Paclitaxel-Coated Versus Sirolimus-Coated Eluting Balloons for Percutaneous Coronary Interventions: Pharmacodynamic Properties, Clinical Evidence, and Future Perspectives
Abstract
1. Introduction
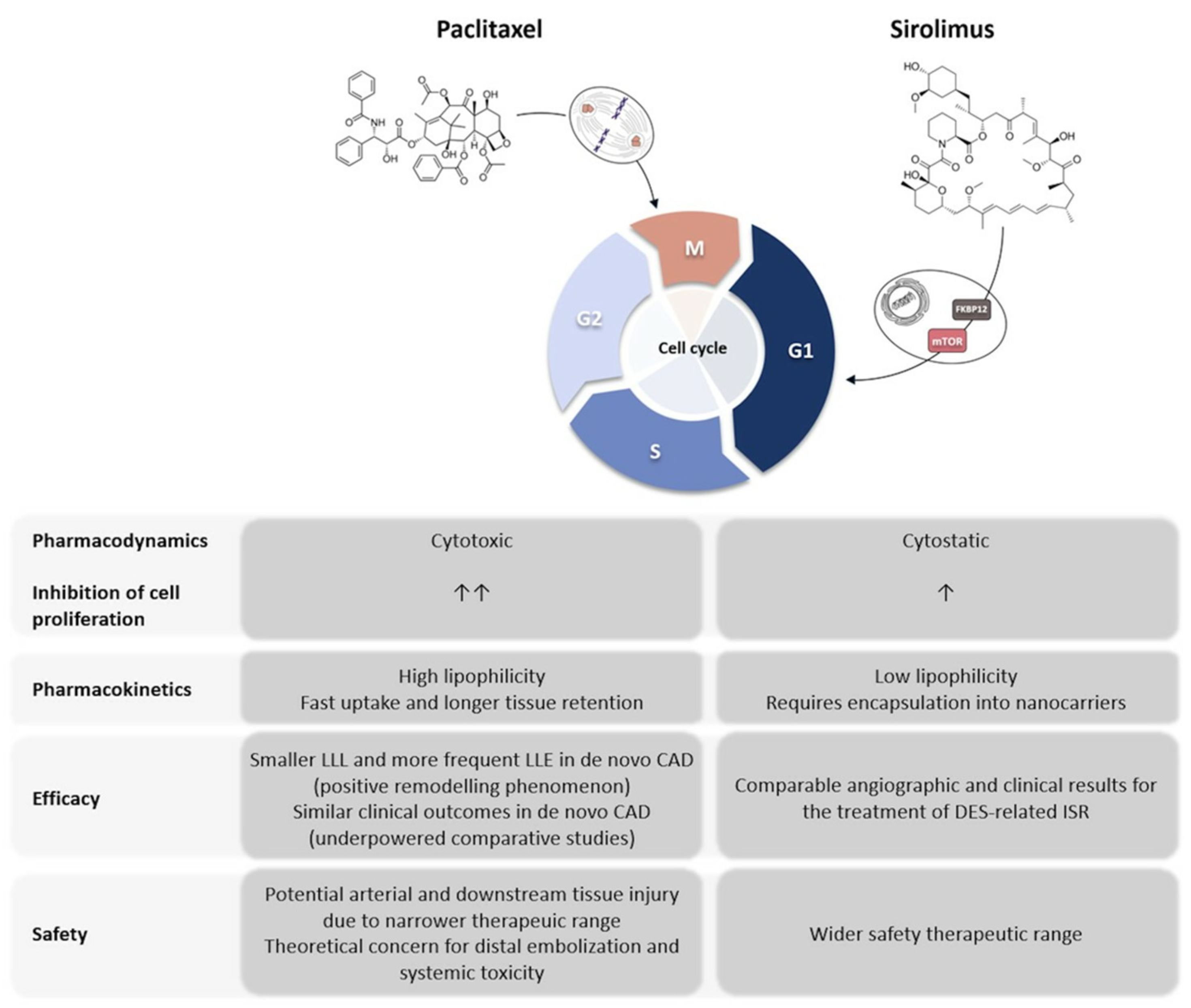
2. Mechanisms of Action of Paclitaxel and Sirolimus
2.1. Paclitaxel
2.2. Sirolimus
3. Preclinical Evidence of Paclitaxel and Sirolimus Use for the Treatment of Atherosclerotic Lesions
4. Clinical Evidence of Paclitaxel- and Sirolimus-Coated Balloons for the Treatment of Coronary and Peripheral Atherosclerotic Lesions
5. Safety Concerns Regarding the Use of Paclitaxel
| First Author, Year [Ref.] | Sample Size | Type of Lesion | Comparison | Results |
|---|---|---|---|---|
| Zeller et al., 2014 [43] | 358 | Infrapopliteal disease | AmPhirion (Paclitaxel) PCB vs. standard PTA | Similar clinical outcomes (17.7% versus 15.8%) Trends towards major amputations at 12 months were observed (8.8% vs. 3.6%; p = 0.080) in PCB group |
| Scheller et al., 2020 [44] | 4590 | Coronary ISR or de novo lesions. | PCB vs. alternative treatment (POBA, uncoated scoring balloon angioplasty, BMS or DES) | At 3 years, all-cause mortality (RR: 0.73; 95% CI: 0.53 to 1.00; p = 0.047) and cardiac mortality (RR: 0.53; 95% CI: 0.33 to 0.85; p = 0.009) were significantly lower in the DCB group when compared with control treatment |
| Katsanos et al., 2018 [45] | 4663 | Femoropopliteal artery disease | DCB or DES (Paclitaxel) vs. standard PTA | Significant association between exposure to paclitaxel (dose-time dependent) and absolute risk of death (0.4 ± 0.1% excess risk of death per paclitaxel mg-year; p < 0.001). |
| Schneider et al., 2019 [47] | 1980 | Femoropopliteal artery disease | PCB vs. standard PTA | No significant difference in all-cause mortality at 5 years |
| Secemsky et al., 2021 [48] | 168,553 | Femoropopliteal artery disease | Drug-coated devices vs. non–drug-coated devices | DCBs were non-inferior to the control group in mortality rate at a median follow-up of 2.72 years (53.8 vs. 55.1%). |
| Parikh et al., 2024 [49] | 2666 | Femoropopliteal artery disease | PCB vs. non-drug coated device | No significant differences in all-cause mortality between the two groups in the intention-to-treat (HR 1.14, 95% CI 0.93–1.40) and as-treated analysis (HR 1.13, 95% CI 0.92–1.39) |
| Zeller et al., 2020 [50] | 358 | Infrapopliteal disease | AmPhirion (Paclitaxel) DCB vs. standard PTA | No differences in freedom from TLR at 5 years (70.9% vs. 76.0%, log-rank p = 0.406) between the two groups. The rate of major amputation was similar between DCBs and PTA (15.4% vs. 10.6%, p = 0.108) |
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCB | biolimus-coated balloon |
| CAD | coronary artery disease |
| DCB | drug-coated balloon |
| DES | drug-eluting stent |
| ISR | in-stent restenosis |
| LLE | late lumen enlargement |
| LLL | late lumen loss |
| MACE | major adverse cardiovascular event |
| mTOR | mammalian target of rapamycin |
| mTORC | mTOR complex 1 |
| PAD | peripheral artery disease |
| PCB | paclitaxel-coated balloon |
| PCI | percutaneous coronary intervention |
| POBA | plain old balloon angioplasty |
| PTX | paclitaxel |
| RCT | randomized controlled trial |
| SCB | sirolimus-coated balloon |
| SRL | sirolimus |
| TLF | target lesion failure |
| VSMC | vascular smooth muscle cell |
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality From Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmad, M.; Mehta, P.; Reddivari, A.K.R.; Mungee, S. Percutaneous Coronary Intervention; In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Moussa, I.D.; Mohananey, D.; Saucedo, J.; Stone, G.W.; Yeh, R.W.; Kennedy, K.F.; Waksman, R.; Teirstein, P.; Moses, J.W.; Simonton, C. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J. Am. Coll. Cardiol. 2020, 76, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Gurgoglione, F.L.; Gattuso, D.; Greco, A.; Donelli, D.; Niccoli, G.; Cortese, B. Angiographic and clinical impact of balloon inflation time in percutaneous coronary interventions with sirolimus-coated balloon: A subanalysis of the EASTBOURNE study. Cardiovasc. Revasc. Med. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Zeller, T. Paclitaxel-coated balloons: The more you gain the more you get. Eur. Hear. J. 2024, 45, 2848–2850. [Google Scholar] [CrossRef] [PubMed]
- Lazar, F.-L.; Onea, H.-L.; Olinic, D.-M.; Cortese, B. A 2024 scientific update on the clinical performance of drug-coated balloons. AsiaIntervention 2024, 10, 15–25. [Google Scholar] [CrossRef]
- Clever, Y.P.; Peters, D.; Calisse, J.; Bettink, S.; Berg, M.C.; Sperling, C.; Stoever, M.; Cremers, B.; Kelsch, B.; Böhm, M.; et al. Novel Sirolimus-Coated Balloon Catheter: In Vivo Evaluation in a Porcine Coronary Model. Circ. Cardiovasc. Interv. 2016, 9, e003543. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Testa, L.; Heang, T.M.; Ielasi, A.; Bossi, I.; Latini, R.A.; Lee, C.Y.; Perez, I.S.; Milazzo, D.; Caiazzo, G.; et al. Sirolimus-Coated Balloon in an All-Comer Population of Coronary Artery Disease Patients: The EASTBOURNE Prospective Registry. JACC Cardiovasc. Interv. 2023, 16, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Donehower, R.C. Paclitaxel (Taxol). N. Engl. J. Med. 1995, 332, 1004–1014. [Google Scholar] [CrossRef]
- Axel, D.I.; Kunert, W.; Goggelmann, C.; Oberhoff, M.; Herdeg, C.; Küttner, A.; Wild, D.H.; Brehm, B.R.; Riessen, R.; Köveker, G.; et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997, 96, 636–645. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Demidenko, Z.N.; Giovino, M.; Szynal, C.; Donskoy, E.; Herrmann, R.A.; Barry, J.J.; Whalen, A.M. Cytostatic activity of Paclitaxel in Coronary Artery Smooth Muscle Cells is Mediated Through Transient Mitotic Arrest Followed by Permanent Post-Mitotic Arrest: Comparison with Cancer Cells. Cell Cycle 2006, 5, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Giannakakou, P.; Robey, R.; Fojo, T.; Blagosklonny, M.V. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: Molecular determinants of paclitaxel-induced cytotoxicity. Oncogene 2001, 20, 3806–3813. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Li, J.Y.; Chen, R.J.; Chen, T.Y.; Hsu, S.H.; Wang, H.H.; Peng, H.Y.; Sun, Y.Y.; Lu, W.J. Paclitaxel exerts antiplatelet and antithrombotic activities: Additional benefit from use of paclitaxel-coated balloons and -eluting stents in coronary revascularization and prevention of in-stent restenosis. Thromb. Res. 2023, 225, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003, 35, S7–S14. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, H.; Guo, L.; Sakamoto, A.; Sato, Y.; Cornelissen, A.; Kawakami, R.; Mori, M.; Torii, S.; Kuntz, S.; Harari, E.; et al. Advances in mammalian target of rapamycin kinase inhibitors: Application to devices used in the treatment of coronary artery disease. Future Med. Chem. 2020, 12, 1181–1195. [Google Scholar] [CrossRef]
- Barilli, A.; Visigalli, R.; Sala, R.; Gazzola, G.C.; Parolari, A.; Tremoli, E.; Bonomini, S.; Simon, A.; Closs, E.I.; Dall’Asta, V.; et al. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc. Res. 2008, 78, 563–571. [Google Scholar] [CrossRef]
- Bieri, M.; Oroszlan, M.; Zuppinger, C.; Mohacsi, P.J. Biosynthesis and expression of VE-cadherin is regulated by the PI3K/mTOR signaling pathway. Mol. Immunol. 2009, 46, 866–872. [Google Scholar] [CrossRef]
- Habib, A.; Karmali, V.; Polavarapu, R.; Akahori, H.; Cheng, Q.; Pachura, K.; Kolodgie, F.D.; Finn, A.V. Sirolimus-FKBP12.6 impairs endothelial barrier function through protein kinase C-alpha activation and disruption of the p120-vascular endothelial cadherin interaction. Arter. Thromb. Vasc. Biol. 2013, 33, 2425–2431. [Google Scholar] [CrossRef]
- Speck, U.; Cremers, B.; Kelsch, B.; Biedermann, M.; Clever, Y.P.; Schaffner, S.; Mahnkopf, D.; Hanisch, U.; Böhm, M.; Scheller, B. Do pharmacokinetics explain persistent restenosis inhibition by a single dose of paclitaxel? Circ. Cardiovasc. Interv. 2012, 5, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Speck, U.; Scheller, B.; Abramjuk, C.; Breitwieser, C.; Dobberstein, J.; Boehm, M.; Hamm, B. Neointima inhibition: Comparison of effectiveness of non-stent-based local drug delivery and a drug-eluting stent in porcine coronary arteries. Radiology 2006, 240, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, Y.; Zhu, X.; Miao, L.; Liang, X.; Duan, J.; Li, H.; Tian, X.; Pang, L.; Wei, Y.; et al. Significant difference between sirolimus and paclitaxel nanoparticles in anti-proliferation effect in normoxia and hypoxia: The basis of better selection of atherosclerosis treatment. Bioact. Mater. 2020, 6, 880–889. [Google Scholar] [CrossRef]
- Aihara, K.; Torii, S.; Ito, M.; Koseki, K.; Shiozaki, M.; Sato, Y.; Nakamura, N.; Yoshikawa, A.; Ikari, Y.; Nakazawa, G. Biological differences of three paclitaxel- and sirolimus-coated balloons on coronary lesions in a rabbit model. EuroIntervention 2024, 20, e389–e398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wessely, R.; Schömig, A.; Kastrati, A. Sirolimus and Paclitaxel on polymer-based drug-eluting stents: Similar but different. J. Am. Coll. Cardiol. 2006, 47, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Camilli, M.; Del Buono, M.G.; Meucci, M.C.; Gurgoglione, F.; Russo, M.; Crea, F.; Niccoli, G. “No-reflow”: Update su diagnosi, fisiopatologia e strategie terapeutiche [No-reflow: Update on diagnosis, pathophysiology and therapeutic strategies]. G. Ital. Cardiol. 2020, 21 (Suppl. S1), 4S–14S. (In Italian) [Google Scholar] [CrossRef] [PubMed]
- Farb, A.; Heller, P.F.; Shroff, S.; Cheng, L.; Kolodgie, F.D.; Carter, A.J.; Scott, D.S.; Froehlich, J.; Virmani, R. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation 2001, 104, 473–479. [Google Scholar] [CrossRef]
- Clever, Y.P.; Cremers, B.; Krauss, B.; Böhm, M.; Speck, U.; Laufs, U.; Scheller, B. Paclitaxel and sirolimus differentially affect growth and motility of endothelial progenitor cells and coronary artery smooth muscle cells. EuroIntervention 2011, 7, K32–K42. [Google Scholar] [CrossRef]
- Finn, A.; Virmani, R.; Cortese, B. Letter: Biological differences of three paclitaxel- and sirolimus-coated balloons on coronary lesions in a rabbit model. EuroIntervention 2024, 20, e954. [Google Scholar] [CrossRef]
- Cortese, B.; Tensol, G.R.P.; Dallan, L.A.P.; Salerno, P.R.V.O.; Finn, A. Navigating challenges in drug-coated balloon technology: The saga between paclitaxel and sirolimus continues. Catheter. Cardiovasc. Interv. 2024, 104, 521–522. [Google Scholar] [CrossRef]
- Ali, R.M.; Abdul Kader, M.A.S.K.; Wan Ahmad, W.A.; Ong, T.K.; Liew, H.B.; Omar, A.F.; Mahmood Zuhdi, A.S.; Nuruddin, A.A.; Schnorr, B.; Scheller, B. Treatment of Coronary Drug-Eluting Stent Restenosis by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc. Interv. 2019, 12, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Mangner, N.; Abdul Kader, M.A.S.K.; Wan Ahmad, W.A.; Jeger, R.; Wöhrle, J.; Ong, T.K.; Liew, H.B.; Gori, T.; Mahfoud, F.; et al. Combined Analysis of Two Parallel Randomized Trials of Sirolimus-Coated and Paclitaxel-Coated Balloons in Coronary In-Stent Restenosis Lesions. Circ. Cardiovasc. Interv. 2022, 15, e012305. [Google Scholar] [CrossRef] [PubMed]
- Briguori, C.; Visconti, G.; Golino, M.; Focaccio, A.; Scarpelli, M.; Nuzzo, S.; Biondi-Zoccai, G. Paclitexel versus sirolimus-coated balloon in the treatment of coronary instent restenosis. Panminerva Med. 2023, 65, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.A.W.; Nuruddin, A.A.; Abdul Kader, M.A.S.K.; Ong, T.K.; Liew, H.B.; Ali, R.M.; Mahmood Zuhdi, A.S.; Ismail, M.D.; Yusof, A.K.M.; Schwenke, C.; et al. Treatment of Coronary De Novo Lesions by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc. Interv. 2022, 15, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Serruys, P.W.; Colombo, A.; Reimers, B.; Basavarajaiah, S.; Sharif, F.; Testa, L.; Di Mario, C.; Nerla, R.; Ding, D.; et al. A Prospective Randomized Trial Comparing Sirolimus-Coated Balloon with Paclitaxel-Coated Balloon in De Novo Small Vessels. JACC Cardiovasc. Interv. 2023, 16, 2884–2896. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B. Treatment of Coronary De-Novo Lesions by a Sirolimus or a Paclitaxel Coated Balloon in a European Population. In Proceedings of the TCT Presentation, San Francisco, CA, USA, 23–26 October 2023. [Google Scholar]
- Cortese, B.; Caiazzo, G.; Di Palma, G.; De Rosa, S. Comparison Between Sirolimus- and Paclitaxel-Coated Balloon for Revascularization of Coronary Arteries: The SIRPAC (SIRolimus-PAClitaxel) Study. Cardiovasc. Revasc. Med. 2021, 28, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sedhom, R.; Hamed, M.; Elbadawi, A.; Mohsen, A.; Swamy, P.; Athar, A.; Bharadwaj, A.S.; Prasad, V.; Elgendy, I.Y.; Alfonso, F. Outcomes with Limus- vs. Paclitaxel-Coated Balloons for Percutaneous Coronary Intervention: Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc. Interv. 2024, 17, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Singh, M.; Shlofmitz, E.; Scheller, B.; Latib, A.; Kandzari, D.E.; Zaman, A.; Mylotte, D.; Dakroub, A.; Malik, S.; et al. Paclitaxel-coated versus sirolimus-coated balloon angioplasty for coronary artery disease: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2024, 104, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, A.; Nakamura, S.; Inoue, N.; Nanto, S.; Nakamura, M.; Iwabuchi, M.; Ando, K.; Asano, R.; Habara, S.; Saito, S.; et al. A multicenter randomized comparison of paclitaxel-coated balloon with plain balloon angioplasty in patients with small vessel disease. Clin. Res. Cardiol. 2017, 106, 824–832. [Google Scholar] [CrossRef]
- Kelsch, B.; Scheller, B.; Biedermann, M.; Clever, Y.P.; Schaffner, S.; Mahnkopf, D.; Speck, U.; Cremers, B. Dose response to Paclitaxel-coated balloon catheters in the porcine coronary overstretch and stent implantation model. Investig. Radiol. 2011, 46, 255–263. [Google Scholar] [CrossRef]
- Radke, P.W.; Joner, M.; Joost, A.; Byrne, R.A.; Hartwig, S.; Bayer, G.; Steigerwald, K.; Wittchow, E. Vascular effects of paclitaxel following drug-eluting balloon angioplasty in a porcine coronary model: The importance of excipients. EuroIntervention 2011, 7, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Pacheco, E.; Yahagi, K.; Mori, H.; Ladich, E.; Virmani, R. Comparison of Particulate Embolization after Femoral Artery Treatment with IN.PACT Admiral versus Lutonix 035 Paclitaxel-Coated Balloons in Healthy Swine. J. Vasc. Interv. Radiol. 2016, 27, 1676–1685.e2. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Romero, M.E.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Comparison of Biologic Effect and Particulate Embolization after Femoral Artery Treatment with Three Drug-Coated Balloons in Healthy Swine Model. J. Vasc. Interv. Radiol. 2018, 30, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Baumgartner, I.; Scheinert, D.; Brodmann, M.; Bosiers, M.; Micari, A.; Peeters, P.; Vermassen, F.; Landini, M.; Snead, D.B.; et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J. Am. Coll. Cardiol. 2014, 64, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Vukadinovic, D.; Jeger, R.; Rissanen, T.T.; Scholz, S.S.; Byrne, R.; Kleber, F.X.; Latib, A.; Clever, Y.P.; Ewen, S.; et al. Survival After Coronary Revascularization with Paclitaxel-Coated Balloons. J. Am. Coll. Cardiol. 2020, 75, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kuntz, S.H.; Surve, D.; Jinnouchi, H.; Sakamoto, A.; Cornelissen, A.; Virmani, R.; Kolodgie, F.; Finn, A.V. What are the Pathological Concerns and Limitations of Current Drug-coated Balloon Technology? Hear. Int. 2019, 13, 15–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, P.A.; Laird, J.R.; Doros, G.; Gao, Q.; Ansel, G.; Brodmann, M.; Micari, A.; Shishehbor, M.H.; Tepe, G.; Zeller, T. Mortality not correlated with paclitaxel exposure: An independent patient-level meta-analysis of a drug-coated balloon. J. Am. Coll. Cardiol. 2019, 73, 2550–2563. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Shen, C.; Schermerhorn, M.; Yeh, R.W. Longitudinal Assessment of Safety of Femoropopliteal Endovascular Treatment with Paclitaxel-Coated Devices Among Medicare Beneficiaries: The SAFE-PAD Study. JAMA Intern. Med. 2021, 181, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parikh, S.A.; Schneider, P.A.; Mullin, C.M.; Rogers, T.; Gray, W.A. Mortality in randomised controlled trials using paclitaxel-coated devices for femoropopliteal interventional procedures: An updated patient-level meta-analysis. Lancet 2023, 402, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Micari, A.; Scheinert, D.; Baumgartner, I.; Bosiers, M.; Vermassen, F.E.; Banyai, M.; Shishehbor, M.H.; Wang, H.; Brodmann, M.; et al. The IN. PACT DEEP Clinical Drug-Coated Balloon Trial. JACC Cardiovasc. Interv. 2020, 13, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Takumi, T.; Mathew, V.; Chung, W.Y.; Barsness, G.W.; Rihal, C.S.; Gulati, R.; McCue, E.T.; Holmes, D.R.; Eeckhout, E.; et al. Plaque characteristics and arterial remodeling in coronary and peripheral arterial systems. Atherosclerosis 2012, 223, 365–371. [Google Scholar] [CrossRef]
- Achim, A.; Péter O, Á.; Cocoi, M.; Serban, A.; Mot, S.; Dadarlat-Pop, A.; Nemes, A.; Ruzsa, Z. Correlation between Coronary Artery Disease with Other Arterial Systems: Similar, Albeit Separate, Underlying Pathophysiologic Mechanisms. J. Cardiovasc. Dev. Dis. 2023, 10, 210. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawai, K.; Rahman, M.T.; Nowicki, R.; Kolodgie, F.D.; Sakamoto, A.; Kawakami, R.; Konishi, T.; Virmani, R.; Labhasetwar, V.; Finn, A.V. Efficacy and Safety of Dual Paclitaxel and Sirolimus Nanoparticle-Coated Balloon. JACC Basic Transl. Sci. 2024, 9, 774–789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, K.; Fu, G.; Tong, Q.; Liu, B.; Han, X.; Zhang, J.; Ma, G.; Yang, Q.; Li, H.; Zhou, Y.; et al. Biolimus-Coated Balloon in Small-Vessel Coronary Artery Disease: The BIO-RISE CHINA Study. JACC Cardiovasc. Interv. 2022, 15, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Traynor, B.P.; Fitzgerald, S.; Alfonso, F.; O’Kane, P.; Sabaté, M.; Tölg, R.; Trevelyan, J.; Hahn, J.Y.; Mylotte, D.; Wöhrle, J.; et al. Design and rationale of a prospective, randomized, non-inferiority trial to determine the safety and efficacy of the Biolimus A9™ drug coated balloon for the treatment of in-stent restenosis: First-in-man trial (REFORM). Cardiovasc. Revascularization Med. 2023, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, L.; Qin, Q.; Zhang, J.; Jia, S.; Wu, M.; He, Y.; Fu, G.; Liu, J.; Chen, H.; et al. Biolimus-coated versus paclitaxel-coated balloons for coronary in-stent restenosis (BIO ASCEND ISR): A randomised, non-inferiority trial. EuroIntervention 2024, 20, e806–e817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katsouras, C.S.; Tousis, A.; Vasilagkos, G.; Semertzioglou, A.; Vratimos, A.; Samara, I.; Karanasiou, G.; Loukas, V.S.; Tsigkas, G.; Fotiadis, D.; et al. Safety and Efficacy of an Innovative Everolimus-Coated Balloon in a Swine Coronary Artery Model. Life 2023, 13, 2053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| First Author, Year [Ref.] | Study Design | Study Population | Type of Lesion | Comparison | Time of Follow-Up | Type of Outcome | Results |
|---|---|---|---|---|---|---|---|
| Ali et al., 2019 [29] | RCT | 50 (25 SCB, 25 PCB) | ISR | SeQuent SCB vs. SeQuent Please Neo PCB | 12 months | LLL/MACE | 0.17 ± 0.55 mm vs. 0.21 ± 0.54 mm (p = NS)/ 3 (12%) vs. 4 (16%) (p > 0.99) |
| Scheller et al., 2022 [30] | RCT | 101 (50 SCB, 51 PCB) | ISR | SeQuent SCB vs. SeQuent Please Neo PCB | 12 months | LLL/MACE | 0.26 ± 0.61 mm vs. 0.20 ± 0.52 mm (p = 0.639)/ 9 (18%) vs. 7 (14%) (p = 0.596) |
| Briguori et al., 2023 [31] | Observational | 372 (186 SCB, 186 PCB) | ISR | Devoi SCB vs. Restore PCB | 12 months | TLF | 29 (15.5%) vs. 32 (17%) (p = 0.29) |
| Ahmad et al., 2022 [32] | RCT | 70 (35 SCB, 35 PCB) | De novo lesions | SeQuent SCB vs. SeQuent Please Neo PCB | 12 months | LLL negative/MACE | 21 (60.0%) vs. 12 (32.4%) (p = 0.019)/ 0 (0%) vs. 2 (6%) ( p = 0.493) |
| Ninomiya et al., 2023 [33] | RCT | 121 (61 SCB, 60 PCB) | De novo SVD (≤2.5 mm). | Magic Touch SCB vs. SeQuent Please Neo PCB | 6 months | LLL | 0.32 mm vs. 0.00 mm; (p < 0.001) |
| Scheller et al., 2023 [34] | RCT | 70 (35 SCB, 35 PCB) | De novo lesions > 2.5 mm | SeQuent SCB vs. SeQuent Please | 12 months | LLL/rate LLE | 0.11 mm vs. 0.04 mm/ 44% vs. 56% |
| Cortese et al., 2021 [35] | Observational | 1090 (596 SCB, 494 PCB) | All lesions | MagicTouch SCB and Elutax SV PCB | 12 months | MACE | 10.7% vs. 10.3% (p = 0.892) |
| Sedhom et al., 2024 [36] | Metanalysis | 821 (446 LCB, 375 PCB) | All lesions | Limus DCB vs. Paclitaxel DCB | 13.4 months | TLR/LLE/MACE | 10.3% vs. 7.8% (p > 0.05)/ 27.5% vs. 50%; (p = 0.0002)/ 14.5% vs. 13.6% (p > 0.05) |
| Shin et al., 2024 [37] | Metanalysis | 1861 (972 SCB, 889 PCB) | All lesions | SCB vs. PCB | 9–12 months | LLL/TLR | mean difference −0.11 (95% CI −0.23–0.02)/no difference (OR 1.01, 95% CI 0.75–1.35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurgoglione, F.L.; De Gregorio, M.; Benatti, G.; Donelli, D.; Vignali, L.; Solinas, E.; Tadonio, I.; Denegri, A.; Covani, M.; Dallaglio, G.; et al. Paclitaxel-Coated Versus Sirolimus-Coated Eluting Balloons for Percutaneous Coronary Interventions: Pharmacodynamic Properties, Clinical Evidence, and Future Perspectives. Future Pharmacol. 2024, 4, 775-787. https://doi.org/10.3390/futurepharmacol4040041
Gurgoglione FL, De Gregorio M, Benatti G, Donelli D, Vignali L, Solinas E, Tadonio I, Denegri A, Covani M, Dallaglio G, et al. Paclitaxel-Coated Versus Sirolimus-Coated Eluting Balloons for Percutaneous Coronary Interventions: Pharmacodynamic Properties, Clinical Evidence, and Future Perspectives. Future Pharmacology. 2024; 4(4):775-787. https://doi.org/10.3390/futurepharmacol4040041
Chicago/Turabian StyleGurgoglione, Filippo Luca, Mattia De Gregorio, Giorgio Benatti, Davide Donelli, Luigi Vignali, Emilia Solinas, Iacopo Tadonio, Andrea Denegri, Marco Covani, Gabriella Dallaglio, and et al. 2024. "Paclitaxel-Coated Versus Sirolimus-Coated Eluting Balloons for Percutaneous Coronary Interventions: Pharmacodynamic Properties, Clinical Evidence, and Future Perspectives" Future Pharmacology 4, no. 4: 775-787. https://doi.org/10.3390/futurepharmacol4040041
APA StyleGurgoglione, F. L., De Gregorio, M., Benatti, G., Donelli, D., Vignali, L., Solinas, E., Tadonio, I., Denegri, A., Covani, M., Dallaglio, G., Cortese, B., & Niccoli, G. (2024). Paclitaxel-Coated Versus Sirolimus-Coated Eluting Balloons for Percutaneous Coronary Interventions: Pharmacodynamic Properties, Clinical Evidence, and Future Perspectives. Future Pharmacology, 4(4), 775-787. https://doi.org/10.3390/futurepharmacol4040041










