Anticancer Activity of 4-Aryl-1,4-Dihydropyridines
Abstract
1. Introduction
2. Materials and Methods
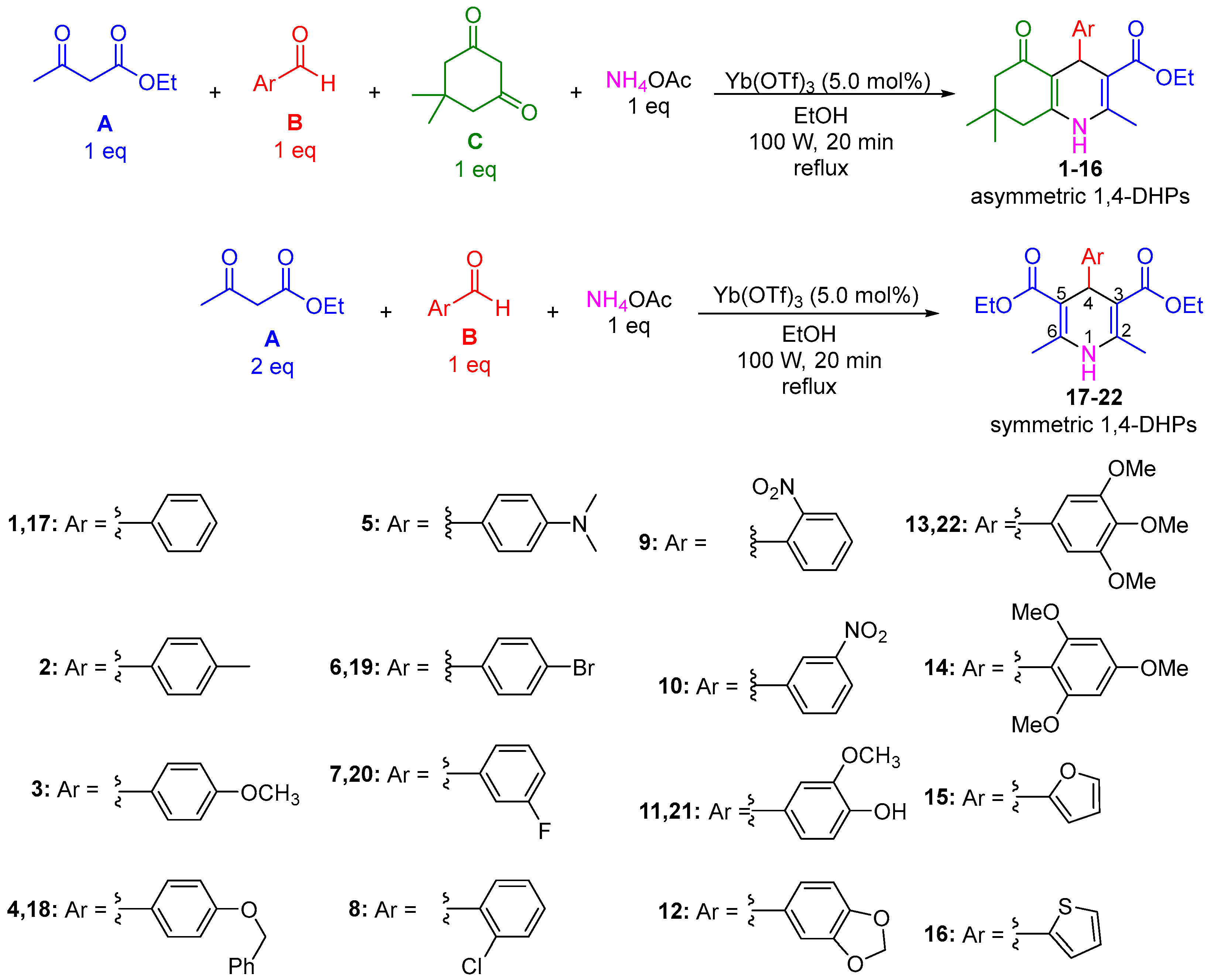
2.1. Synthesis of 1,4-DHPs 1–22
2.2. Cell Viability Analysis
3. Results
3.1. Synthesis of 4-Aryl-1,4-Dihydropyridines (1,4-DHPs) 1–22
3.2. Anticancer Activity of 1,4-DHPs 1–22
4. Discussion
4.1. Synthesis of Compounds 1–22
4.2. Cytotoxicity of 1,4-DHPs 1–22 to Cancer Cell Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anaikutti, P.; Makam, P. 1,4-Dihydropyridine: Synthetic advances, medicinal and insecticidal properties. RCS Adv. 2022, 12, 29253–29290. [Google Scholar] [CrossRef]
- Gonzalez, A.; Casado, J.; Gunduz, M.G.; Santos, B.; Velazquez-Campoy, A.; Sarasa-Buisan, C.; Fillat, M.F.; Montes, M.; Piazuelo, E.; Lanas, A. 1,4-Dihydropyridine as a promising scaffold for novel antimicrobials against Helicobacter pylori. Front. Microbiol. 2022, 13, 874709. [Google Scholar] [CrossRef] [PubMed]
- Pattan, S.R.; Rasal, V.P.; Venkatramana, N.V.; Khade, A.B.; Butle, S.R.; Jadhav, S.G.; Desai, B.G.; Manvi, F.V. Synthesis and evaluation of some 1,4-dihydropyridine and their derivatives as antihypertensive agents. Indian J. Chem. B 2007, 46, 698–701. [Google Scholar] [CrossRef]
- Prasanthi, G.; Prasad, K.; Bharathi, K. Design, synthesis and evaluation of dialkyl 4-(benzo d-1,3 dioxol-6-yl)-1,4-dihydro-2,6-dimethyl-1-substituted pyridine-3,5-dicarboxylates as potential anticonvulsants and their molecular properties prediction. Eur. J. Med. Chem. 2013, 66, 516–525. [Google Scholar] [CrossRef]
- Akbar, I.; Radhakrishnan, S.; Meenakshisundaram, K.; Manilal, A.; Hatamleh, A.A.; Alnafisi, B.K.; Ahamed, A.; Balasubramani, R. Design of 1,4-dihydropyridine hybrid benzamide derivatives: Synthesis and evaluation of analgesic activity and their molecular docking studies. Drug Des. Dev. Ther. 2022, 16, 4021–4039. [Google Scholar] [CrossRef]
- Anaikutti, P.; Makam, P. Dual active 1,4-dihydropyridine derivatives: Design, green synthesis and in vitro anti-cancer and anti-oxidant studies. Bioorg. Chem. 2020, 105, 104379. [Google Scholar] [CrossRef] [PubMed]
- Miri, R.; Motamedi, R.; Rezaei, M.R.; Firuzi, O.; Javidnia, A.; Shafiee, A. Design, synthesis and evaluation of cytotoxicity of novel chromeno[4,3-b]quinoline derivatives. Arch. Pharm. Chem. Life Sci. 2011, 2, 111–118. [Google Scholar] [CrossRef]
- Aswin, K.; Logaiya, K.; Sudhan, P.N.; Mansoor, S.S. An efficient one-pot synthesis of 1,4-dihydropyridine derivatives through Hantzsch reaction catalysed by melamine trisulfonic acid. J. Taibah Univ. Sci. 2012, 6, 1–9. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.; Yadav, N.; Jain, H.; Deswal, N.; Upadhyay, R.K.; Leekha, A.; Verma, A.K.; Kareem, A.; Chikati, R.; Kumar, L.S. Microwave-assisted synthesis of 4-aryl-1,4-dihydropyridines as potent anticancer agent and their in-silico studies. ChemistrySelect 2022, 7, e202104129. [Google Scholar] [CrossRef]
- Balaboina, R.; Thirukovela, N.S.; Kankala, S.; Balasubramanian, S.; Bathula, S.R.; Vadde, R.; Jonnalagadda, S.B.; Vasam, C.S. Synergistic catalysis of Ag(I) and organo-N-heterocyclic carbenes: One-pot synthesis of new anticancer spirooxindole-1,4-dihydropyridines. ChemistrySelect 2019, 4, 2562–2567. [Google Scholar] [CrossRef]
- Vieira, T.M.; Barco, J.G.; Paula, L.A.L.; Felix, P.C.A.; Bastos, J.K.; Magalhaes, L.G.; Crotti, A.E.M. In vitro evaluation of the antileishmanial and antischistosomal activities of p-coumaric acid prenylated derivatives. Chem. Biodiv. 2024, 21, e202400491. [Google Scholar] [CrossRef] [PubMed]
- Pagotti, M.C.; Dias, H.J.; Candido, A.; Oliveira, T.A.S.; Borges, A.; Oliveira, N.D.; Lopes, C.D.; Orenha, R.P.; Parreira, R.L.T.; Crotti, A.E.M.; et al. Exploring synthetic dihydrobenzofuran and benzofuran neolignans as antiprotozoal agents against Trypanosoma cruzi. Pharmaceutics 2023, 15, 754. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.M.; dos Santos, I.A.; Silva, T.S.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial activity of monoketone curcuminoids against cariogenic bacteria. Chem. Biodivers. 2018, 15, e1800216. [Google Scholar] [CrossRef] [PubMed]
- De Melo, N.I.; de Carvalho, C.E.; Fracarolli, L.; Cunha, W.R.; Veneziani, R.C.S.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial activity of the essential oil of Tetradenia riparia (Hochst.) Codd. (Lamiaceae) against cariogenic bacteria. Braz. J. Microbiol. 2015, 46, 519–525. [Google Scholar] [CrossRef]
- De Andrade, P.M.; De Melo, D.C.; Alcoba, A.E.T.; Ferreira, W.G.; Pagotti, M.C.; Magalhaes, L.G.; Dos Santos, T.C.L.; Crotti, A.E.M.; Alves, C.C.F.; Miranda, M.L.D. Chemical composition and evaluation of antileishmanial and cytotoxic activities of the essential oil from leaves of Cryptocarya aschersoniana Mez. (Lauraceae Juss.). An. Acad. Bras. Cienc. 2018, 90, 2671–2678. [Google Scholar] [CrossRef]
- Ghoorbannejad, S.; Akbari, D.; Nikoo, A. Synthesis and assessment of the cytotoxic effect of some of 1,4-dihydropyridine derivatives which contain azole moiety. J. Serb. Chem. Soc. 2021, 86, 1013–1021. [Google Scholar] [CrossRef]
- Kumar, R.; Gahlyan, P.; Vema, A.; Jain, R.; Das, S.; Konwar, R.; Prasad, A.K. Design and synthesis of fluorescent symmetric bis-triazolylated-1,4-dihydropyridines as potent antibreast cancer agents. Synth. Commun. 2018, 48, 778–785. [Google Scholar] [CrossRef]
- Kumar, R.S.; Idhayadhulla, A.; Nasser, A.J.A.; Murali, K. Synthesis and anticancer activity of some new series of 1,4-dihydropyridine derivatives. Indian J. Chem. Sect. B. 2011, 50, 1140–1144. [Google Scholar]
- Magalhães, C.M.; González-Berdullas, P.; Pereira, M.; Duarte, D.; Vale, N.; Da Silva, J.C.G.E.; Silva, L.P. Investigation of the anticancer and drug combination potential of brominated coelenteramines toward breast and prostate cancer. Int. J. Mol. Sci. 2022, 23, 13981. [Google Scholar] [CrossRef]
- Manna, D.; Akhtar, S.; Maiti, P.; Mondal, S.; Mandal, T.K.; Ghosh, R. Anticancer activity of a 1,4-dihydropyridine in DMBA-induced mouse skin tumor model. Anti-Cancer Drugs 2020, 31, 394–402. [Google Scholar] [CrossRef]
- Manna, D.; Bhuyan, R.; Saikh, F.; Ghosh, S.; Basak, J.; Ghosh, R. Novel 1,4-dihydropyridine induces apoptosis in human cancer cells through overexpression of Sirtuin1. Apoptosis 2018, 23, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Shamsara, J.; Iman, M.; Hadizadeh, F. Docking and QSAR studies of 1,4-dihydropyridine derivatives as anti-cancer agent. Rec. Pat. Anti-Cancer Drug Disc. 2017, 12, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Radadiya, A.; Khedkar, V.; Bavishi, A.; Vala, H.; Thakrar, S.; Bhavsar, D.; Shah, A.; Coutinho, E. Synthesis and 3D-QSAR study of 1,4-dihydropyridine derivatives as MDR cancer reverters. Eur. J. Med. Chem. 2014, 74, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Miri, R.; Firuzi, O. Assessment of the cytotoxic effect of a series of 1,4-dihydropyridine derivatives against human cancer cells. Iran. J. Pharm. Res. 2016, 15, 413–420. [Google Scholar]
- Shekari, F.; Sadeghpour, H.; Javidnia, K.; Saso, L.; Nazari, F.; Firuzi, O.; Miri, R. Cytotoxic and multidrug resistance reversal activities of novel 1,4-dihydropyridines against human cancer cells. Eur. J. Pharmacol. 2015, 746, 233–244. [Google Scholar] [CrossRef]
- Viradiya, D.; Mirza, S.; Shaikh, F.; Kakadiya, R.; Rathod, A.; Jain, N.; Rawal, R.; Shah, A. Design and synthesis of 1,4-dihydropyridine derivatives as anti-cancer agent. Anti-Cancer Agents Med. Chem. 2017, 17, 1003–1013. [Google Scholar] [CrossRef]
- Wang, L.-M.; Sheng, J.; Zhang, L.; Han, J.-W.; Fan, Z.-Y.; Tian, H.; Qian, C.-T. Facile Yb(OTf)3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reaction. Tetrahedron 2005, 61, 1539–1543. [Google Scholar] [CrossRef]
- Chang, C.C.; Cao, S.; Kang, S.; Kai, L.; Tian, X.Y.; Pandey, P.; Dunne, S.F.; Luan, C.H.; Surmeier, D.J.; Silverman, R.B. Antagonism of 4-substituted 1,4-dihydropyridine-3,5-dicarboxylates toward voltage-dependent L-type Ca2+ channels Ca(V)1.3 and Ca(V)1.2. Bioorg. Med. Chem. 2010, 18, 3147–3158. [Google Scholar] [CrossRef]
- Debache, A.; Ghalem, W.; Boulcina, R.; Belfaitah, A.; Rhouati, S.; Carboni, B. An efficient one-step synthesis of 1,4-dihydropyridines via a triphenylphosphine-catalyzed three-component Hantzsch reaction under mild conditions. Tetrahedron Lett. 2009, 50, 5248–5250. [Google Scholar] [CrossRef]
- Wang, X.-K.; Li, P.-W.; Yan, B.; Wang, B.-J. 1,4-Dihydropyridine derivatives: Synthesis and anti-hepatoma cancer activity. Latin Am. J. Pharm. 2016, 35, 1692–1695. [Google Scholar]
- Harale, R.R.; Shitre, P.V.; Sathe, B.R.; Shingare, M.S. Visible light motivated synthesis of polyhydroquinoline derivatives using CdS nanowires. Res. Chem. Intermed. 2017, 43, 3237–3249. [Google Scholar] [CrossRef]
- Ji, S.J.; Jiang, Z.Q.; Lu, J.; Loh, T.P. Facile ionic liquids-promoted one-pot synthesis of polyhydroquinoline derivatives under solvent free conditions. Synlett 2004, 831–835. [Google Scholar] [CrossRef]
- Karade, N.N.; Budhewar, V.H.; Shinde, S.V.; Jadhav, W.N. L-proline as an efficient organo-catalyst for the synthesis of polyhydroquinoline via multicomponent Hantzsch reaction. Lett. Org. Chem. 2007, 4, 16–19. [Google Scholar] [CrossRef]
- Kumbhar, S.D.; Gore, A.H.; Choudhari, P.B.; Barooah, N.; Anbhule, P.V.; Sonavane, Y.S.; Kolekar, G.B.; Bodake, A.J. In vitro study of ethyl-4-(3,4.5-trimethoxyphenyl)-2,7,7-trimethyl-5-oxo1,4,5,6,7,8-hexahydroquinoline-3-carboxylate and bovine serum albumin using multi-spectroscopic techniques and molecular docking. Macromol. Symp. 2019, 387, 1800206. [Google Scholar] [CrossRef]
- Maheswara, M.; Siddaiah, V.; Damu, G.L.V.; Rao, C.V. An efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using a heterogeneous catalyst under solvent-free conditions. Arkivoc 2006, 201–206. [Google Scholar] [CrossRef]
- Murthy, Y.L.N.; Rajack, A.; Ramji, M.T.; Babu, J.J.; Praveen, C.; Lakshmi, K.A. Design, solvent free synthesis, and antimicrobial evaluation of 1,4 dihydropyridines. Bioorg. Med. Chem. Lett. 2012, 22, 6016–6023. [Google Scholar] [CrossRef]
- Paidepala, H.; Nagendra, S.; Saddanappu, V.; Addlagatta, A.; Das, B. Catalyst-free efficient synthesis of polyhydroquinolines using polyethylene glycol as a solvent and evaluation of their cytotoxicity. Med. Chem. Res. 2014, 23, 1031–1036. [Google Scholar] [CrossRef]
- Perumal, M.; Sengodu, P.; Venkatesan, S.; Perumal, S.; Antony, S.; Paramsivam, M. Polybenzimidazole-triphenylphosphene-catalyzed one-pot synthesis and evaluation of dihydropyridine derivative as antibiotics and antifungals. ChemistrySelect 2017, 2, 7489–7496. [Google Scholar] [CrossRef]
- Shashi, R.; Prasad, N.L.; Begum, N.S. One-Pot synthesis of 1,4-dihydropyridine derivatives and their X-ray crystal structures: Role of fluorine in weak interactions. J. Struct. Chem. 2020, 61, 938–947. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, Z.Y.; Zhang, J.C.; Li, J.T. The solvent-free synthesis of 1,4-dihydropyridines under ultrasound irradiation without catalyst. Ultrason. Sonochem. 2008, 15, 677–680. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesha, MD, USA, 2016. [Google Scholar]
- Baydar, E.; Gündüz, M.G.; Krishna, V.S.; Simsek, R.; Sriram, D.; Yildirim, S.O.; Butcher, R.J.; Safak, C. Synthesis, crystal structure and antimycobacterial activities of 4-indolyl-1,4-dihydropyridine derivatives possessing various ester groups. Res. Chem. Intermed. 2017, 43, 7471–7489. [Google Scholar] [CrossRef]
- Agrwal, A.; Kasana, V. Fesipmim Cl as highly efficient and reusable catalyst for solventless synthesis of dihydropyridine derivatives through Hantzsch reaction. J. Chem. Sci. 2020, 132, 67. [Google Scholar] [CrossRef]
- Devarajan, N.; Suresh, P. MIL-101-SO3H metal-organic framework as a Bronsted acid catalyst in Hantzsch reaction: An efficient and sustainable methodology for one-pot synthesis of 1,4-dihydropyridine. New J. Chem. 2019, 43, 6806–6814. [Google Scholar] [CrossRef]
- Jafari-Chermahini, M.T.; Tavakol, H. One-pot synthesis of Hantzsch 1,4-dihydropyridines by a series of iron oxide nanoparticles: Putative synthetic TRPV6 calcium channel blockers. ChemistrySelect 2021, 6, 2360–2365. [Google Scholar] [CrossRef]
- Kiasat, A.R.; Almasi, H.; Saghanezhad, S.J. One-pot synthesis of Hantzsch esters and polyhydroquinoline derivatives catalyzed by gamma-Al2O3-nanoparticles under solvent-free thermal conditions. Rev. Roum. Chim. 2014, 59, 61–66. [Google Scholar]
- Kumar, G.; Bhargava, G.; Kumar, R. Trio role of deep eutectic solvents in the green synthesis of 1,4-dihydropyridine synthesis via Hantzsch reaction. Polyc. Arom. Comp. 2023, 43, 7238–7251. [Google Scholar] [CrossRef]
- Mirzaei, H.; Davoodnia, A. Microwave assisted sol-gel synthesis of MgO nanoparticles and their catalytic activity in the synthesis of Hantzsch 1,4-dihydropyridines. Chin. J. Catal. 2012, 33, 1502–1507. [Google Scholar] [CrossRef]
- Salem, M.E.; Fares, I.M.Z.; Ghozlan, S.A.S.; Elwahy, A.H.M.; Abdelhamid, I.A. Hantzsch-like three-component synthesis of bis(1,4-dihydropyridines) and bis(fused-1,4-dihydropyridines) linked to piperazine core via 2-phenoxyethanone linkage: Novel hybrid molecules. Synth. Commun. 2022, 52, 1981–1997. [Google Scholar] [CrossRef]
- Sharma, M.G.; Rajani, D.P.; Patel, H.M. Green approach for synthesis of bioactive Hantzsch 1,4-dihydropyridine derivatives based on thiophene moiety via multicomponent reaction. R. Soc. Open Sci. 2017, 4, 170006. [Google Scholar] [CrossRef]
- Sunkara, P.; Keshavulu, M.; Puppala, V.; Kumar, P.V.; Basude, M. Hantzsch synthesis of 1,4-dihydropyridine derivatives over ZnO/ZrO2 catalyst under solvent free condition. Indian J. Chem. Sect. A. 2021, 60, 1055–1063. [Google Scholar]
- Abbas, H.A.S.; El Sayed, W.A.; Fathy, N.M. Synthesis and antitumor activity of new dihydropyridine thioglycosides and their corresponding dehydrogenated forms. Eur. J. Med. Chem. 2010, 45, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Deswal, N.; Shrivastava, A.; Hossain, M.S.; Gahlyan, P.; Bawa, R.; Gupta, R.D.; Kumar, R. Design, synthesis, evaluation and molecular docking studies of novel triazole linked 1,4-dihydropyridine-isatin scaffolds as potent anticancer agents. ChemistrySelect 2021, 6, 717–725. [Google Scholar] [CrossRef]
- Chen, X.H.; Niu, N.; Li, D.; Zhang, Z.C.; Zhuang, Z.; Yan, D.Y.; Li, J.; Zhao, Z.J.; Wang, D.; Tang, B.Z. The golden touch by light: A finely engineered luminogen empowering high photoactivatable and photodynamic efficiency for cancer phototheranostics. Adv. Func. Mater. 2023, 33, 2211571. [Google Scholar] [CrossRef]
- Singh, R.K.; Prasad, D.N.; Bhardwaj, T.R. Hybrid pharmacophore-based drug design, synthesis, and antiproliferative activity of 1,4-dihydropyridines-linked alkylating anticancer agents. Med. Chem. Res. 2015, 24, 1534–1545. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry; Hostettmann, K., Ed.; Academic Press: London, UK, 1991; pp. 71–133. [Google Scholar]
- Langle, D.; Marquardt, V.; Heider, E.; Vigante, B.; Duburs, G.; Luntena, I.; Flotgen, D.; Golz, C.; Strohmann, C.; Koch, O.; et al. Design, synthesis and 3D-QSAR studies of novel 1,4-dihydropyridines as TGFb/Smad inhibitors. Eur. J. Med. Chem. 2015, 95, 249–266. [Google Scholar] [CrossRef]
- González-Berdullas, P.; Pereira, R.B.; Teixeira, C.; Silva, J.P.; Magalhães, C.M.; Rodroigues-Borges, J.E.; Pereira, D.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Discovery of the anticancer activity for lung and gastric cancer of a brominated coelenteramine analog. Int. J. Mol. Sci. 2022, 23, 8271. [Google Scholar] [CrossRef]
- Ohishi, K.; Morinaga, Y.; Ohsumi, K.; Nakagawa, R.; Suga, Y.; Tsuji, T.; Akiyama, Y.; Tsuruo, T. Potentiation of antitumor and antimetastatic activities of adriamycin by a novel N-alkylated dihydropyridine, AC394, and its enantiomers in colon cancer-bearing mice. Cancer Chemother. Pharmacol. 1996, 38, 446–452. [Google Scholar] [CrossRef]
- Engi, H.; Sakagami, H.; Kawase, M.; Parecha, A.; Manvar, D.; Kothari, H.; Adlakha, P.; Shah, A.; Motohashi, N.; Ocsovszki, I.; et al. Tumour-specific cytotoxicity and MDR-reversal activity of dihydropyridines. In Vivo 2006, 20, 637–644. [Google Scholar]
- Foroughinia, F.; Javidnia, K.; Amirghofran, Z.; Mehdipour, A.; Miri, R. Design and synthesis of new symmetrical derivatives of dihydropyridine containing a pyridyl group on the 3, 5-positions and evaluation of their cytotoxic and multidrug resistance reversal activity. J. Pharm. Pharmacol. 2008, 60, 1481–1489. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, Y.; Park, J.; Lee, J.; Shin, S.Y.; Lee, Y.H.; Koh, D.; Lim, Y. Synthetic diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylates induce apoptosis. Med. Chem. 2018, 14, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Matiichuk, Y.E.; Horak, Y.I.; Chaban, T.I.; Horishny, V.Y.; Tymoshuk, O.S.; Matiychuk, V.S. 5-(1,3-Benzothiazol-2-yl)furan-2-carbaldehyde in the design of antitumor agents. Russ. J. Org. Chem. 2020, 56, 1720–1727. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; El-Reedy, A.A.M. Intramolecular ring transformation of bis-oxadiazoles to bis-thiadiazoles and investigation of their anticancer activities. J. Heterocycl. Chem. 2018, 55, 2360–2367. [Google Scholar] [CrossRef]
- Gong, X.; Liang, Z.; Yang, Y.; Liu, H.; Li, J.; Fan, Y. A resazurin-based, nondestructive assay for monitoring cell proliferation during a scaffold-based 3D culture process. Regen. Biomater. 2020, 7, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, S.; Hou, W.; Xiao, Y.; Liu, P.; Shi, X.; Shen, M. Comparative study of resazurin reduction and MTT assays for cytocompatibility evaluation of nanofibrous materials. Anal. Methods 2019, 11, 483–489. [Google Scholar] [CrossRef]
- Clara, T.H.; Muthu, S.; Prasana, J.C. Quantum mechanical, spectroscopic and docking studies of (2E)-1-(4-aminophenyl)-3-(4-benzyloxyphenyl)-prop-2-en-1-one chalcone derivative by density functional theory—A prospective respiratory drug. Mater. Today Proc. 2022, 50, 2816–2825. [Google Scholar] [CrossRef]
- Datar, P.A.; Auti, P.B. Design and synthesis of novel 4-substituted 1,4-dihydropyridine derivatives as hypotensive agents. J. Saudi Chem. Soc. 2016, 20, 510–516. [Google Scholar] [CrossRef]
- Gangireddy, M.R.; Mantipally, M.; Gundla, R.; Badavath, V.N.; Paidikondala, K.; Yamala, A. Design and synthesis of piperazine-linked imidazo 1,2-a-pyridine derivatives as potent anticancer agents. ChemistrySelect 2019, 4, 13622–13629. [Google Scholar] [CrossRef]
- Kleczewska, N.; Ruszkowski, P.; Singh, A.; Trznadel, R.; Celewicz, L. Synthesis and anticancer activity of 3′-4-fluoroaryl-(1,2,3-triazol-1-yl)-3′-deoxythymidine analogs and their phosphoramidates. Nucleosides Nucleotides Nucleic Acids 2019, 38, 605–641. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications in fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Faizan, S.; Talath, S.; Wali, A.F.; Hani, U.; Haider, N.; Mandal, S.P.; Kumar, B.R.P. Anticancer potential of novel symmetrical and asymmetrical dihydropyridines against breast cancer via EGFR inhibition: Molecular design, synthesis, analysis and screening. RSC Adv. 2024, 14, 11368–11387. [Google Scholar] [CrossRef]
- Bijani, S.; Iqbal, D.; Mirza, S.; Jain, V.; Jahan, S.; Alsaweed, M.; Madkhali, Y.; Alsagaby, S.A.; Banawas, S.; Algarni, A.; et al. Green synthesis and anticancer potential of 1,4-dihydropyridines-based triazole derivatives: In silico and in vitro study. Life 2022, 12, 519. [Google Scholar] [CrossRef] [PubMed]

| 1,4-DHP | Cell Lines | |||||
|---|---|---|---|---|---|---|
| GM07492A | HeLa | SI | MCF-7 | SI | U-251MG | |
| 1 | >100 | >100 | NC | >100 | NC | >100 |
| 2 | >100 | >100 | NC | >100 | NC | >100 |
| 3 | >100 | >100 | NC | >100 | NC | >100 |
| 4 | >100 | >100 | NC | >100 | NC | >100 |
| 5 | >100 | >100 | NC | >100 | NC | >100 |
| 6 | >100 | >100 | NC | 62.10 ± 2.41 * | NC | >100 |
| 7 | >100 | >100 | NC | >100 | NC | >100 |
| 8 | 6.48 ± 2.44 | >100 | NC | >100 | NC | >100 |
| 9 | >100 | >100 | NC | >100 | NC | >100 |
| 10 | >100 | >100 | NC | >100 | NC | >100 |
| 11 | >100 | >100 | NC | >100 | NC | >100 |
| 12 | >100 | >100 | NC | >100 | NC | >100 |
| 13 | >100 | >100 | NC | >100 | NC | >100 |
| 14 | >100 | >100 | NC | >100 | NC | >100 |
| 15 | >100 | >100 | NC | >100 | NC | >100 |
| 16 | 84.42 ± 12.17 | 51.83 ± 0.03 * | 1.62 | >100 | NC | >100 |
| 17 | >100 | 59.00 ± 10.23 * | >1.69 | >100 | NC | >100 |
| 18 | 9.69 ± 0.25 | 3.60 ± 0.96 * | 2.69 | 5.19 ± 1.60 * | 1.86 | >100 |
| 19 | 3.88 ± 0.37 | 2.31 ± 0.28 * | 1.67 | 5.74 ± 0.88 | 0.67 | >100 |
| 20 | 11.00 ± 0.22 | 4.10 ± 1.24 * | 2.68 | 11.95 ± 0.91 | 0.92 | >100 |
| 21 | >100 | 39.67 ± 2.60 * | 2.54 | >100 | NC | >100 |
| 22 | >100 | >100 | NC | >100 | NC | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.A.S.; Silva, J.B.A.; Esperandim, T.R.; Acésio, N.O.; Tavares, D.C.; Crotti, A.E.M. Anticancer Activity of 4-Aryl-1,4-Dihydropyridines. Future Pharmacol. 2024, 4, 564-573. https://doi.org/10.3390/futurepharmacol4030031
Oliveira TAS, Silva JBA, Esperandim TR, Acésio NO, Tavares DC, Crotti AEM. Anticancer Activity of 4-Aryl-1,4-Dihydropyridines. Future Pharmacology. 2024; 4(3):564-573. https://doi.org/10.3390/futurepharmacol4030031
Chicago/Turabian StyleOliveira, Thaís A. S., Jackson B. A. Silva, Tábata R. Esperandim, Nathália O. Acésio, Denise C. Tavares, and Antônio E. M. Crotti. 2024. "Anticancer Activity of 4-Aryl-1,4-Dihydropyridines" Future Pharmacology 4, no. 3: 564-573. https://doi.org/10.3390/futurepharmacol4030031
APA StyleOliveira, T. A. S., Silva, J. B. A., Esperandim, T. R., Acésio, N. O., Tavares, D. C., & Crotti, A. E. M. (2024). Anticancer Activity of 4-Aryl-1,4-Dihydropyridines. Future Pharmacology, 4(3), 564-573. https://doi.org/10.3390/futurepharmacol4030031







