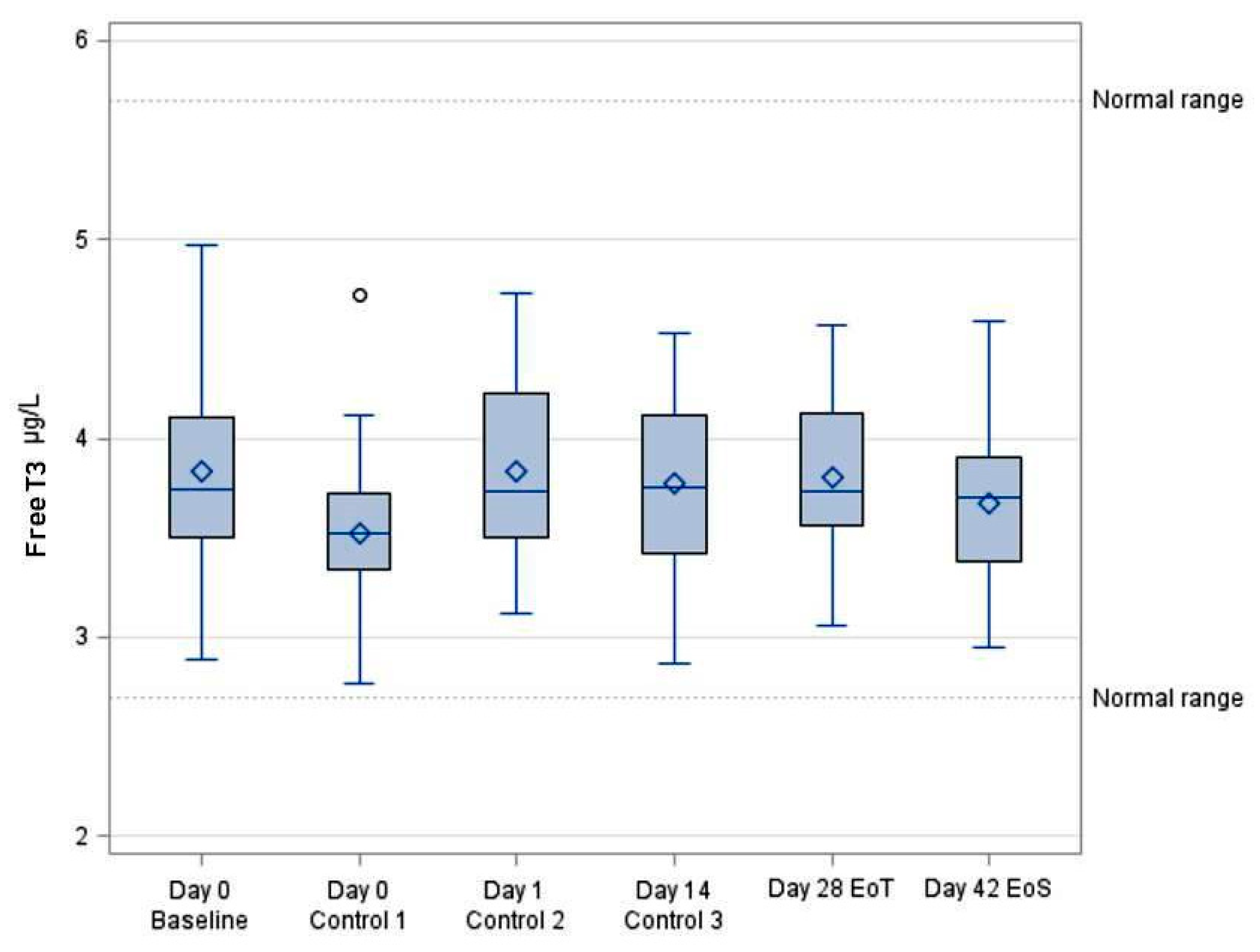
3.3. Systemic Bioavailability of FT3
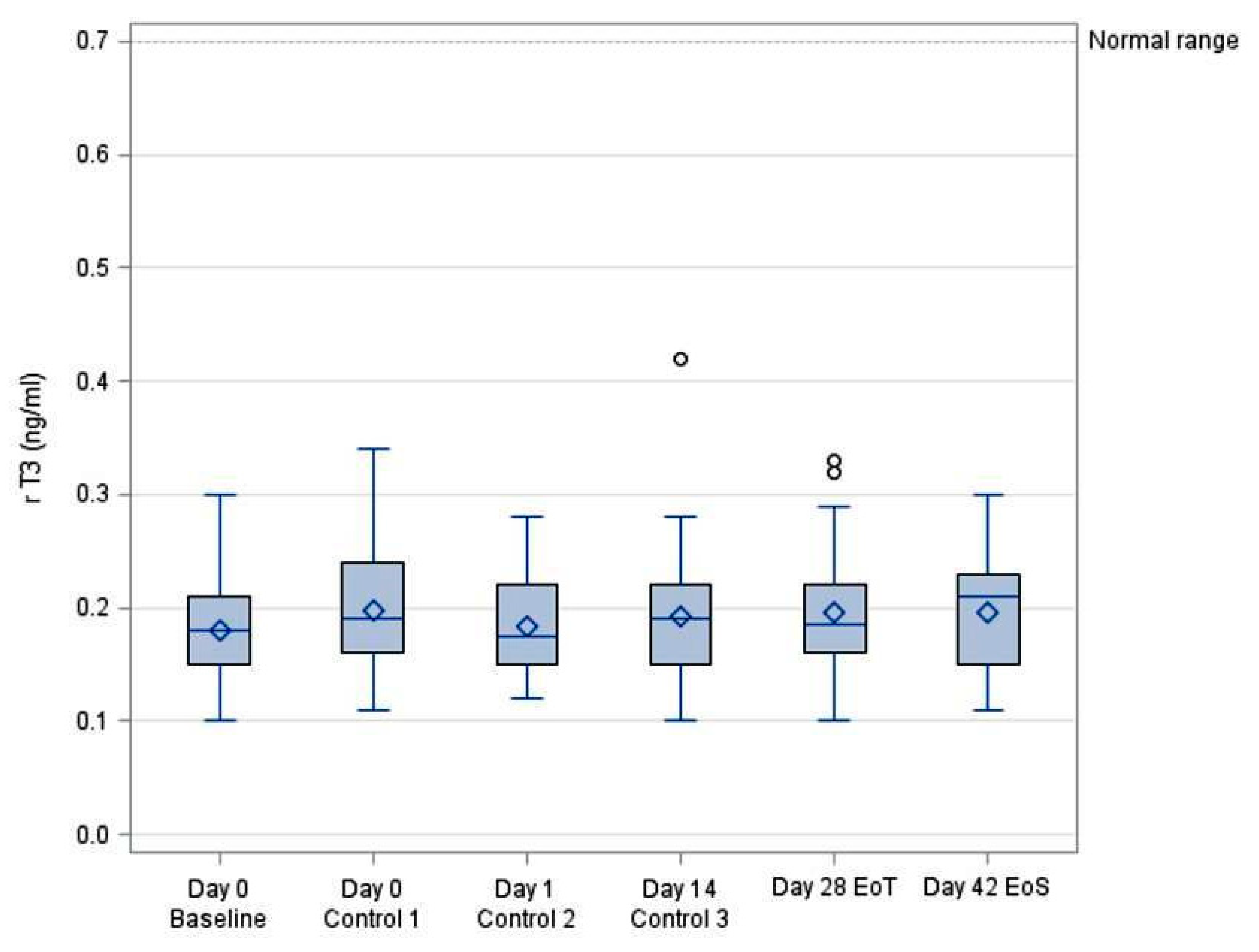
3.4. Systemic Bioavailability of rT3
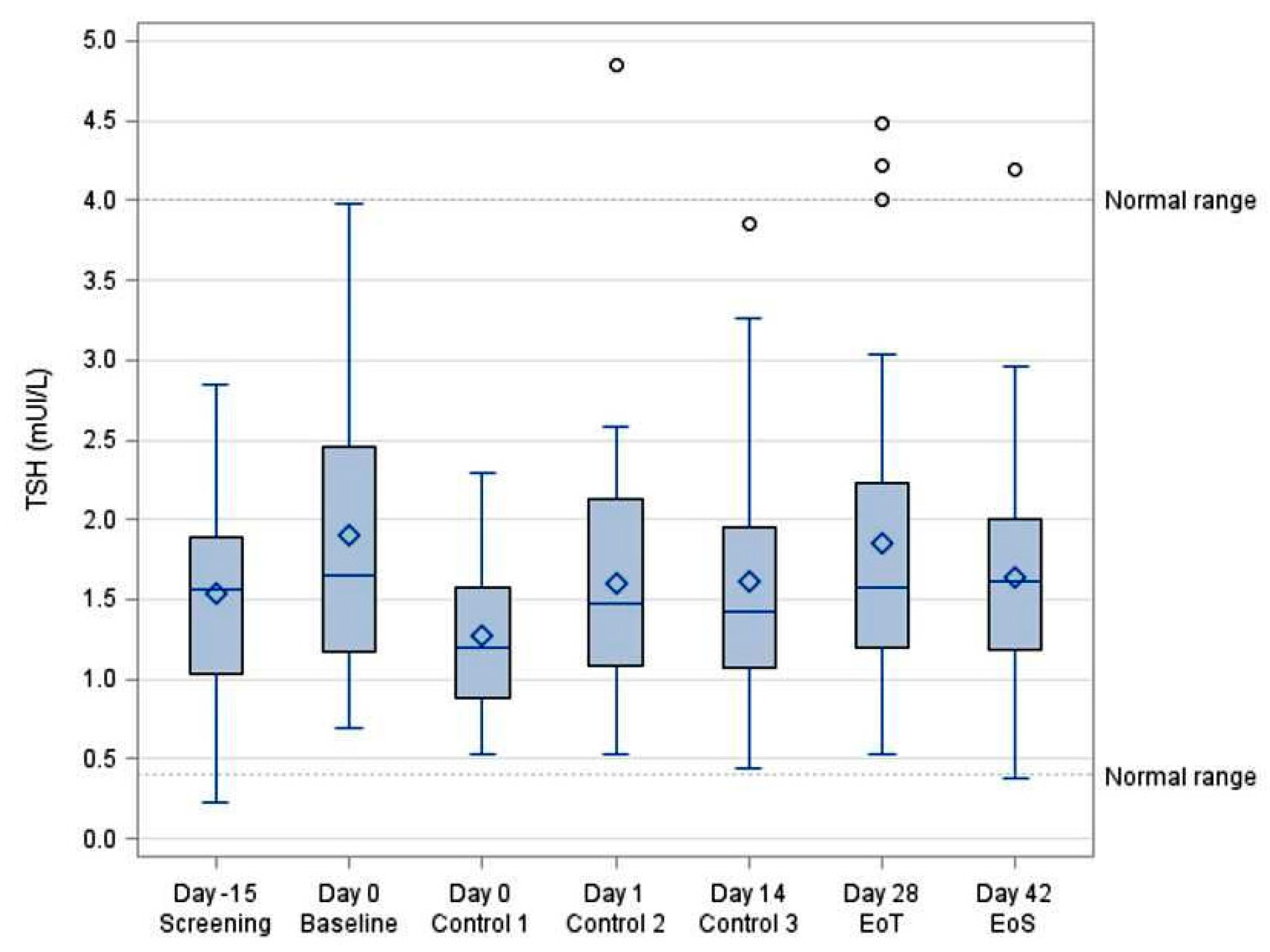
3.5. TSH Concentrations
3.6. Extent of Exposure and Compliance
3.7. Adverse Events
3.8. Vital Signs and Safety Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Autorizzazione all’Immissione in Commercio |
| AIFA | Agenzia Italiana del Farmaco |
| ECG | Electrocardiogram |
| EoS | End of Study |
| EoT | End of Treatment |
| FT3 | Free Triiodothyronine |
| FT4 | Free Thyroxine |
| GCP | Good Clinical Practice |
| IMP | Investigational Medicinal Product |
| ITT | Intention-to-Treat |
| L-T3 | Levotriiodothyronine |
| L-T4 | Levothyroxine |
| PP | Per Protocol |
| rT3 | Reverse Triiodothyronine |
| TSH | Thyroid-Stimulating Hormone |
References
- Safer, J.D. Thyroid hormone action on skin. Dermato-Endocrinology 2011, 3, 211–215. [Google Scholar] [CrossRef]
- Paus, R.; Ramot, Y.; Kirsner, R.S.; Tomic-Canic, M. Topical L-thyroxine: The Cinderella among hormones waiting to dance on the floor of dermatological therapy? Exp. Dermatol. 2020, 29, 910–923. [Google Scholar] [CrossRef]
- Törmä, H.; Rollman, O.; Vahlquist, A. Detection of mRNA transcripts for retinoic acid, vitamin D3, and thyroid hormone (c-erb-A) nuclear receptors in human skin using reverse transcription and polymerase chain reaction. Acta Derm. Venereol. 1993, 73, 102–107. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Kohn, L.; Ain, K.B.; Venkataraman, G.M.; Pisarchik, A.; Chung, J.H.; Giuliani, C.; Thornton, M.; Slugocki, G.; et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J. Investig. Dermatol. 2002, 119, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, F.; Baldini, E.; Cavalli, A.; Marchioni, E.; Lembo, L.; Teson, M.; Persechino, S.; Zambruno, G.; Ulisse, S.; Odorisio, T.; et al. TSH receptor and thyroid-specific gene expression in human skin. J. Investig. Dermatol. 2010, 130, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Langan, E.A.; Meier, N.T.; Funk, W.; Siemers, F.; Paus, R. Thyroxine (T4) may promote re-epithelialisation and angiogenesis in wounded human skin ex vivo. PLoS ONE 2019, 14, e0212659. [Google Scholar] [CrossRef] [PubMed]
- Post, H.; Hundt, J.E.; Zhang, G.; Depping, R.; Rose, C.; Langan, E.A.; Paus, R. Thyroxine restores severely impaired cutaneous re-epithelialisation and angiogenesis in a novel preclinical assay for studying human skin wound healing under “pathological” conditions ex vivo. Arch. Dermatol. Res. 2021, 313, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Shishido, M.; Kuroda, K.; Tsukifuji, R.; Fujita, M.; Shinkai, H. A case of pretibial myxedema associated with Graves’ disease: An immunohistochemical study of serum-derived hyaluronan-associated protein. J. Dermatol. 1995, 22, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Kronick, P.L.; Sacks, M.S. Matrix macromolecules that affect the viscoelasticity of calfskin. J. Biomech. Eng. 1994, 116, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.; Vitti, P.; Chiovato, L.; Ceccarini, G.; Macchia, M.; Montanelli, L.; Gatti, G.; Rosellini, V.; Mammoli, C.; Martino, E.; et al. Role for inner ring deiodination preventing transcutaneous passage of thyroxine. J. Clin. Endocrinol. Metab. 2003, 88, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Certan, D.; Righini, V.; Oliva, M.; Fioravanti, P.; Bevilacqua, M. Bioavailability of L-thyroxine and its metabolites after topical treatment with an emulsion containing 0.1% micronised L-thyroxine. G. Ital. Dermatol. Venereol. 2013, 148, 287–292. [Google Scholar] [PubMed]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. National Cancer Institute, NIH. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 27 November 2017).
- Comi, R.; Bertolotti, M. La Somatoline nel trattamento degli inestetismi da accumulo adiposo sottocutaneo. Il Policlin. Sez. Medica 1994, 101, 1–21. [Google Scholar]
- Lisi, P. Sperimentazione clinica di una crema contenente levo-tiroxina ed escina. G. Ital. Dermatol. Venereol. 1973, 108, 1–7. [Google Scholar]
- Dini, D.; Bianchini, M.; Massa, T.; Fassio, T. Trattamento del linfedema dell’arto superiore conseguente a mastectomia con escina e levo-tiroxina. Minerva Med. 1981, 72, 2319–2322. [Google Scholar] [PubMed]
- European Medicines Agency (EMA). Guideline on the Bioequivalence of Levothyroxine Oral Products. EMA/CHMP/176098/2020, Committee for Medicinal Products for Human Use (CHMP). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/levothyroxine-tablets-125-mcg-25-mcg-50-mcg-75-mcg-100-mcg-and-200-mcg-and-additional-strengths-within-range-product-specific-bioequivalence-guidance_en.pdf (accessed on 10 December 2020).
- Norbiato, G.; Endocrinology Service, “L. Sacco” Hospital, Milan, Italy. Valutazione dell’eventuale biodisponibilità sistemica di l-T4 esogena dopo ripetuta applicazione topica di Somatoline bustine nel volontario sano—Confronto cross-over tra due formulazioni di emulsione, Final Report, Study SOMA 001.98. 1998; Unpublished internal document.
- Buraglio, M.; LCG Bioscience Clinical Pharmacology Unit, Bourn Hall Clinic, Bourn, Cambridge, UK. Somatoline Cream Repeat Topical Application in Women. Evaluation of Absorption, Study Report, Protocol No. LCGCPU 0197 E. 1997; Unpublished internal document.
- Righini, V.; Renzetti, D.; Bevilacqua, M. Studio sulla biodisponibilità sistemica di L-tiroxina4 dopo ripetuta applicazione cutanea di un farmaco topico in commercio. G. Ital. Dermatol. Venereol. 2003, 138, 333–340. [Google Scholar]
- European Medicines Agency (EMA). Guideline on the Investigation of Bioequivalence. EMA/CPMP/EWP/QWP/1401/98 Rev. 1. Committee for Medicinal Products for Human Use (CHMP); London, 20 January 2010, Effective from 1 August 2010. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 16 June 2025).
- Köhrle, J.; Frädrich, C. Deiodinases control local cellular and systemic thyroid hormone availability. Free Radic. Biol. Med. 2022, 193 Pt 1, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Haus, E. Chronobiology in the endocrine system. Adv. Drug Deliv. Rev. 2007, 59, 985–1014. [Google Scholar] [CrossRef] [PubMed]
- Philippe, J.; Dibner, C. Thyroid circadian timing: Roles in physiology and thyroid malignancies. J. Biol. Rhythms 2015, 30, 76–83. [Google Scholar] [CrossRef]
- Russell, W.; Harrison, R.F.; Smith, N.; Darzy, K.; Shalet, S.; Weetman, A.P.; Ross, R.J. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J. Clin. Endocrinol. Metab. 2008, 93, 2300–2306. [Google Scholar] [CrossRef] [PubMed]

| Procedure Timing | Screening | Baseline | Day 0 | Day 1 | Day 14 | EOT | EOS |
|---|---|---|---|---|---|---|---|
| Day—15 → 0 | Day 0 | 5 h | 24 h | Day 14 | Day 28 | Day 42 | |
| Informed Consent | x | ||||||
| Eligibility | x | x | |||||
| Demography and Lifestyle | x | ||||||
| Hematology | x | x | |||||
| Blood chemistry | x | x | |||||
| Urine Drug Screening | x | ||||||
| Urinalysis | x | x | |||||
| Urinary iodine | x | x | |||||
| Virology | x | ||||||
| Alcohol/Nicotine Consumption | x | ||||||
| TSH concentration (a) | x | x | x | x | x | x | x |
| Free T4, rT3, T3 (a) | x | x | x | x | x | x | |
| Pregnancy Test | x | x | |||||
| Treatment (b) | x | x | x | x | x | ||
| Vital Sign (SBP, DBP, HR, RR, BT) | x | x | x | x | x | x | x |
| ECG | x | x | |||||
| Adverse Events | x | x | x | x | x | x | |
| Local Tolerability assessed by investigators | x | x | x | x | x | ||
| Local Tolerability assessed by subject (diary) | x | x | x | x | x |
| Variable | Category | Statistic | Enrolled Subjects |
|---|---|---|---|
| N (%) | |||
| Gender | Female | n (%) | 30 (100.0%) |
| Ethnicity | Caucasian | n (%) | 30 (100.0%) |
| Age (years) | Mean ± SD | 33.3 ± 8.61 | |
| Height (cm) | Mean ± SD | 165.1 ± 4.9 | |
| Weight (kg) | Mean ± SD | 61.2 ± 6.3 | |
| BMI (kg/m2) | Mean ± SD | 22.4 ± 2.2 |
| FT4 (ng/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| VISIT | N | Mean | SD | Min | Median | Max | 95% LCL | 95% UCL |
| Day 0 Baseline | 30 | 1.13 | 0.15 | 0.82 | 1.17 | 1.44 | 1.08 | 1.19 |
| Day 0 (5 h) Control 1 | 30 | 1.09 | 0.15 | 0.82 | 1.12 | 1.46 | 1.03 | 1.14 |
| Day 1 (24 h) Control 2 | 30 | 1.14 | 0.12 | 0.90 | 1.15 | 1.51 | 1.10 | 1.19 |
| Day 14 Control 3 | 30 | 1.10 | 0.12 | 0.86 | 1.10 | 1.39 | 1.06 | 1.15 |
| Day 28 EoT | 30 | 1.11 | 0.13 | 0.91 | 1.12 | 1.40 | 1.07 | 1.16 |
| Day 42 EoS | 30 | 1.13 | 0.14 | 0.87 | 1.12 | 1.52 | 1.08 | 1.18 |
| FT3 (ng/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| VISIT | N | Mean | SD | Min | Median | Max | 95% LCL | 95% UCL |
| Day 0 Baseline | 30 | 3.83 | 0.52 | 2.89 | 3.75 | 4.97 | 3.64 | 4.03 |
| Day 0 (5 h) Control 1 | 30 | 3.52 | 0.44 | 2.77 | 3.52 | 4.72 | 3.36 | 3.69 |
| Day 1 (24 h) Control 2 | 30 | 3.83 | 0.44 | 3.12 | 3.74 | 4.73 | 3.67 | 3.99 |
| Day 14 Control 3 | 30 | 3.77 | 0.42 | 2.87 | 3.75 | 4.53 | 3.62 | 3.93 |
| Day 28 EoT | 30 | 3.80 | 0.40 | 3.06 | 3.74 | 4.57 | 3.65 | 3.95 |
| Day 42 EoS | 30 | 3.67 | 0.40 | 2.95 | 3.70 | 4.59 | 3.52 | 3.82 |
| rT3 (ng/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| VISIT | N | Mean | SD | Min | Median | Max | 95% LCL | 95% UCL |
| Day 0 Baseline | 30 | 0.18 | 0.04 | 0.10 | 0.18 | 0.30 | 0.16 | 0.20 |
| Day 0 (5 h) Control 1 | 30 | 0.20 | 0.05 | 0.11 | 0.19 | 0.34 | 0.18 | 0.22 |
| Day 1 (24 h) Control 2 | 30 | 0.18 | 0.04 | 0.12 | 0.18 | 0.28 | 0.17 | 0.20 |
| Day 14 Control 3 | 30 | 0.19 | 0.06 | 0.10 | 0.19 | 0.42 | 0.17 | 0.22 |
| Day 28 EoT | 30 | 0.20 | 0.06 | 0.10 | 0.19 | 0.33 | 0.18 | 0.22 |
| Day 42 EoS | 30 | 0.20 | 0.05 | 0.11 | 0.21 | 0.30 | 0.18 | 0.21 |
| TSH (mUI/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| VISIT | N | Mean | SD | Min | Median | Max | 95% LCL | 95% UCL |
| Day −15 Screening | 30 | 1.54 | 0.69 | 0.23 | 1.56 | 2.85 | 1.29 | 1.80 |
| Day 0 Baseline | 30 | 1.90 | 0.91 | 0.69 | 1.65 | 3.98 | 1.56 | 2.24 |
| Day 0 (5 h) Control 1 | 30 | 1.27 | 0.48 | 0.53 | 1.47 | 2.29 | 1.10 | 1.45 |
| Day 1 (24 h) Control 2 | 30 | 1.60 | 0.82 | 0.53 | 1.47 | 4.85 | 1.29 | 1.90 |
| Day 14 Control 3 | 30 | 1.61 | 0.81 | 0.44 | 1.42 | 3.85 | 1.31 | 1.92 |
| Day 28 EoT | 30 | 1.85 | 1.00 | 0.53 | 1.58 | 4.48 | 1.48 | 2.23 |
| Day 42 EoS | 30 | 1.63 | 0.75 | 0.38 | 1.62 | 4.20 | 1.35 | 1.91 |
| System Organ Class | Number (%) of Subjects | |||
|---|---|---|---|---|
| Preferred Term | Not Related N = 30 | Related N = 29 | Overall N = 32 | |
| Gastrointestinal disorders | ||||
| Dyspepsia | 1 (6.3%) | 1 (5.3%) | ||
| Injury, poisoning, and procedural complications | ||||
| Limb injury | 1 (6.3%) | 1 (5.3%) | ||
| Musculoskeletal and connective tissue disorders | ||||
| Back pain | 1 (6.3%) | 1 (5.3%) | ||
| Nervous system disorders | ||||
| Headache | 9 (56.3%) | 9 (47.4%) | ||
| Psychiatric disorders | ||||
| Anxiety | 2 (12.5%) | 2 (10.5%) | ||
| Insomnia | 1 (6.3%) | 1 (5.3%) | ||
| Reproductive system and breast disorders | ||||
| Premenstrual syndrome | 1 (6.3%) | 1 (5.3%) | ||
| Skin and subcutaneous tissue disorders | ||||
| Erythema | 2 (66.7%) | 2 (10.5%) | ||
| Pruritus | 1 (33.3%) | 1 (5.3%) | ||
| ALL | 16 (100%) | 3 (100%) | 19 (100%) | |
| Baseline | Change from Baseline | |||||
|---|---|---|---|---|---|---|
| Day 0 | 5 h | Day 1 | Day 14 | Day 28 | Day 42 | |
| N = 30 | N = 30 | N = 30 | N = 30 | N = 30 | N = 30 | |
| SBP (mmHg) | 111.6 ± 9.0 | −4.1 ± 7.5 | −1.4 ± 8.6 | −1.1 ± 9.0 | −1.8 ± 7.9 | 0.1 ± 10.4 |
| DBP (mmHg) | 75.0 ± 7.5 | −2.7 ± 7.4 | −1.4 ± 7.3 | −1.5 ± 6.9 | −2.4 ± 6.5 | −5.1 ± 5.9 |
| HR (bpm) | 75.5 ± 10.4 | −6.8 ± 7.6 | 2.1 ± 9.1 | −0.2 ± 14.8 | −0.8 ± 11.3 | −2.3 ± 10.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gori, G.; De Negri, F.; Fioravanti, A.; De Feo, F.; Castiglioni, C.; Fini, E. A Single-Group, Open-Label Study on the Systemic Bioavailability, Safety, and Local Tolerability of a New L-Thyroxine/Escin Gel Formulation in Healthy Women. Future Pharmacol. 2025, 5, 33. https://doi.org/10.3390/futurepharmacol5030033
Gori G, De Negri F, Fioravanti A, De Feo F, Castiglioni C, Fini E. A Single-Group, Open-Label Study on the Systemic Bioavailability, Safety, and Local Tolerability of a New L-Thyroxine/Escin Gel Formulation in Healthy Women. Future Pharmacology. 2025; 5(3):33. https://doi.org/10.3390/futurepharmacol5030033
Chicago/Turabian StyleGori, Giovanni, Ferdinando De Negri, Anna Fioravanti, Francesca De Feo, Catia Castiglioni, and Elisabetta Fini. 2025. "A Single-Group, Open-Label Study on the Systemic Bioavailability, Safety, and Local Tolerability of a New L-Thyroxine/Escin Gel Formulation in Healthy Women" Future Pharmacology 5, no. 3: 33. https://doi.org/10.3390/futurepharmacol5030033
APA StyleGori, G., De Negri, F., Fioravanti, A., De Feo, F., Castiglioni, C., & Fini, E. (2025). A Single-Group, Open-Label Study on the Systemic Bioavailability, Safety, and Local Tolerability of a New L-Thyroxine/Escin Gel Formulation in Healthy Women. Future Pharmacology, 5(3), 33. https://doi.org/10.3390/futurepharmacol5030033






