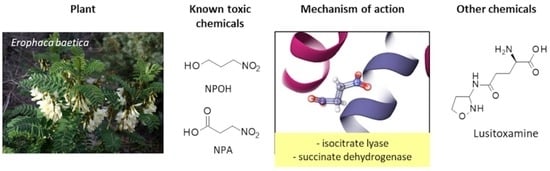
On the Molecular Origin of the Toxicity of Erophaca baetica (L.) Boiss.
Abstract
1. Introduction
2. Toxicity of Erophaca baetica
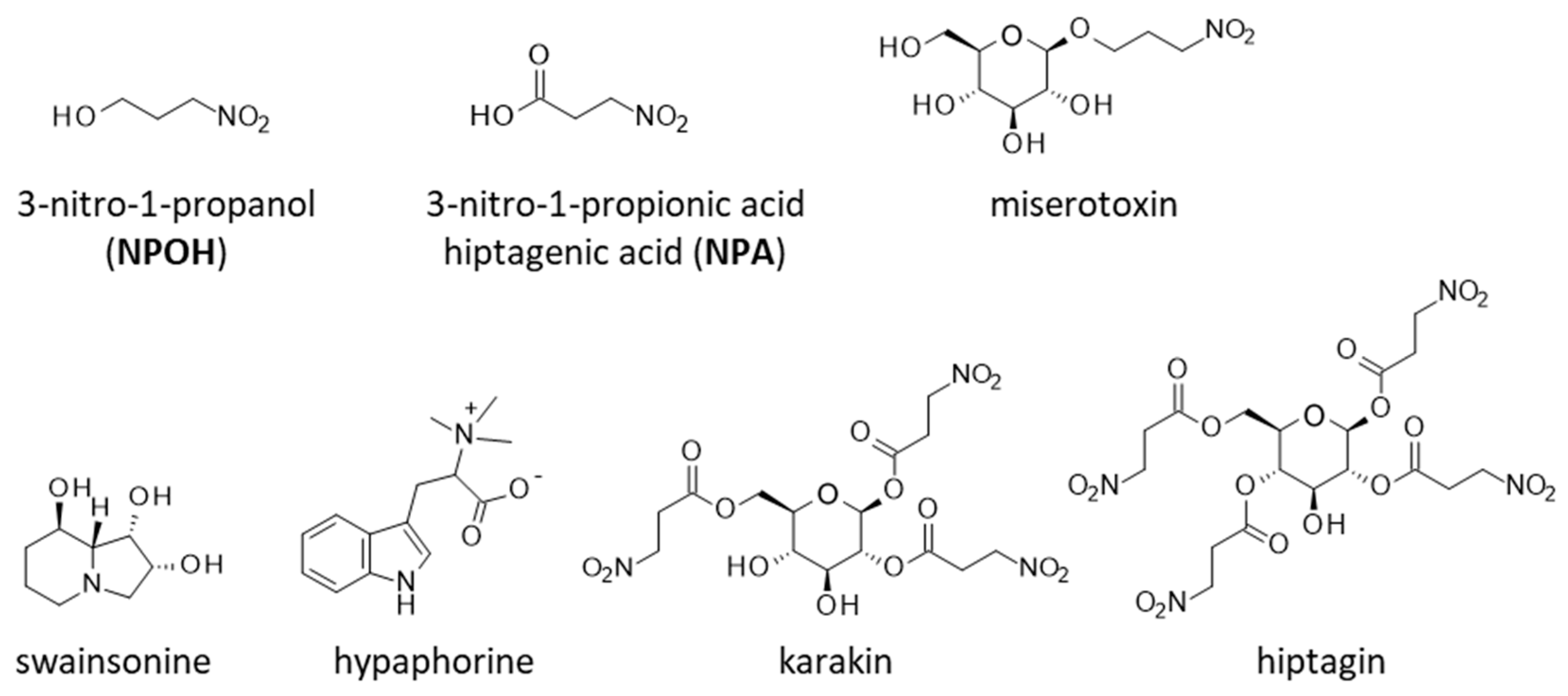
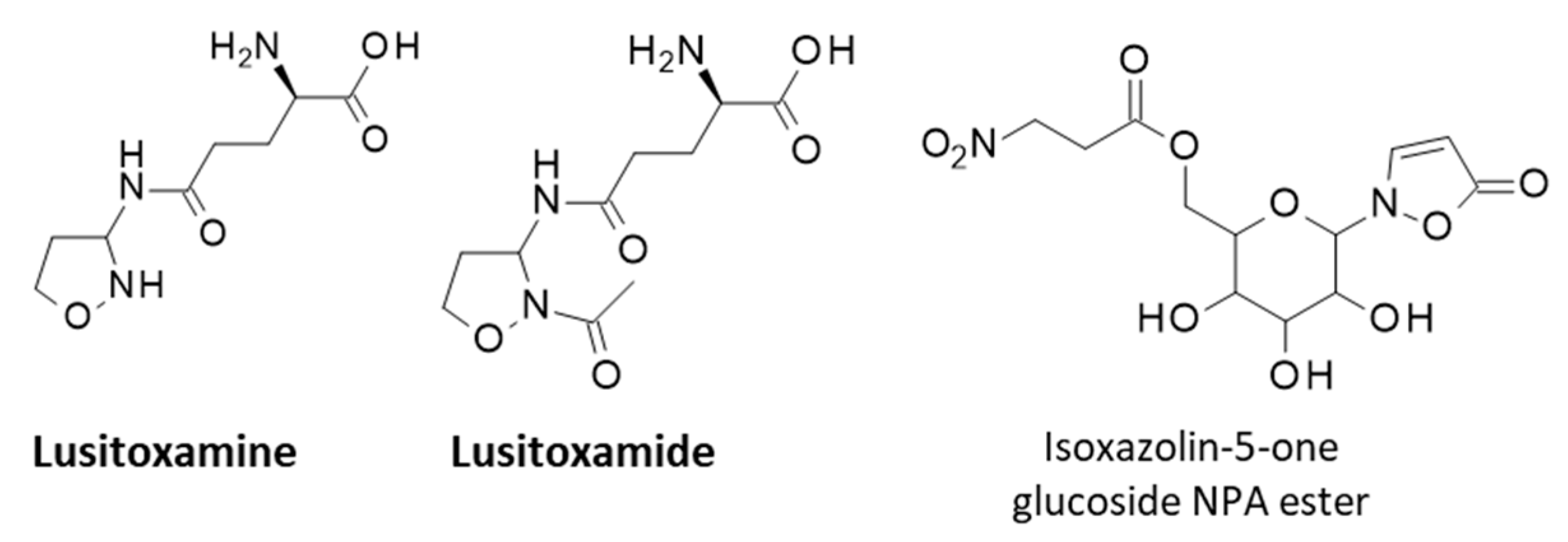
3. Toxic Phytochemicals from E. baetica: The Nitrotoxin Hypothesis
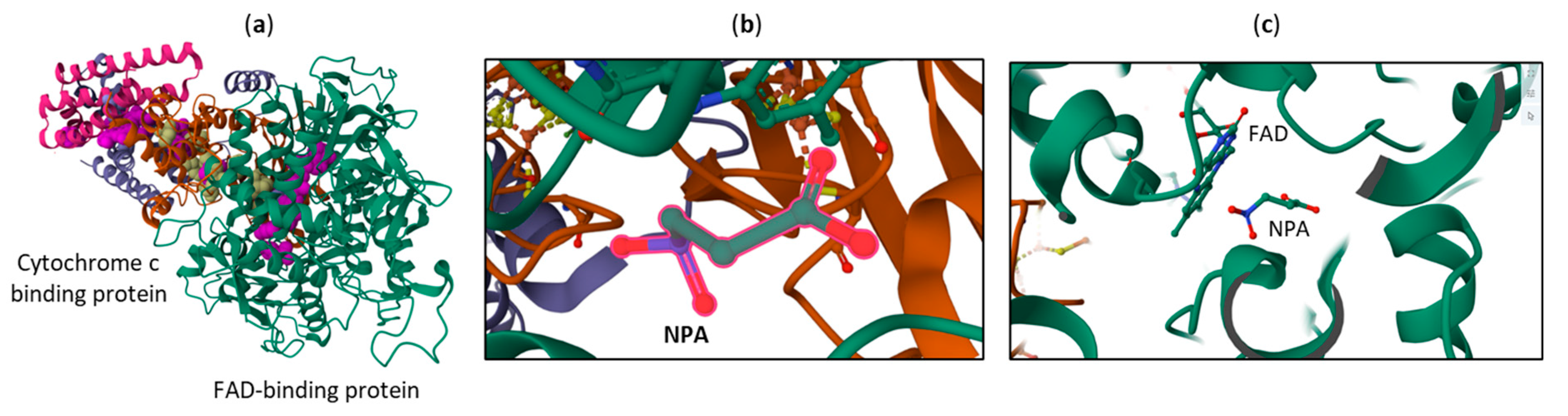
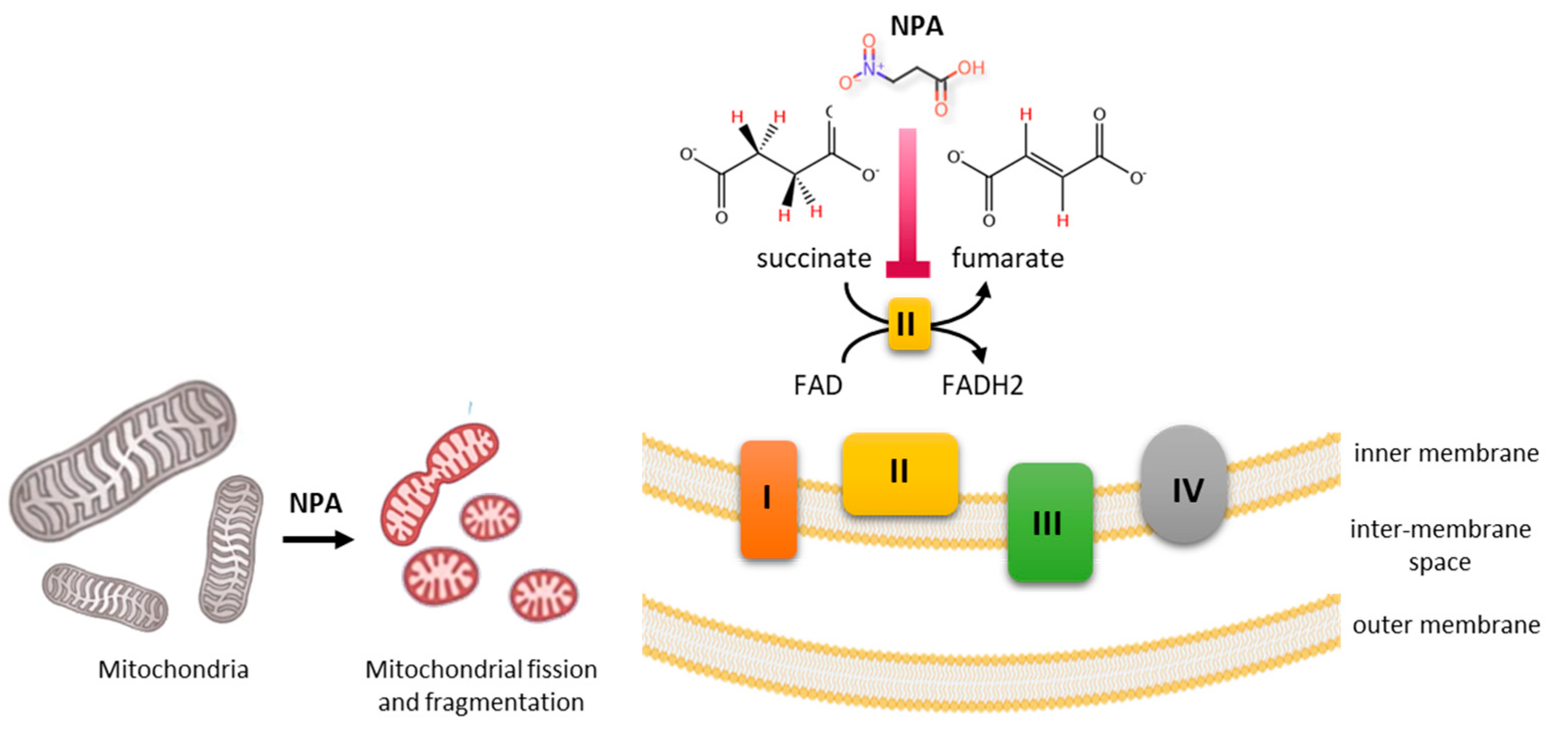
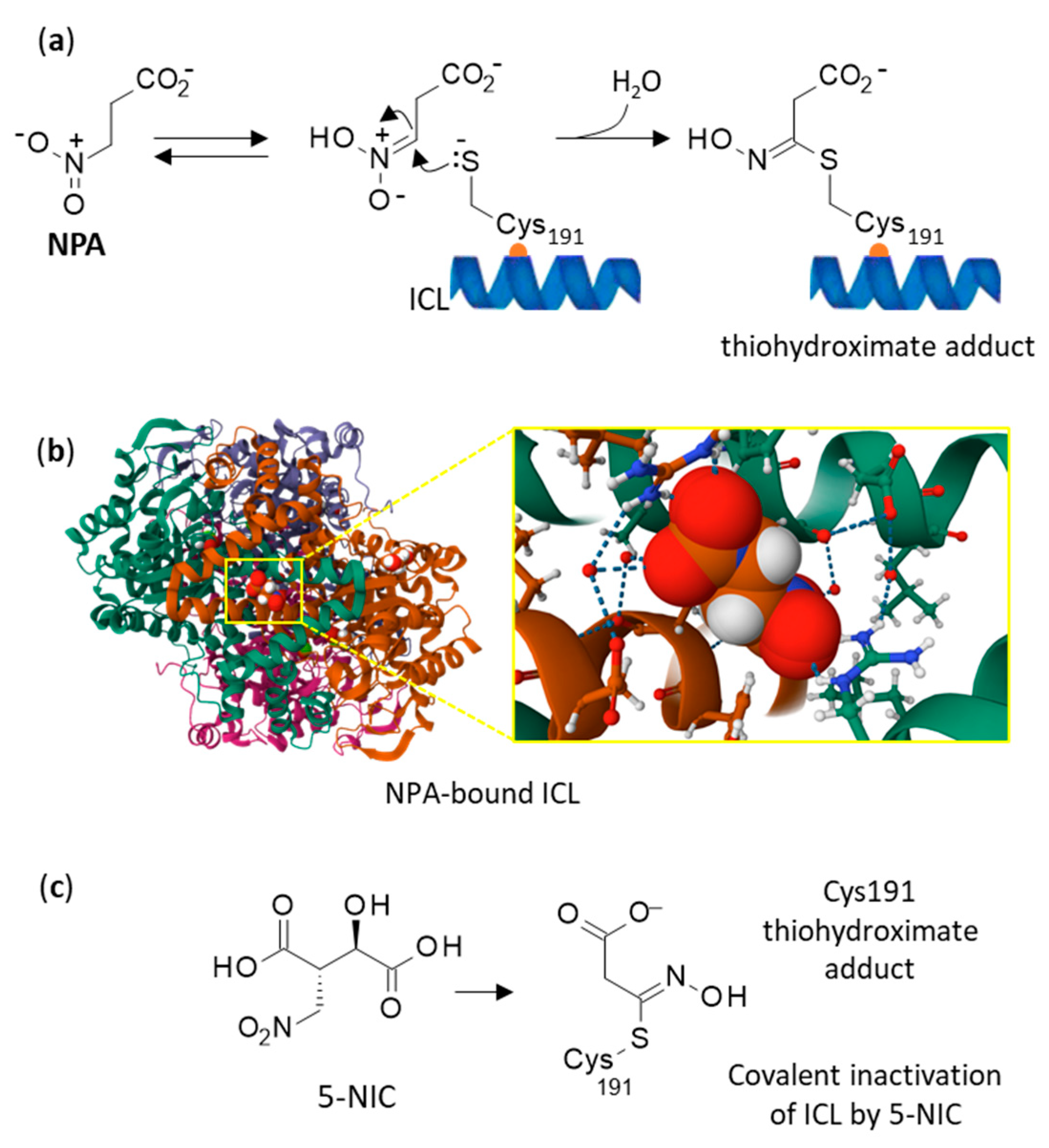
4. Toxicity and Mechanism of Action NPA and NPOH
5. Conclusions
6. Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ICL | isocitrate lyase |
| NPA | 3-nitropropionic acid |
| NPOH | 3-nitropropanol |
References
- Rivera, D.; Verde, A.; Fajardo Rodríguez, J.; Ríos, S.; Alcaraz, F.; Cárceles, C.; Ortíz, J.; Valdés, A.; Ruíz-Gallardo, J.R.; García-Flores, A.; et al. Ethnoveterinary Medicine and Ethnopharmacology in the Main Transhumance Areas of Castilla-La Mancha (Spain). Front. Vet. Sci. 2022, 9, 866132. [Google Scholar] [CrossRef] [PubMed]
- Najem, M.; Nassiri, L.; Ibijbijen, J. Vernacular names of toxic plants used as medicine in the central Middle Atlas-Morocco. Ethnobot. Res. Appl. 2020, 20, 48. [Google Scholar]
- Najem, M.; Nassiri, L.; Ibijbijen, J. Origin of vernacular names of plants: Case of toxic plants for medicinal use in the central Middle Atlas—Morocco. Interdiscip. Sci. Rev. 2021, 47, 40–61. [Google Scholar] [CrossRef]
- Casimiro-Soriguer, R.; Talavera, M.; Balao, F.; Terrab, A.; Herrera, J.; Talavera, S. Phylogeny and genetic structure of Erophaca (Leguminosae), an East-West Mediterranean disjunct genus from the Tertiary. Mol. Phylogenet. Evol. 2010, 56, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Duval, J. Perceptions et Usages de la Diversité éCologique des Parcours Présahariens par les éLeveurs Camelins dans la Zone Rurale de M’Hamid El Ghizlane au Maroc. Mémoire de Fin d’éTudes. 2019. Available online: https://camed.cirad.fr/fr/content/download/4186/31380/version/1/file/Duval+2019+Perceptions+et+usages+diversit%C3%A9+%C3%A9colo+parcours+pr%C3%A9sahariens+par+%C3%A9leveurs+camelins+Zone+M%27Hamid+El+Ghizlane+Maroc+%28M%C3%A9moire%29.pdf (accessed on 15 April 2025).
- Casimiro-Soriguer, R.; Herrera, J.; Talavera, S. Andromonoecy in an Old World Papilionoid legume, Erophaca baetica. Plant Biol. 2013, 15, 353–359. [Google Scholar] [CrossRef]
- Lyu, S.T.; Zou, T.T.; Jiang, Q.L.; Wang, X.F. Maintenance of andromonoecy in an autogamous species: Superior male function in male flowers of the endangered Sagittaria guayanensis. Plant Divers. 2024, 46, 783–790. [Google Scholar] [CrossRef]
- Murakami, K.; Katsuhara, K.R.; Ushimaru, A. Intersexual flower differences in an andromonoecious species: Small pollen-rich staminate flowers under resource limitation. Plant Biol. 2022, 24, 259–265. [Google Scholar] [CrossRef]
- Rios, J.L.; Waterman, P.G. A Review of the Pharmacology and Toxicology of Astragalus. Phytother. Res. 1997, 11, 411–418. [Google Scholar] [CrossRef]
- Wagstaff, D.J. International Poisonous Plants Checklist. An Evidence-Based Reference; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2008. [Google Scholar]
- Abdennebi, E.H.; el Ouazzani, N.; Lamnaouer, D. Clinical and analytical studies of sheep dosed with various preparations of Astragalus lusitanicus. Vet. Hum. Toxicol. 1998, 40, 327–331. [Google Scholar]
- Ouazzani, N.; Lamnaouer, D.; Abdennebi, E.H. Toxicology of Astragalus lusitanicus Lam. Therapie 1999, 54, 707–710. [Google Scholar]
- El-Hamidi, M.; Leipold, H.W. Poisoning of Sheep by Astragalus lusitanicus in Morocco: Field and Experimental Studies. J. Vet. Med. A 1989, 36, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.E.; Gardner, D.R.; Armien, A.G.; Fortunato, R.H.; Uzal, F.A. Intoxication of llamas by Astragalus punae in Argentina. J. Vet. Diagn. Investig. 2022, 34, 674–678. [Google Scholar] [CrossRef]
- Garcia Roman, A.; Moyano Salvago, M.R.; Soler Rodriguez, F.; Infante Miranda, F. Physiopathologic changes in lambs fed with Astragalus lusitanicus Lam. Vet. Hum. Toxicol. 1987, 29, 387–389. [Google Scholar] [PubMed]
- Tarazona, J.V.; Sanz, F. Toxicity of fractions obtained from the legume species Astragalus lusitanicus Lam. lusitanicus. Toxicon. 1990, 28, 235–237. [Google Scholar] [CrossRef]
- Bel-Kassaoui, H.; Lamnaouer, D.; Abdennebi, E.H.; Jossang, A. Experimental poisoning by extracts and fractions of Astragalus lusitanicus Lam. in sheep. Rev. Med. Vet. 2007, 158, 269–273. [Google Scholar]
- Poyato, J. Estudio fitoquimico, toxicologico y farmacologico del Astragalus lusitanicus. Rev. Patron. Biol. Anim. 1969, XII, 67–118. [Google Scholar]
- Gonzalez, J.M.; Tovar, P.; Castejon, F.J.; Gonzalez, M. Intoxicacion experimental por Astragalus lusitanicus Lam. I. Alteraciones cardio-respiratorias. Archos Zootec. 1980, 29, 23–30. [Google Scholar]
- Quintas, H.; Aguiar, C.; Ramos Antón, J.J.; Lacasta Lozano, D.; Ferrer Mayayo, L.M. Intoxicación por plantas en rumiantes: Bases para el diagnóstico clínico. In Agrárias: Pesquisa e Inovação nas Ciências que Alimentam o Mundo VII; Editora Artemis: Curitiba, Brazil, 2021; pp. 291–301. ISBN 978-65-87396-51-4. [Google Scholar]
- Pascual, J.; Hernandez, J.C.; Grande, M. Componentes de Astragalus lusitanicus. Lam. II. Flavonoides. An. Quim. 1979, 75, 1005–1007. [Google Scholar]
- Pascual, J.; Hernandez, J.C.; Sanchez, J.J. Componentes de Astragalus lusitanicus Lam. I. Selenio, pinitol y derivados nitrados. An. Quiet. 1979, 75, 775–777. [Google Scholar]
- Pascual, J.; Hernandez, J.C.; San Feliciano, A.; Miguel, J.M. Saccharinic acid lactone from Astragalus lusitanicus Lam. (-)-2-C-methyl-D-erythrono-1,4-lactone. Tetrahedron Lett. 1980, 21, 1359–1360. [Google Scholar]
- Cook, D.; Gardner, D.R.; Pfister, J.A. Swainsonine-containing plants and their relationship to endophytic fungi. J. Agric. Food Chem. 2014, 62, 7326–7334. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Gardner, D.R.; Lee, S.T.; Pfister, J.A.; Stonecipher, C.A.; Welsh, S.L. A swainsonine survey of North American Astragalus and Oxytropis taxa implicated as locoweeds. Toxicon 2016, 118, 104–111. [Google Scholar] [CrossRef]
- Cook, D.; Gardner, D.R.; Martinez, A.; Robles, C.A.; Pfister, J.A. Screening for swainsonine among South American Astragalus species. Toxicon 2017, 139, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Pistán, M.E.; Cook, D.; Gutiérrez, S.A.; Schnittger, L.; Gardner, D.R.; Cholich, L.A.; Gonzalez, A.M. Identification and distribution of a fungal endosymbiotic Alternaria species (Alternaria section Undifilum sp.) in Astragalus garbancillo tissues. Mycologia 2024, 116, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Abdennebi, E.H.; Ouazzani, N.; Jossang, A.; Benkouka, F.; Lamnaouer, D. Inhibition of glycosidases by Astragalus lusitanicus and correlation with toxicity. Vet. Hum. Toxicol. 2001, 43, 266–269. [Google Scholar] [PubMed]
- Bel-Kassaoui, H.; Lamnaouer, D.; Jossang, A.; Abdennebi el, H.; Charrouf, Z.; Bodo, B. Role of hypaphorine in the toxicity of Astragalus lusitanicus. Nat. Prod. Res. 2008, 22, 453–457. [Google Scholar] [CrossRef]
- Mohamed, I.I.; Hassan, H.E.; Ahmed, S.A.; Bahaa, S.M. Alkaloids of Astragalus kahiricus DC. plant roots. Bull. Fac. Agric. Cairo Univ. 2009, 60, 366–370. [Google Scholar] [CrossRef]
- Hou, B.; Wen, Y.; Zhu, X.; Qi, M.; Cai, W.; Du, B.; Sun, H.; Qiu, L. Preparation and characterization of vaccarin, hypaphorine and chitosan nanoparticles and their promoting effects on chronic wounds healing. Int. J. Biol. Macromol. 2022, 221, 1580–1592. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Gan, L.; Wu, R.; Jin, J.; Wang, T.; Sun, S.; Zhang, Z.; Li, L.; Zheng, X.; et al. Hepatoprotective effects of Niudali (Callerya speciosa) root aqueous extracts against tetrachloromethane-induced acute liver injury and inflammation. Food Sci. Nutr. 2023, 11, 7026–7038. [Google Scholar] [CrossRef]
- Ivanov, I.A.; Siniavin, A.E.; Palikov, V.A.; Senko, D.A.; Shelukhina, I.V.; Epifanova, L.A.; Ojomoko, L.O.; Belukhina, S.Y.; Prokopev, N.A.; Landau, M.A.; et al. Analogs of 6-Bromohypaphorine with Increased Agonist Potency for α7 Nicotinic Receptor as Anti-Inflammatory Analgesic Agents. Mar. Drugs 2023, 21, 368. [Google Scholar] [CrossRef]
- Tarazona, J.V.; Sanz, F. Aliphatic nitro compounds in Astragalus lusitanicus Lam. Vet. Hum. Toxicol. 1987, 29, 437–439. [Google Scholar] [PubMed]
- Williams, M.C. Nitro compounds in foreign species of Astragalus. Weed Sci. 1981, 29, 261–269. [Google Scholar] [CrossRef]
- Williams, M.C. 3-Nitropropionic acid and 3-nitro-1-propanol in species of Astragalus. Can. J. Bot. 1982, 60, 1956–1963. [Google Scholar] [CrossRef]
- Hamilton, B.F.; Gould, D.H.; Gustine, D.L. History of 3-Nitropropionic Acid. Occurrence and Role in Human and Animal Disease. In Mitochondrial Inhibitors and Neurodegenerative Disorders. Contemporary Neuroscience; Sanberg, P.R., Nishino, H., Borlongan, C.V., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 21–33. [Google Scholar] [CrossRef]
- Liu, H.; Shao, S.; Schellenberg, M. A Simple and Fast Procedure to Determine 3-Nitropropanoic Acid and 3-Nitropropanol in Freeze Dried Canadian Milkvetch (Astragalus canadensis). Toxins 2017, 9, 204. [Google Scholar] [CrossRef]
- Tarazona Lafarga, J.V. Origin of the Toxicicity of the Astrafalus lusitanicus Lam. and Its Relation with Acid 3-Nitropropanol and Other Extracts Spanish. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1988. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=198174 (accessed on 15 April 2025).
- Carter, C.L.; McChesney, W.J. Hiptagenic acid identified as beta-nitropropionic acid. Nature 1949, 164, 575. [Google Scholar] [CrossRef]
- Morris, M.P.; Pagán, C.; Warmke, H.E. Hiptagenic Acid, a Toxic Component of Indigofera endecaphylla. Science 1954, 119, 322–323. [Google Scholar] [CrossRef]
- Becker, T.; Pasteels, J.; Weigel, C.; Dahse, H.M.; Voigt, K.; Boland, W. A tale of four kingdoms—Isoxazolin-5-one- and 3-nitropropanoic acid-derived natural products. Nat. Prod. Rep. 2017, 34, 343–360. [Google Scholar] [CrossRef]
- Raistrick, H.; Stössl, A. Studies in the biochemistry of micro-organisms. 104. Metabolites of Penicillium atrovenetum G. Smith: β-nitropropionic acid, a major metabolite. Biochem. J. 1958, 68, 647–653. [Google Scholar]
- Anzai, K.; Suzuki, S. A New Antibiotic Bovinocidin, Identified as β-Nitropropionic Acid. J. Antibiot. 1960, 13, 133–136. [Google Scholar]
- Rotilio, L.; Boverio, A.; Nguyen, Q.T.; Mannucci, B.; Fraaije, M.W.; Mattevi, A. A biosynthetic aspartate N-hydroxylase performs successive oxidations by holding intermediates at a site away from the catalytic center. J. Biol. Chem. 2023, 299, 104904. [Google Scholar] [CrossRef]
- Chomcheon, P.; Wiyakrutta, S.; Sriubolmas, N.; Ngamrojanavanich, N.; Isarangkul, D.; Kittakoop, P. 3-Nitropropionic acid (3-NPA), a potent antimycobacterial agent from endophytic fungi: Is 3-NPA in some plants produced by endophytes? J. Nat. Prod. 2005, 68, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.D.; McCloskey, J.A. Biosynthesis of nitro compounds. II. Studies on potential precursors for the nitro group of beta-nitropropionic acid. Biochemistry 1967, 6, 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.L.; Smith, S.L.; Martin, J.R.; Hanley, A.B. The fungal biosynthesis of 3-nitropropionic acid: Is the decarboxylation of L-nitrosuccinate an enzymatic reaction? J. Chem. Soc. Perkin Trans. 1 1994, 16, 2297–2299. [Google Scholar] [CrossRef]
- Johnson, C.W.; Ohashi, M.; Tang, Y. How Fungi Biosynthesize 3-Nitropropanoic Acid: The Simplest yet Lethal Mycotoxin. Org. Lett. 2024, 26, 3158–3163. [Google Scholar] [CrossRef]
- Francis, K.; Smitherman, C.; Nishino, S.F.; Spain, J.C.; Gadda, G. The biochemistry of the metabolic poison propionate 3-nitronate and its conjugate acid, 3-nitropropionate. IUBMB Life 2013, 65, 759–768. [Google Scholar] [CrossRef]
- Takács, O.; Nagyné Nedves, A.; Boldizsár, I.; Höhn, M.; Béni, S.; Gampe, N. Analysis of 3-nitropropionic acid in Fabaceae plants by HPLC-MS/MS. Phytochem. Anal. 2022, 33, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- MacAskill, J.J.; Manley-Harris, M.; Field, R.J. Quantification of nitropropanoyl glucosides in karaka nuts before and after treatment. Food Chem. 2015, 175, 543–548. [Google Scholar] [CrossRef]
- Johnson, D.L.; Majak, W.; Benn, M.H. Excretion of miserotoxin and detoxification of the aglycone by grasshoppers (Orthoptera: Acrididae). Phytochemistry 2001, 58, 739–742. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Norris, F.A.; Williams, M.C. Miserotoxin, new naturally occurring nitro compound. J. Am. Chem. Soc. 1969, 91, 4599–4600. [Google Scholar] [CrossRef]
- Patocka, J.; Bielavský, J.; Cabal, J.; Fusek, J. 3-Nitropropionic acid and similar nitrotoxins. Acta Medica 2000, 43, 9–13. [Google Scholar] [CrossRef]
- Coles, C.J.; Edmondson, D.E.; Singer, T.P. Inactivation of succinate dehydrogenase by 3-nitropropionate. J. Biol. Chem. 1979, 254, 5161–5167. [Google Scholar] [CrossRef]
- Huang, L.S.; Sun, G.; Cobessi, D.; Wang, A.C.; Shen, J.T.; Tung, E.Y.; Anderson, V.E.; Berry, E.A. 3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 2006, 281, 5965–5972. [Google Scholar] [CrossRef]
- Becker, T.; Ploss, K.; Boland, W. Biosynthesis of isoxazolin-5-one and 3-nitropropanoic acid containing glucosides in juvenile Chrysomelina. Org. Biomol. Chem. 2016, 14, 6274–6280. [Google Scholar] [CrossRef]
- Liot, G.; Bossy, B.; Lubitz, S.; Kushnareva, Y.; Sejbuk, N.; Bossy-Wetzel, E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009, 16, 899–909. [Google Scholar] [CrossRef]
- He, F.; Zhang, S.; Qian, F.; Zhang, C. Delayed dystonia with striatal CT lucencies induced by a mycotoxin (3-nitropropionic acid). Neurology 1995, 45, 2178–2183. [Google Scholar] [CrossRef]
- Brouillet, E.; Guyot, M.C.; Mittoux, V.; Altairac, S.; Condé, F.; Palfi, S.; Hantraye, P. Partial inhibition of brain succinate dehydrogenase by 3-nitropropionic acid is sufficient to initiate striatal degeneration in rat. J. Neurochem. 1998, 70, 794–805. [Google Scholar] [CrossRef]
- Bouillaud, F. Inhibition of Succinate Dehydrogenase by Pesticides (SDHIs) and Energy Metabolism. Int. J. Mol. Sci. 2023, 24, 4045. [Google Scholar] [CrossRef]
- Upadhayay, S.; Yedke, N.G.; Rahi, V.; Singh, S.; Kumar, S.; Arora, A.; Chandolia, P.; Kaur, P.; Kumar, M.; Koshal, P.; et al. An Overview of the Pathophysiological Mechanisms of 3-Nitropropionic Acid (3-NPA) as a Neurotoxin in a Huntington’s Disease Model and Its Relevance to Drug Discovery and Development. Neurochem. Res. 2023, 48, 1631–1647. [Google Scholar] [CrossRef]
- Noureldeen, M.E.; Shahin, N.N.; Amin, H.A.A.; El-Sawalhi, M.M.; Ghaiad, H.R. Parthenolide ameliorates 3-nitropropionic acid-induced Huntington’s disease-like aberrations via modulating NLRP3 inflammasome, reducing microglial activation and inducing astrocyte shifting. Mol. Med. 2024, 30, 158. [Google Scholar] [CrossRef]
- Lum, P.T.; Sekar, M.; Seow, L.J.; Shaikh, M.F.; Arulsamy, A.; Retinasamy, T.; Gan, S.H.; Gnanaraj, C.; Esa, N.M.; Ramachawolran, G.; et al. Neuroprotective potency of mangiferin against 3-nitropropionic acid induced Huntington’s disease-like symptoms in rats: Possible antioxidant and anti-inflammatory mechanisms. Front. Pharmacol. 2023, 14, 1189957. [Google Scholar] [CrossRef]
- Túnez, I.; Tasset, I.; Pérez-De La Cruz, V.; Santamaría, A. 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: Past, present and future. Molecules 2010, 15, 878–916. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Biel, N.; Camins, A.; Pelegrí, C.; Vilaplana, J.; Pallàs, M.; Canudas, A.M. 3-Nitropropionic acid activates calpain/cdk5 pathway in rat striatum. Neurosci. Lett. 2007, 421, 77–81. [Google Scholar] [CrossRef]
- Sun, F.; Huo, X.; Zhai, Y.; Wang, A.; Xu, J.; Su, D.; Bartlam, M.; Rao, Z. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 2005, 121, 1043–1057. [Google Scholar] [CrossRef]
- Majak, W.; Pass, M.A.; Madryga, F.J. Toxicity of miserotoxin and its aglycone (3-nitropropanol) to rats. Toxicol. Lett. 1983, 19, 171–178. [Google Scholar] [CrossRef]
- Muir, A.D.; Majak, W.; Pass, M.A.; Yost, G.S. Conversion of 3-nitropropanol (miserotoxin aglycone) to 3-nitropropionic acid in cattle and sheep. Toxicol. Lett. 1984, 20, 137–141. [Google Scholar] [CrossRef]
- Teng, P.Y.; Kim, W.K. Roles of Nitrocompounds in Inhibition of Foodborne Bacteria, Parasites, and Methane Production in Economic Animals. Animals 2021, 11, 923. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, R.; Warzecha, C.M.; Coverdale, J.A.; Latham, E.A.; Hume, M.E.; Callaway, T.R.; O’Neil, M.R.; Beier, R.C.; Anderson, R.C.; et al. Characterization of bovine ruminal and equine cecal microbial populations enriched for enhanced nitro-toxin metabolizing activity. Anaerobe 2014, 26, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Rasmussen, M.A.; Jensen, N.S.; Allison, M.J. Denitrobacterium detoxificans gen. nov., sp. nov., a ruminal bacterium that respires on nitrocompounds. Int. J. Syst. Evol. Microbiol. 2000, 50, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Coolen, S.; Rogowska-van der Molen, M.A.; Kwakernaak, I.; van Pelt, J.A.; Postma, J.L.; van Alen, T.; Jansen, R.S.; Welte, C.U. Microbiota of pest insect Nezara viridula mediate detoxification and plant defense repression. ISME J. 2024, 18, wrae097. [Google Scholar] [CrossRef]
- Rogowska-van der Molen, M.A.; Nagornîi, D.; Coolen, S.; de Graaf, R.M.; Berben, T.; van Alen, T.; Janssen, M.A.C.H.; Rutjes, F.P.J.T.; Jansen, R.S.; Welte, C.U. Insect Gut Isolate Pseudomonas sp. Strain Nvir Degrades the Toxic Plant Metabolite Nitropropionic Acid. Appl. Environ. Microbiol. 2022, 88, e0071922. [Google Scholar] [CrossRef]
- Rogowska-van der Molen, M.A.; Savova, H.V.; Janssen, E.A.T.; van Alen, T.; Coolen, S.; Jansen, R.S.; Welte, C.U. Unveiling Detoxifying Symbiosis and Dietary Influence on the Southern Green Shield Bug Microbiota. FEMS Microbiol. Ecol. 2024, 100, fiae150. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, A.; Becker, T.; Pauls, G.; von Reuß, S.H.; Boland, W. Spodoptera littoralis detoxifies neurotoxic 3-nitropropanoic acid by conjugation with amino acids. Insect Biochem. Mol. Biol. 2015, 63, 97–103. [Google Scholar] [CrossRef]
- Ray, S.; Kreitler, D.F.; Gulick, A.M.; Murkin, A.S. The Nitro Group as a Masked Electrophile in Covalent Enzyme Inhibition. ACS Chem. Biol. 2018, 13, 1470–1473. [Google Scholar] [CrossRef] [PubMed]
- Mellott, D.M.; Torres, D.; Krieger, I.V.; Cameron, S.A.; Moghadamchargari, Z.; Laganowsky, A.; Sacchettini, J.C.; Meek, T.D.; Harris, L.D. Mechanism-Based Inactivation of Mycobacterium tuberculosis Isocitrate Lyase 1 by (2R,3S)-2-Hydroxy-3-(nitromethyl)succinic acid. J. Am. Chem. Soc. 2021, 143, 17666–17676. [Google Scholar] [CrossRef]
- Ray, S.; Murkin, A.S. New Electrophiles and Strategies for Mechanism-Based and Targeted Covalent Inhibitor Design. Biochemistry 2019, 58, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, M.M.; Murkin, A.S. Cysteine is the general base that serves in catalysis by isocitrate lyase and in mechanism-based inhibition by 3-nitropropionate. Biochemistry 2014, 53, 178–187. [Google Scholar] [CrossRef]
- Torres-Guzman, J.C.; Padilla-Guerrero, I.E.; Cervantes-Quintero, K.Y.; Martinez-Vazquez, A.; Ibarra-Guzman, M.; Gonzalez-Hernandez, G.A. Peculiarities of nitronate monooxygenases and perspectives for in vivo and in vitro applications. Appl. Microbiol. Biotechnol. 2021, 105, 8019–8032. [Google Scholar] [CrossRef]
- Richter, A.; Rudolph, I.; Möllmann, U.; Voigt, K.; Chung, C.W.; Singh, O.M.P.; Rees, M.; Mendoza-Losana, A.; Bates, R.; Ballell, L.; et al. Novel insight into the reaction of nitro, nitroso and hydroxylamino benzothiazinones and of benzoxacinones with Mycobacterium tuberculosis DprE1. Sci. Rep. 2018, 8, 13473. [Google Scholar] [CrossRef]
- James, L.F.; Hartley, W.J.; Williams, M.C.; Van Kampen, K.R. Field and experimental studies in cattle and sheep poisoned by nitro-bearing Astragalus or their toxins. Am. J. Vet. Res. 1980, 41, 377–382. [Google Scholar] [CrossRef]
- Johnson, J.R.; Robinson, B.L.; Ali, S.F.; Binienda, Z. Dopamine toxicity following long term exposure to low doses of 3-nitropropionic acid (3-NPA) in rats. Toxicol. Lett. 2000, 116, 113–118. [Google Scholar] [CrossRef]
- Birkelund, T.; Johansen, R.F.; Illum, D.G.; Dyrskog, S.E.; Østergaard, J.A.; Falconer, T.M.; Andersen, C.; Fridholm, H.; Overballe-Petersen, S.; Jensen, J.S. Fatal 3-Nitropropionic Acid Poisoning after Consuming Coconut Water. Emerg. Infect. Dis. 2021, 27, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Bendiksen Skogvold, H.; Yazdani, M.; Sandås, E.M.; Østeby Vassli, A.; Kristensen, E.; Haarr, D.; Rootwelt, H.; Elgstøen, K.B.P. A pioneer study on human 3-nitropropionic acid intoxication: Contributions from metabolomics. J. Appl. Toxicol. 2022, 42, 818–829. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G.; Soni, M.G. Safety assessment of β-nitropropionic acid: A monograph in support of an acceptable daily intake in humans. Food Chem. 2001, 75, 1–27. [Google Scholar] [CrossRef]
- Milutinović, A. Lithium chloride could aggravate brain injury caused by 3-nitropropionic acid. Bosn. J. Basic Med. Sci. 2016, 16, 261–267. [Google Scholar] [CrossRef][Green Version]
- Somer, G.; Caliskan, A.C. Selenium and Trace Element Distribution in Astragalus Plants: Developing a Differential Pulse Polarographic Method for Their Determination. Turk. J. Chem. 2007, 31, 3. [Google Scholar]
- Lahsissene, H.; Kahouadji, A.; Tijane, M.; Hseine, S. Catalogue des plantes médicinales utilisées dans la région de Zaër (Maroc Oriental). Rev. Bot. 2009, 186, 2. [Google Scholar]
- Naciri, K.; Aboukhalaf, A.; Kalili, A.; Moujabbir, S.; Essaih, S.; Tbatou, M.; Belahyane, A.; Belahsen, R. Ethnobotanical knowledge of wild food plants in Khenifra, a province in the Middle Atlas region of Morocco. GSC Adv. Res. Rev. 2022, 13, 180–200. [Google Scholar] [CrossRef]
- Obregón, R.; Shaw, M.R.; Fernández-Haeger, J.; Jordano, D. Parasitoid and ant interactions of some Iberian butterflies (Insecta: Lepidoptera). SHILAP Rev. Lepidopterol. 2015, 43, 439–454. [Google Scholar]
- Cortés-Giraldo, I.; Alaiz, M.; Girón-Calle, J.; Megías, C.; Vioque, J. Nutritional and functional characteristics of Erophaca baetica seeds, a legume endemic to the Mediterranean region. Grasas y Aceites 2013, 64, 229–236. [Google Scholar] [CrossRef][Green Version]
- Silva, P.C.; Costa, J.S.; Pereira, V.L.P. An expeditious synthesis of 3-nitropropionic acid and its methyl esters. Synth. Commun. 2001, 31, 595–600. [Google Scholar] [CrossRef]
- Bel Kassaoui, H. Etude phytochimique et toxicologique d’Astragalus lusitanicus Lam. et élucidation structurale d’un nouveau produit toxique pour les ruminants. Ph.D. Thesis, Université Mohammed V—Agdal, Faculté des Sciences, Maroc, Rabat, Morocco, 2007. [Google Scholar]
- Pasteels, J.M.; Braekman, J.C.; Daloze, D.; Ottinger, R. Chemical defence in Chrysomelid larvae and adults. Tetrahedron 1982, 38, 1891. [Google Scholar] [CrossRef]
- Benn, M.H.; Majak, W.; Aplin, R. A nitropropanoyl isoxazolinone derivative in two species of Astragalus. Biochem. System Ecol. 1997, 25, 467–468. [Google Scholar] [CrossRef]
- Becker, T.; Kartikeya, P.; Paetz, C.; von Reuss, S.H.; Boland, W. Synthesis and photosensitivity of isoxazolin-5-one glycosides. Org. Biomol. Chem. 2015, 13, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Pauls, G.; Becker, T.; Rahfeld, P.; Gretscher, R.R.; Paetz, C.; Pasteels, J.; von Reuss, S.H.; Burse, A.; Boland, W. Two Defensive Lines in Juvenile Leaf Beetles; Esters of 3-nitropropionic Acid in the Hemolymph and Aposematic Warning. J. Chem. Ecol. 2016, 42, 240–248. [Google Scholar] [CrossRef]
- Tebayashi, S.; Moriyama, R.; Arakawa, R.; Sato, M. Induction of 2-cyanoethyl-isoxazolin-5-one as an antifeedant against the tobacco cutworm (Spodoptera litura) by jasmonic acid in sweet pea leaf. Biosci. Biotechnol. Biochem. 2020, 84, 1105–1112. [Google Scholar] [CrossRef]
- Fu, N.; Becker, T.; Brandt, W.; Kunert, M.; Burse, A.; Boland, W. Involvement of CYP347W1 in neurotoxin 3-nitropropionic acid-based chemical defense in mustard leaf beetle Phaedon cochleariae. Insect Sci. 2022, 29, 453–466. [Google Scholar] [CrossRef]
- Pasteéls, J.M.; Daloze, D.; de Biseau, J.C.; Termonia, A.; Windsor, D.M. Patterns in host-plant association and defensive toxins produced by neotropical chrysomeline beetles. In New Developments in the Biology of Chrysomelidae; Brill: Leiden, The Netherlands, 2004; pp. 669–676. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chroho, M.; Bouissane, L.; Bailly, C. On the Molecular Origin of the Toxicity of Erophaca baetica (L.) Boiss. Future Pharmacol. 2025, 5, 28. https://doi.org/10.3390/futurepharmacol5020028
Chroho M, Bouissane L, Bailly C. On the Molecular Origin of the Toxicity of Erophaca baetica (L.) Boiss. Future Pharmacology. 2025; 5(2):28. https://doi.org/10.3390/futurepharmacol5020028
Chicago/Turabian StyleChroho, Mounia, Latifa Bouissane, and Christian Bailly. 2025. "On the Molecular Origin of the Toxicity of Erophaca baetica (L.) Boiss." Future Pharmacology 5, no. 2: 28. https://doi.org/10.3390/futurepharmacol5020028
APA StyleChroho, M., Bouissane, L., & Bailly, C. (2025). On the Molecular Origin of the Toxicity of Erophaca baetica (L.) Boiss. Future Pharmacology, 5(2), 28. https://doi.org/10.3390/futurepharmacol5020028