Preclinical Pharmacology of the Low-Impact Ampakine CX717
Abstract
1. Introduction
2. Materials and Methods
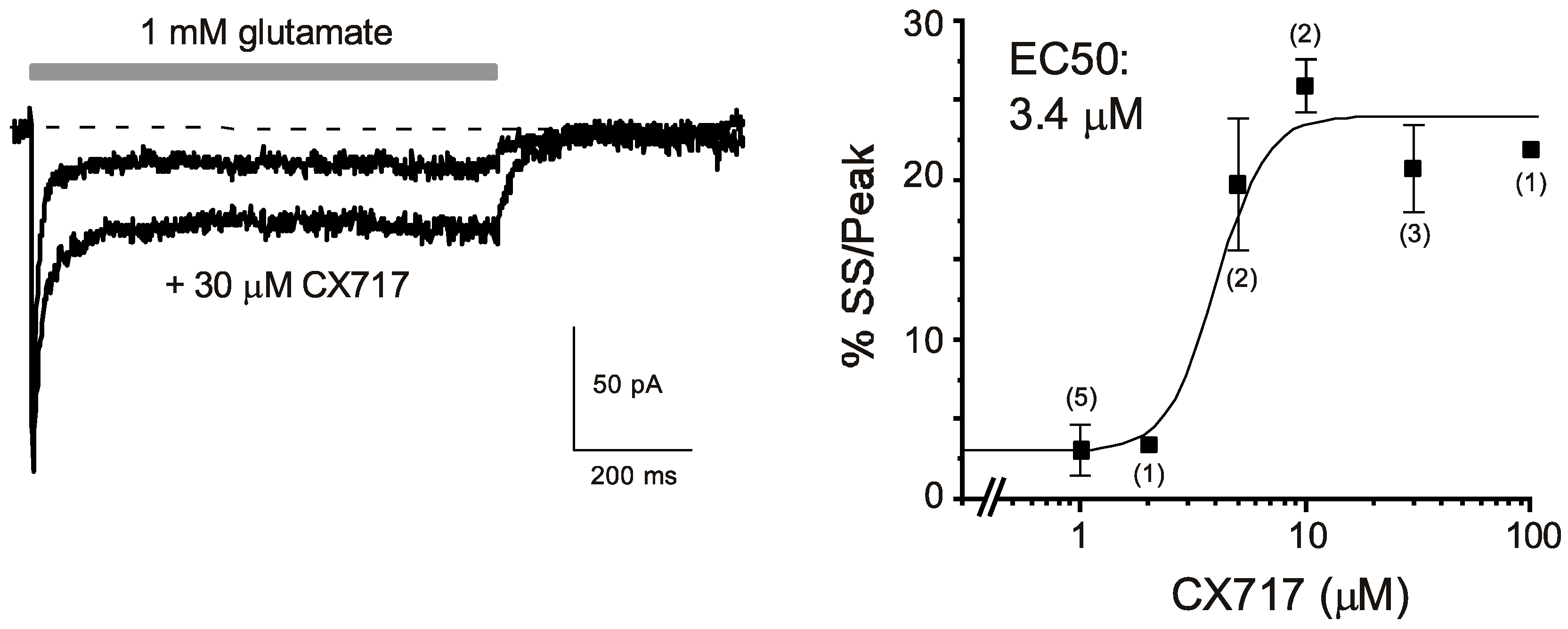
2.1. Hippocampal Slice Patch Clamp Experiments
2.2. Electrically Evoked Field Excitatory Postsynaptic Potentials (fEPSPs)
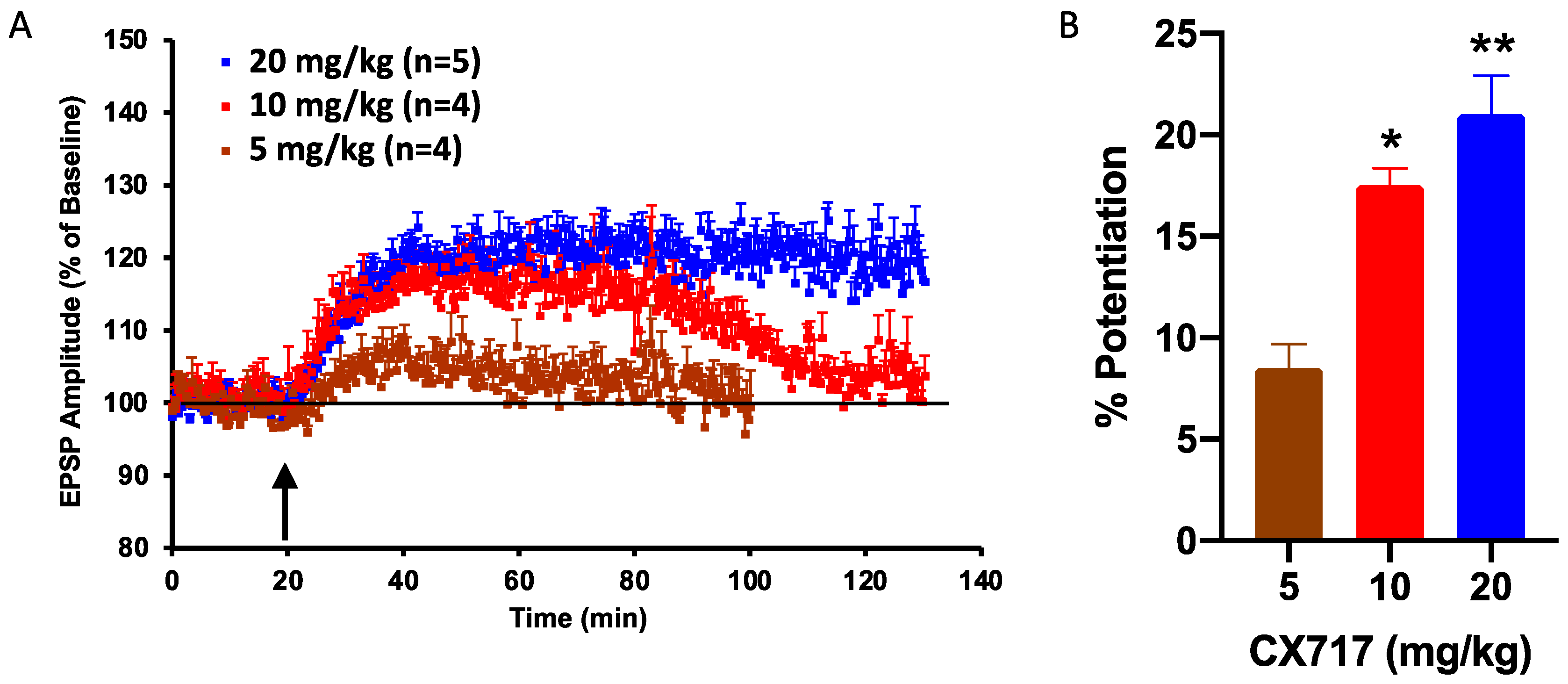
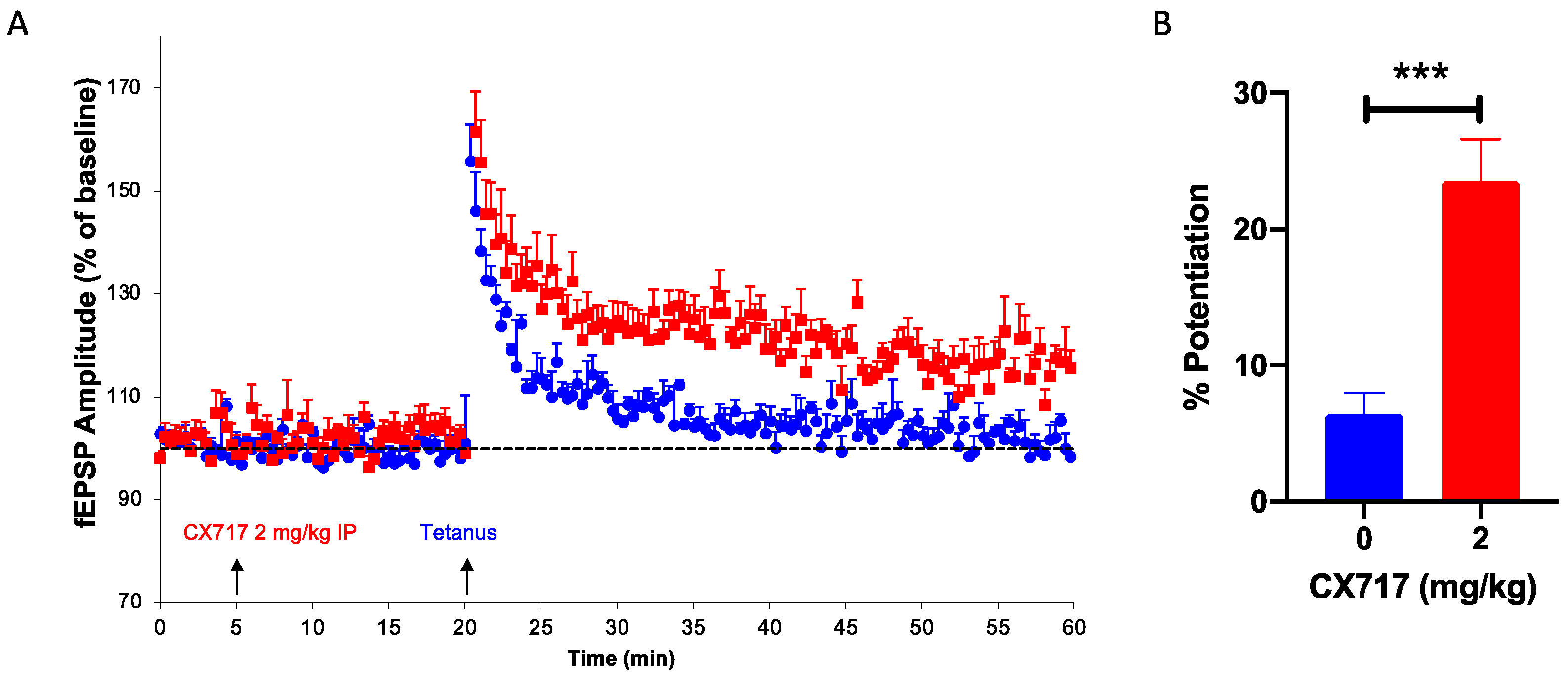
2.3. In Vivo LTP Experiments
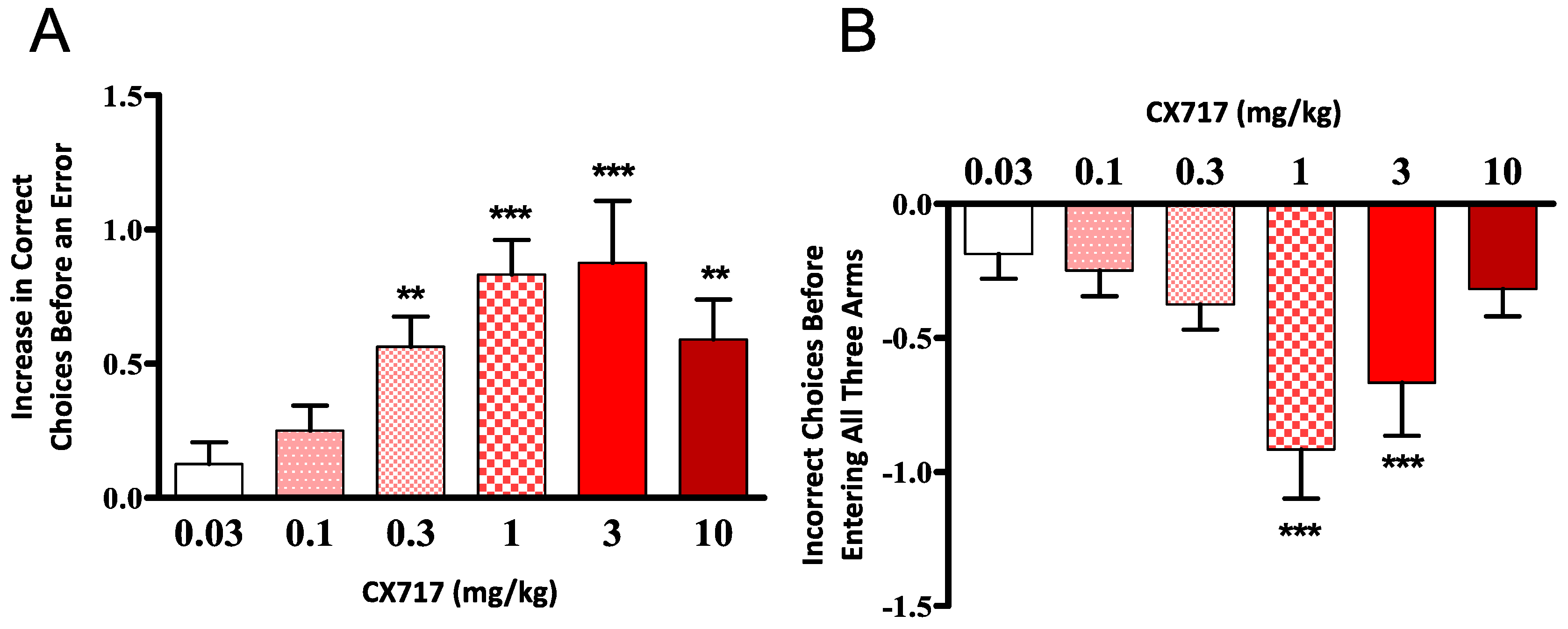
2.4. Eight-Arm Radial Maze
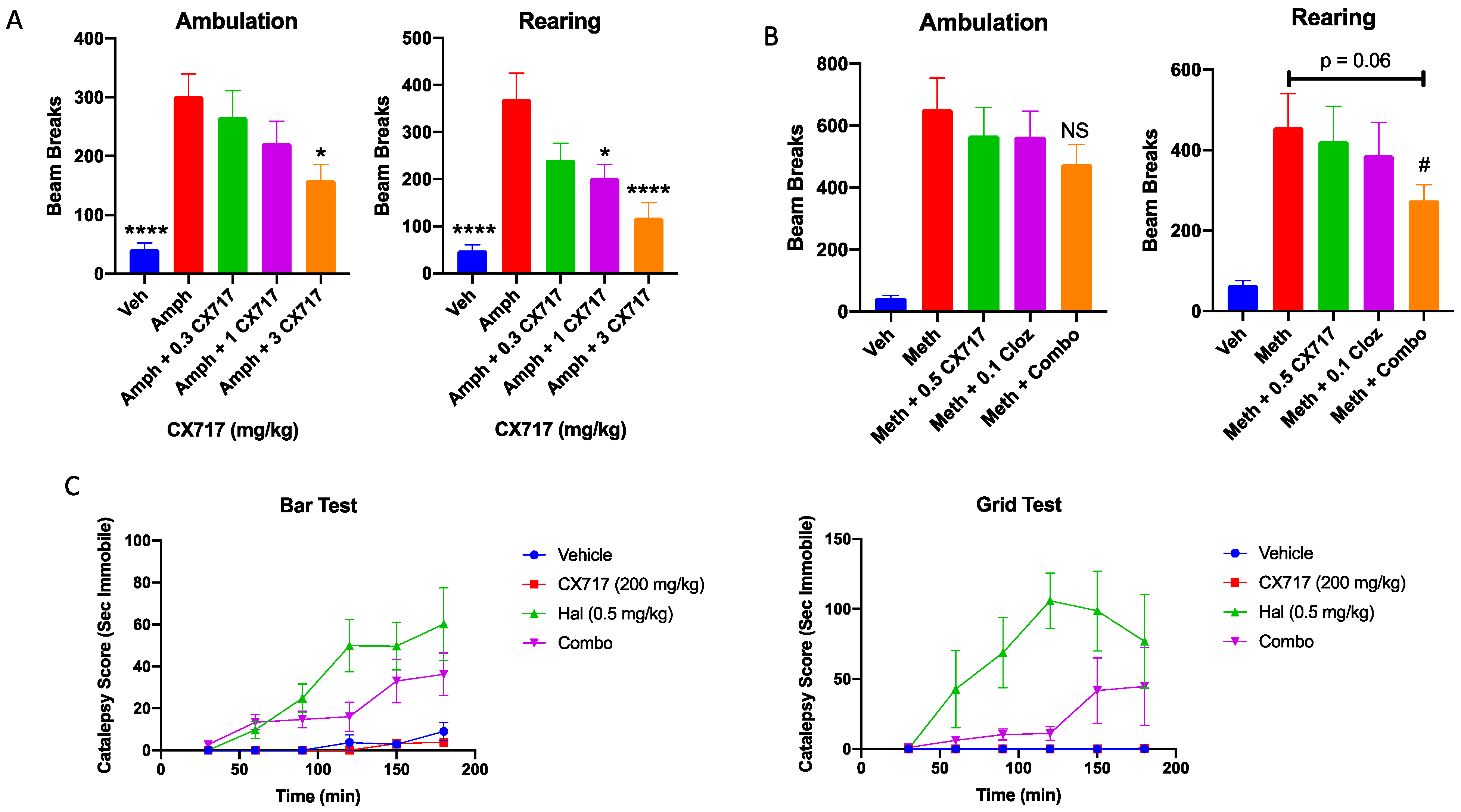
2.5. Amphetamine-Induced Locomotor Activity
2.6. Catalepsy Studies
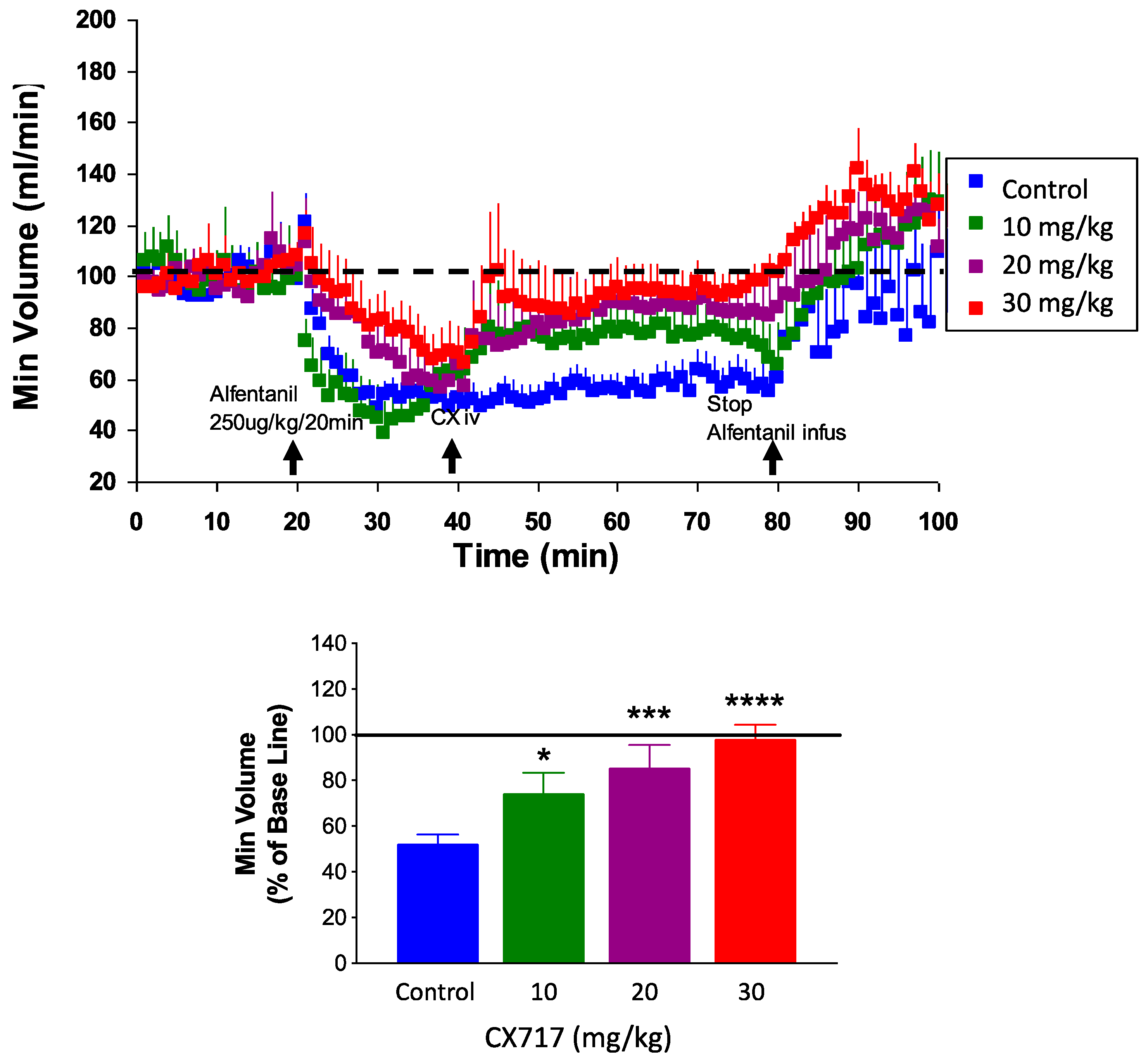
2.7. In Vivo Plethysmography Studies
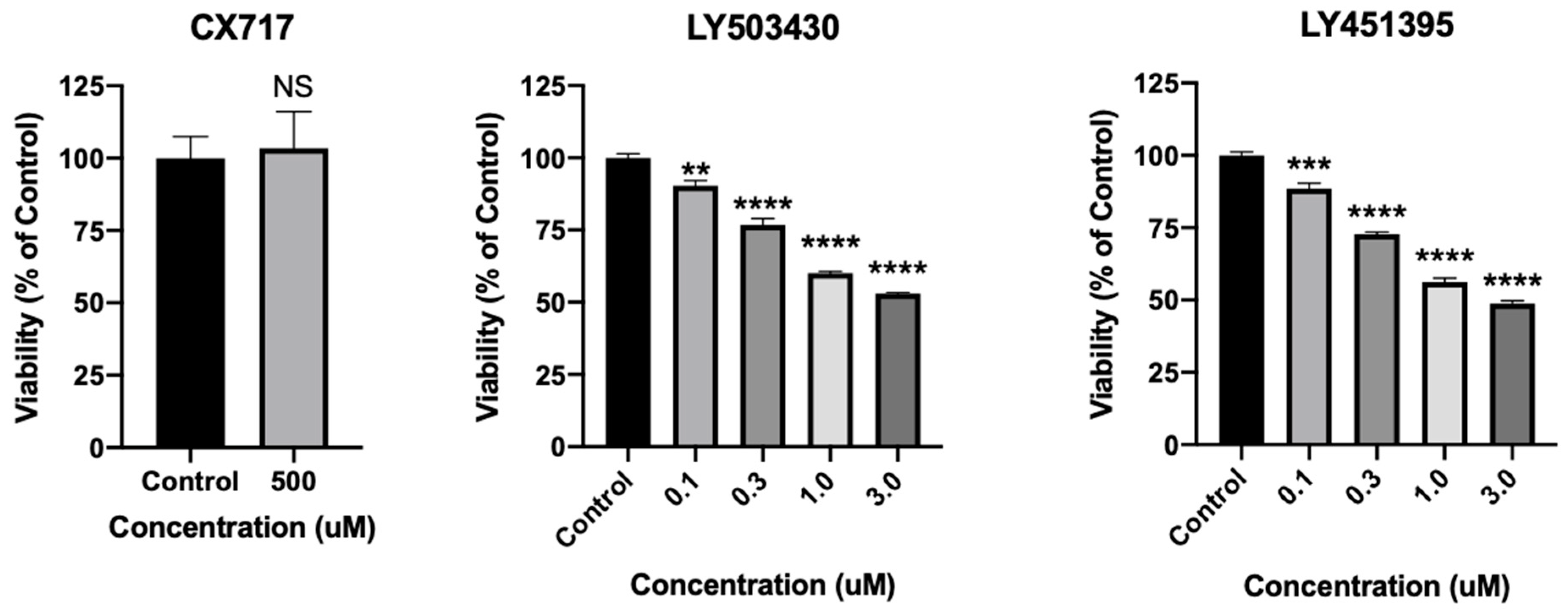
2.8. Assessment of CX717 Toxicity in Cultured Neurons
2.9. Single Dose Toxicity in Adult Mice
2.10. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bredt, D.S.; Nicoll, R.A. AMPA receptor trafficking at excitatory synapses. Neuron 2003, 40, 361–379. [Google Scholar] [CrossRef]
- Naaijen, J.; Bralten, J.; Poelmans, G.; Consortium, I.; Glennon, J.C.; Franke, B.; Buitelaar, J.K. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: Association to overlapping traits in ADHD and autism. Transl. Psychiatry 2017, 7, e999. [Google Scholar] [CrossRef] [PubMed]
- Medin, T.; Jensen, V.; Skare, O.; Storm-Mathisen, J.; Hvalby, O.; Bergersen, L.H. Altered alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function and expression in hippocampus in a rat model of attention-deficit/hyperactivity disorder (ADHD). Behav. Brain Res. 2019, 360, 209–215. [Google Scholar] [CrossRef]
- Bai, W.J.; Luo, X.G.; Jin, B.H.; Zhu, K.S.; Guo, W.Y.; Zhu, X.Q.; Qin, X.; Yang, Z.X.; Zhao, J.J.; Chen, S.R.; et al. Deficiency of transmembrane AMPA receptor regulatory protein gamma-8 leads to attention-deficit hyperactivity disorder-like behavior in mice. Zool. Res. 2022, 43, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.B.; Tucholski, J.; Haroutunian, V.; Meador-Woodruff, J.H. Transmembrane AMPA receptor regulatory protein (TARP) dysregulation in anterior cingulate cortex in schizophrenia. Schizophr. Res. 2013, 147, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tucholski, J.; Simmons, M.S.; Pinner, A.L.; Haroutunian, V.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr. Res. 2013, 146, 177–183. [Google Scholar] [CrossRef]
- Zeppillo, T.; Schulmann, A.; Macciardi, F.; Hjelm, B.E.; Focking, M.; Sequeira, P.A.; Guella, I.; Cotter, D.; Bunney, W.E.; Limon, A.; et al. Functional impairment of cortical AMPA receptors in schizophrenia. Schizophr. Res. 2022, 249, 25–37. [Google Scholar] [CrossRef]
- Benesh, J.L.; Mueller, T.M.; Meador-Woodruff, J.H. AMPA receptor subunit localization in schizophrenia anterior cingulate cortex. Schizophr. Res. 2022, 249, 16–24. [Google Scholar] [CrossRef]
- Singh, T.; Poterba, T.; Curtis, D.; Akil, H.; Al Eissa, M.; Barchas, J.D.; Bass, N.; Bigdeli, T.B.; Breen, G.; Bromet, E.J.; et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 2022, 604, 509–516. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Mizukami, K.; Davies, P.; Hamilton, R.; Sheffield, R.; Armstrong, D.M. The loss of GluR2(3) immunoreactivity precedes neurofibrillary tangle formation in the entorhinal cortex and hippocampus of Alzheimer brains. J. Neuropathol. Exp. Neurol. 1997, 56, 1018–1027. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Nocera, R.; Mizukami, K.; Armstrong, D.M. Age-related loss of the AMPA receptor subunits GluR2/3 in the human nucleus basalis of Meynert. Exp. Neurol. 2000, 166, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Han, H.; Li, H.; Bai, Y.; Wang, W.; Tu, M.; Peng, Y.; Zhou, L.; He, W.; Wu, X.; et al. Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. J. Clin. Investig. 2015, 125, 234–247. [Google Scholar] [CrossRef]
- Suzuki, K.; Elegheert, J.; Song, I.; Sasakura, H.; Senkov, O.; Matsuda, K.; Kakegawa, W.; Clayton, A.J.; Chang, V.T.; Ferrer-Ferrer, M.; et al. A synthetic synaptic organizer protein restores glutamatergic neuronal circuits. Science 2020, 369, eabb4853. [Google Scholar] [CrossRef]
- Alfaro-Ruiz, R.; Aguado, C.; Martin-Belmonte, A.; Moreno-Martinez, A.E.; Merchan-Rubira, J.; Hernandez, F.; Avila, J.; Fukazawa, Y.; Lujan, R. Alteration in the Synaptic and Extrasynaptic Organization of AMPA Receptors in the Hippocampus of P301S Tau Transgenic Mice. Int. J. Mol. Sci. 2022, 23, 13527. [Google Scholar] [CrossRef]
- Wright, A.L.; Konen, L.M.; Mockett, B.G.; Morris, G.P.; Singh, A.; Burbano, L.E.; Milham, L.; Hoang, M.; Zinn, R.; Chesworth, R.; et al. The Q/R editing site of AMPA receptor GluA2 subunit acts as an epigenetic switch regulating dendritic spines, neurodegeneration and cognitive deficits in Alzheimer's disease. Mol. Neurodegener. 2023, 18, 65. [Google Scholar] [CrossRef]
- Prinkey, K.; Thompson, E.; Saikia, J.; Cid, T.; Dore, K. Fluorescence lifetime imaging of AMPA receptor endocytosis in living neurons: Effects of Abeta and PP1. Front. Mol. Neurosci. 2024, 17, 1409401. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Da Ros, L.U.; Machado, L.S.; Rocha, A.; Lazzarotto, G.; Carello-Collar, G.; De Bastiani, M.A.; Ferrari-Souza, J.P.; Lussier, F.Z.; Souza, D.O.; et al. The glutamatergic system in Alzheimer's disease: A systematic review with meta-analysis. Mol. Psychiatry 2024. [Google Scholar] [CrossRef]
- Restivo, L.; Ferrari, F.; Passino, E.; Sgobio, C.; Bock, J.; Oostra, B.A.; Bagni, C.; Ammassari-Teule, M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 11557–11562. [Google Scholar] [CrossRef]
- Chojnacka, M.; Beroun, A.; Magnowska, M.; Stawikowska, A.; Cysewski, D.; Milek, J.; Dziembowska, M.; Kuzniewska, B. Impaired synaptic incorporation of AMPA receptors in a mouse model of fragile X syndrome. Front. Mol. Neurosci. 2023, 16, 1258615. [Google Scholar] [CrossRef]
- Suresh, A.; Dunaevsky, A. Impaired AMPARs Translocation into Dendritic Spines with Motor Skill Learning in the Fragile X Mouse Model. eNeuro 2023, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, N.; Shamsizadeh, A.; Fatemi, I.; Allahtavakoli, M.; Moghadam-Ahmadi, A.; Kaviani, E.; Kaeidi, A. CX691, as an AMPA receptor positive modulator, improves the learning and memory in a rat model of Alzheimer's disease. Iran. J. Basic. Med. Sci. 2018, 21, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.K.; Whalley, K.; Robinson, C.S.; Ward, M.A.; Hicks, C.A.; Lodge, D.; Vandergriff, J.L.; Baumbarger, P.; Siuda, E.; Gates, M.; et al. LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson's disease. J. Pharmacol. Exp. Ther. 2003, 306, 752–762. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Murray, T.K.; Whalley, K.; Ward, M.A.; Hicks, C.A.; Woodhouse, S.; Osborne, D.J.; Skolnick, P. Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson's disease. Eur. J. Pharmacol. 2004, 486, 163–174. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Murray, T.K.; Clay, M.P.; Lindstrom, T.; Yang, C.R.; Nisenbaum, E.S. LY503430: Pharmacology, pharmacokinetics, and effects in rodent models of Parkinson's disease. CNS Drug Rev. 2005, 11, 77–96. [Google Scholar] [CrossRef]
- Jourdi, H.; Hamo, L.; Oka, T.; Seegan, A.; Baudry, M. BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology 2009, 56, 876–885. [Google Scholar] [CrossRef]
- Ogier, M.; Wang, H.; Hong, E.; Wang, Q.; Greenberg, M.E.; Katz, D.M. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 10912–10917. [Google Scholar] [CrossRef] [PubMed]
- Degano, A.L.; Park, M.J.; Penati, J.; Li, Q.; Ronnett, G.V. MeCP2 is required for activity-dependent refinement of olfactory circuits. Mol. Cell. Neurosci. 2014, 59, 63–75. [Google Scholar] [CrossRef]
- Scaramuzza, L.; De Rocco, G.; Desiato, G.; Cobolli Gigli, C.; Chiacchiaretta, M.; Mirabella, F.; Pozzi, D.; De Simone, M.; Conforti, P.; Pagani, M.; et al. The enhancement of activity rescues the establishment of Mecp2 null neuronal phenotypes. EMBO Mol. Med. 2021, 13, e12433. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Overman, J.J.; Zhong, S.; Mueller, R.; Lynch, G.; Carmichael, S.T. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 3766–3775. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Parker, K.; Nilsson, M.; Walker, F.R.; Gowing, E.K. Combined ampakine and BDNF treatments enhance poststroke functional recovery in aged mice via AKT-CREB signaling. J. Cereb. Blood Flow. Metab. 2015, 35, 1272–1279. [Google Scholar] [CrossRef]
- Simmons, D.A.; Rex, C.S.; Palmer, L.; Pandyarajan, V.; Fedulov, V.; Gall, C.M.; Lynch, G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc. Natl. Acad. Sci. USA 2009, 106, 4906–4911. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, C.; Cummings, D.M.; Hickey, M.A.; Kleiman-Weiner, M.; Chen, J.Y.; Watson, J.B.; Levine, M.S. Rescuing the Corticostriatal Synaptic Disconnection in the R6/2 Mouse Model of Huntington's Disease: Exercise, Adenosine Receptors and Ampakines. PLoS Curr. 2010, 2. [Google Scholar] [CrossRef]
- Simmons, D.A.; Mehta, R.A.; Lauterborn, J.C.; Gall, C.M.; Lynch, G. Brief ampakine treatments slow the progression of Huntington's disease phenotypes in R6/2 mice. Neurobiol. Dis. 2011, 41, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Kramar, E.; Xu, X.; Zadran, H.; Moreno, S.; Lynch, G.; Gall, C.; Bi, X. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol. Dis. 2012, 47, 210–215. [Google Scholar] [CrossRef]
- Ren, J.; Ding, X.; Funk, G.D.; Greer, J.J. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 2009, 110, 1364–1370. [Google Scholar] [CrossRef]
- Greer, J.J.; Ren, J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir. Physiol. Neurobiol. 2009, 168, 153–157. [Google Scholar] [CrossRef]
- Lorier, A.R.; Funk, G.D.; Greer, J.J. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS ONE 2010, 5, e8766. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Meng, F.H.; Dai, W.; Yong, Z.; Liu, J.Q.; Zhou, X.B.; Li, S. Design, Synthesis and Biological Evaluation of Brain-Targeted Thiamine Disulfide Prodrugs of Ampakine Compound LCX001. Molecules 2016, 21, 488. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Cerne, R.; Shoaib, M.; Witkin, J.M.; Lippa, A. Low-Impact Ampakine CX1739 Exerts Pro-Cognitive Effects and Reverses Opiate-Induced Respiratory Depression in Rodents. Future Pharmacol. 2024, 4, 173–187. [Google Scholar] [CrossRef]
- Oertel, B.G.; Felden, L.; Tran, P.V.; Bradshaw, M.H.; Angst, M.S.; Schmidt, H.; Johnson, S.; Greer, J.J.; Geisslinger, G.; Varney, M.A.; et al. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin. Pharmacol. Ther. 2010, 87, 204–211. [Google Scholar] [CrossRef]
- Lasztoczi, B.; Kardos, J. Cyclothiazide prolongs low [Mg2+]-induced seizure-like events. J. Neurophysiol. 2006, 96, 3538–3544. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Qian, B.; Liu, J.; Fan, M.; Chen, G.; Wang, Y. Cyclothiazide induces seizure behavior in freely moving rats. Brain Res. 2010, 1355, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, C.L.; Hurst, R.S.; Scialis, R.J.; Osgood, S.M.; Bryce, D.K.; Hoffmann, W.E.; Lazzaro, J.T.; Hanks, A.N.; Lotarski, S.; Weber, M.L.; et al. Positive allosteric modulation of AMPA receptors from efficacy to toxicity: The interspecies exposure-response continuum of the novel potentiator PF-4778574. J. Pharmacol. Exp. Ther. 2013, 347, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Cheng, Z.; Liu, J.; Wang, Y. Downregulated GABA and BDNF-TrkB pathway in chronic cyclothiazide seizure model. Neural Plast. 2014, 2014, 310146. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, A.; Tanaka, M.; Suzuki, A.; Tajima, Y.; Suzuki, N.; Suzuki, M.; Nakamura, S.; Kuno, H.; Yokota, A.; Sogabe, S.; et al. TAK-137, an AMPA-R potentiator with little agonistic effect, has a wide therapeutic window. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Luu, N.T.; Herbst, T.A.; Knapp, R.; Lutz, D.; Arai, A.; Rogers, G.A.; Lynch, G. Synergistic interactions between ampakines and antipsychotic drugs. J. Pharmacol. Exp. Ther. 1999, 289, 392–397. [Google Scholar] [PubMed]
- Arai, A.C.; Xia, Y.F.; Rogers, G.; Lynch, G.; Kessler, M. Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J. Pharmacol. Exp. Ther. 2002, 303, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Marenco, S.; Egan, M.F.; Goldberg, T.E.; Knable, M.B.; McClure, R.K.; Winterer, G.; Weinberger, D.R. Preliminary experience with an ampakine (CX516) as a single agent for the treatment of schizophrenia: A case series. Schizophr. Res. 2002, 57, 221–226. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Krause, S.E.; Block, S.S.; Guter, S.; Wuu, J.; Leurgans, S.; Decle, P.; Potanos, K.; Cook, E.; Salt, J.; et al. Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: A controlled trial. J. Child. Adolesc. Psychopharmacol. 2006, 16, 525–540. [Google Scholar] [CrossRef]
- Turner, S.M.; ElMallah, M.K.; Hoyt, A.K.; Greer, J.J.; Fuller, D.D. Ampakine CX717 potentiates intermittent hypoxia-induced hypoglossal long-term facilitation. J. Neurophysiol. 2016, 116, 1232–1238. [Google Scholar] [CrossRef]
- Wollman, L.B.; Streeter, K.A.; Fusco, A.F.; Gonzalez-Rothi, E.J.; Sandhu, M.S.; Greer, J.J.; Fuller, D.D. Ampakines stimulate phrenic motor output after cervical spinal cord injury. Exp. Neurol. 2020, 334, 113465. [Google Scholar] [CrossRef]
- Rana, S.; Sunshine, M.D.; Greer, J.J.; Fuller, D.D. Ampakines Stimulate Diaphragm Activity after Spinal Cord Injury. J. Neurotrauma 2021, 38, 3467–3482. [Google Scholar] [CrossRef]
- Witkin, J.M.; Radin, D.P.; Rana, S.; Fuller, D.D.; Fusco, A.F.; Demers, J.C.; Pradeep Thakre, P.; Smith, J.L.; Lippa, A.; Cerne, R. AMPA receptors play an important role in the biological consequences of spinal cord injury: Implications for AMPA receptor modulators for therapeutic benefit. Biochem. Pharmacol. 2024, 116302. [Google Scholar] [CrossRef]
- Rana, S.; Alom, F.; Martinez, R.C.; Fuller, D.D.; Mickle, A.D. Acute ampakines increase voiding function and coordination in a rat model of SCI. eLife 2024, 12, 1–19. [Google Scholar] [CrossRef]
- Rana, S.; Thakre, P.P.; Fuller, D.D. Ampakines increase diaphragm activation following mid-cervical contusion injury in rats. Exp. Neurol. 2024, 376, 114769. [Google Scholar] [CrossRef]
- Porrino, L.J.; Daunais, J.B.; Rogers, G.A.; Hampson, R.E.; Deadwyler, S.A. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005, 3, e299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Balabhadrapatruni, S.; Masumura, C.; Darlington, C.L.; Smith, P.F. Effects of the putative cognitive-enhancing ampakine, CX717, on attention and object recognition memory. Curr. Alzheimer Res. 2011, 8, 876–882. [Google Scholar] [CrossRef]
- Ren, J.; Ding, X.; Greer, J.J. Respiratory depression in rats induced by alcohol and barbiturate and rescue by ampakine CX717. J. Appl. Physiol. 2012, 113, 1004–1011. [Google Scholar] [CrossRef]
- Ren, J.; Lenal, F.; Yang, M.; Ding, X.; Greer, J.J. Coadministration of the AMPAKINE CX717 with propofol reduces respiratory depression and fatal apneas. Anesthesiology 2013, 118, 1437–1445. [Google Scholar] [CrossRef]
- Gordillo-Salas, M.; Pascual-Anton, R.; Ren, J.; Greer, J.; Adell, A. Antidepressant-Like Effects of CX717, a Positive Allosteric Modulator of AMPA Receptors. Mol. Neurobiol. 2020, 57, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Wollman, L.B.; Streeter, K.A.; Fuller, D.D. Ampakine pretreatment enables a single brief hypoxic episode to evoke phrenic motor facilitation. J. Neurophysiol. 2020, 123, 993–1003. [Google Scholar] [CrossRef]
- Thakre, P.P.; Sunshine, M.D.; Fuller, D.D. Ampakine pretreatment enables a single hypoxic episode to produce phrenic motor facilitation with no added benefit of additional episodes. J. Neurophysiol. 2021, 126, 1420–1429. [Google Scholar] [CrossRef]
- Thakre, P.P.; Sunshine, M.D.; Fuller, D.D. Spinally delivered ampakine CX717 increases phrenic motor output in adult rats. Respir. Physiol. Neurobiol. 2022, 296, 103814. [Google Scholar] [CrossRef]
- Thakre, P.P.; Fuller, D.D. Pattern sensitivity of ampakine-hypoxia interactions for evoking phrenic motor facilitation in anesthetized rat. J. Neurophysiol. 2023, 131, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.A.; Stein, M.; Mansbach, H. Treatment of adult ADHD with the novel Ampakine CX717. In Proceedings of the 53rd Annual Meeting of the American Academy of Child and Adolescent Psychiatry, San Diego, CA, USA, 24–29 October 2006; p. A42. [Google Scholar]
- Purcell, R.; Lynch, G.; Gall, C.; Johnson, S.; Sheng, Z.; Stephen, M.R.; Cook, J.; Garman, R.H.; Jortner, B.; Bolon, B.; et al. Brain Vacuolation Resulting From Administration of the Type II Ampakine CX717 Is An Artifact Related to Molecular Structure and Chemical Reaction With Tissue Fixative Agents. Toxicol. Sci. 2018, 162, 383–395. [Google Scholar] [CrossRef]
- Yamada, K.A. Therapeutic potential of positive AMPA receptor modulators in the treatment of neurological disease. Expert. Opin. Investig. Drugs 2000, 9, 765–778. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Bleakman, D.; Zimmerman, D.M.; Nisenbaum, E.S. AMPA receptor potentiators for the treatment of CNS disorders. Curr. Drug Targets. CNS Neurol. Disord. 2004, 3, 181–194. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Dix, S. AMPA receptor potentiators as cognitive enhancers. IDrugs Investig. Drugs J. 2007, 10, 185–192. [Google Scholar]
- Woolley, M.L.; Waters, K.A.; Gartlon, J.E.; Lacroix, L.P.; Jennings, C.; Shaughnessy, F.; Ong, A.; Pemberton, D.J.; Harries, M.H.; Southam, E.; et al. Evaluation of the pro-cognitive effects of the AMPA receptor positive modulator, 5-(1-piperidinylcarbonyl)-2,1,3-benzoxadiazole (CX691), in the rat. Psychopharmacology 2009, 202, 343–354. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Mohn, A.R.; Bohn, L.M.; Caron, M.G. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc. Natl. Acad. Sci. USA 2001, 98, 11047–11054. [Google Scholar] [CrossRef]
- Hess, U.S.; Whalen, S.P.; Sandoval, L.M.; Lynch, G.; Gall, C.M. Ampakines reduce methamphetamine-driven rotation and activate neocortex in a regionally selective fashion. Neuroscience 2003, 121, 509–521. [Google Scholar] [CrossRef]
- Arai, A.; Kessler, M.; Xiao, P.; Ambros-Ingerson, J.; Rogers, G.; Lynch, G. A centrally active drug that modulates AMPA receptor gated currents. Brain Res. 1994, 638, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.; Kessler, M.; Ambros-Ingerson, J.; Quan, A.; Yigiter, E.; Rogers, G.; Lynch, G. Effects of a centrally active benzoylpyrrolidine drug on AMPA receptor kinetics. Neuroscience 1996, 75, 573–585. [Google Scholar] [CrossRef]
- Arai, A.; Kessler, M.; Rogers, G.; Lynch, G. Effects of a memory-enhancing drug on DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J. Pharmacol. Exp. Ther. 1996, 278, 627–638. [Google Scholar]
- Arai, A.; Lynch, G. Response to repetitive stimulation of AMPA receptors in patches excised from fields CA1 and CA3 of the hippocampus. Brain Res. 1996, 716, 202–206. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Purcell, R.; Lippa, A. Acute ampakine treatment ameliorates age-related deficits in long-term potentiation. Biomed. Pharmacother. 2016, 84, 806–809. [Google Scholar] [CrossRef]
- Hoffman, D.C.; Donovan, H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology 1995, 120, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Radin, D.P.; Zhong, S.; Cerne, R.; Witkin, J.; Lippa, A. High impact AMPAkines induce a Gq-protein coupled endoplasmic calcium release in cortical neurons: A possible mechanism for explaining the toxicity of high impact AMPAkines. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ryder, J.W.; Falcone, J.F.; Manro, J.R.; Svensson, K.A.; Merchant, K.M. Pharmacological characterization of cGMP regulation by the biarylpropylsulfonamide class of positive, allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. J. Pharmacol. Exp. Ther. 2006, 319, 293–298. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Witkin, J.M. AMPA receptor potentiators: Application for depression and Parkinson's disease. Curr. Drug Targets 2007, 8, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Jhee, S.S.; Chappell, A.S.; Zarotsky, V.; Moran, S.V.; Rosenthal, M.; Kim, E.; Chalon, S.; Toublanc, N.; Brandt, J.; Coutant, D.E.; et al. Multiple-dose plasma pharmacokinetic and safety study of LY450108 and LY451395 (AMPA receptor potentiators) and their concentration in cerebrospinal fluid in healthy human subjects. J. Clin. Pharmacol. 2006, 46, 424–432. [Google Scholar] [CrossRef]
- Jones, N.; Messenger, M.J.; O'Neill, M.J.; Oldershaw, A.; Gilmour, G.; Simmons, R.M.; Iyengar, S.; Libri, V.; Tricklebank, M.; Williams, S.C. AMPA receptor potentiation can prevent ethanol-induced intoxication. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 1713–1723. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Cummings, J.; Konechnik, T.; Forrester, T.D.; Chang, C.; Dennehy, E.B.; Willis, B.A.; Shuler, C.; Tabas, L.B.; Lyketsos, C. Mibampator (LY451395) randomized clinical trial for agitation/aggression in Alzheimer's disease. Int. Psychogeriatr. 2013, 25, 707–719. [Google Scholar] [CrossRef]
- Kunugi, A.; Tajima, Y.; Kuno, H.; Sogabe, S.; Kimura, H. HBT1, a Novel AMPA Receptor Potentiator with Lower Agonistic Effect, Avoided Bell-Shaped Response in In Vitro BDNF Production. J. Pharmacol. Exp. Ther. 2018, 364, 377–389. [Google Scholar] [CrossRef]
- Miraucourt, L.S.; Accardi, M.V.; Asin, K.E.; Pugsley, M.K.; Curtis, M.J.; Authier, S. The application of electrophysiological methods to characterize AMPA receptors in dissociated adult rat and non-human primate cerebellar neurons for use in neuronal safety pharmacology assessments of the central nervous system. J. Pharmacol. Toxicol. Methods 2020, 105, 106883. [Google Scholar] [CrossRef]
- Ishii, T.; Stolz, J.R.; Swanson, G.T. Auxiliary Proteins are the Predominant Determinants of Differential Efficacy of Clinical Candidates Acting as AMPA Receptor Positive Allosteric Modulators. Mol. Pharmacol. 2020, 97, 336–350. [Google Scholar] [CrossRef]
- Radin, D.P.; Smith, G.; Moushiaveshi, V.; Wolf, A.; Bases, R.; Tsirka, S.E. Lucanthone Targets Lysosomes to Perturb Glioma Proliferation, Chemoresistance and Stemness, and Slows Tumor Growth In Vivo. Front. Oncol. 2022, 12, 852940. [Google Scholar] [CrossRef]
- Goff, D.C.; Leahy, L.; Berman, I.; Posever, T.; Herz, L.; Leon, A.C.; Johnson, S.A.; Lynch, G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J. Clin. Psychopharmacol. 2001, 21, 484–487. [Google Scholar] [CrossRef]
- Goff, D.C.; Lamberti, J.S.; Leon, A.C.; Green, M.F.; Miller, A.L.; Patel, J.; Manschreck, T.; Freudenreich, O.; Johnson, S.A. A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 465–472. [Google Scholar] [CrossRef]
- Wan, L.; Liu, X.; Wu, Z.; Ren, W.; Kong, S.; Dargham, R.A.; Cheng, L.; Wang, Y. Activation of extrasynaptic GABA(A) receptors inhibits cyclothiazide-induced epileptiform activity in hippocampal CA1 neurons. Neurosci. Bull. 2014, 30, 866–876. [Google Scholar] [CrossRef]
- Hampson, R.E.; Rogers, G.; Lynch, G.; Deadwyler, S.A. Facilitative effects of the ampakine CX516 on short-term memory in rats: Correlations with hippocampal neuronal activity. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 2748–2763. [Google Scholar] [CrossRef]
- Hampson, R.E.; Rogers, G.; Lynch, G.; Deadwyler, S.A. Facilitative effects of the ampakine CX516 on short-term memory in rats: Enhancement of delayed-nonmatch-to-sample performance. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 2740–2747. [Google Scholar] [CrossRef]
- Arai, A.; Lynch, G. The waveform of synaptic transmission at hippocampal synapses is not determined by AMPA receptor desensitization. Brain Res. 1998, 799, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Sun, Y.; Wu, Z.; Wan, L.; Chen, L.; Kong, S.; Zhang, B.; Zhang, F.; Wang, Z.Y.; Wang, Y. Epileptiform response of CA1 neurones to convulsant stimulation by cyclothiazide, kainic acid and pentylenetetrazol in anaesthetized rats. Seizure 2011, 20, 312–319. [Google Scholar] [CrossRef]
- Radin, D.P.; Johnson, S.; Purcell, R.; Lippa, A.S. Effects of chronic systemic low-impact ampakine treatment on neurotrophin expression in rat brain. Biomed. Pharmacother. 2018, 105, 540–544. [Google Scholar] [CrossRef]
- Jordan, G.R.; McCulloch, J.; Shahid, M.; Hill, D.R.; Henry, B.; Horsburgh, K. Regionally selective and dose-dependent effects of the ampakines Org 26576 and Org 24448 on local cerebral glucose utilisation in the mouse as assessed by 14C-2-deoxyglucose autoradiography. Neuropharmacology 2005, 49, 254–264. [Google Scholar] [CrossRef]
- Vanover, K.E. Effects of AMPA receptor positive modulators on amphetamine- and dizocilpine-induced locomotion. Eur. J. Pharmacol. 1997, 332, 115–119. [Google Scholar] [CrossRef]
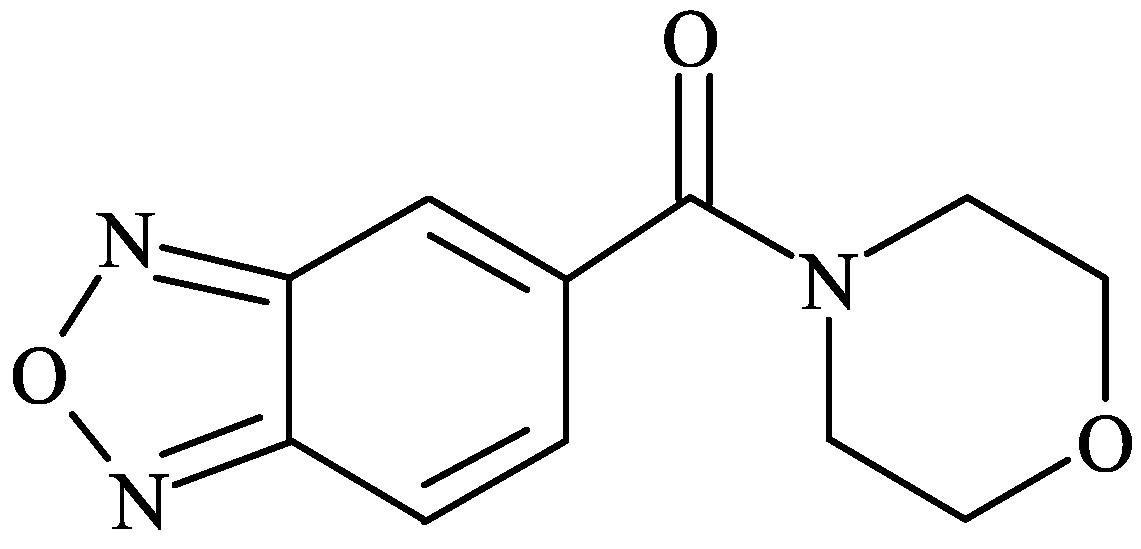
| Dose (mg/kg) | # of Mice Seizing | # of Mice Surviving to 14 Days |
|---|---|---|
| 2000 | 0/3 | 3/3 |
| 2500 | 0/2 | 0/2 |
| 5000 | 1/2 | 1/2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radin, D.P.; Zhong, S.; Cerne, R.; Smith, J.L.; Witkin, J.M.; Lippa, A. Preclinical Pharmacology of the Low-Impact Ampakine CX717. Future Pharmacol. 2024, 4, 494-509. https://doi.org/10.3390/futurepharmacol4030028
Radin DP, Zhong S, Cerne R, Smith JL, Witkin JM, Lippa A. Preclinical Pharmacology of the Low-Impact Ampakine CX717. Future Pharmacology. 2024; 4(3):494-509. https://doi.org/10.3390/futurepharmacol4030028
Chicago/Turabian StyleRadin, Daniel P., Sheng Zhong, Rok Cerne, Jodi L. Smith, Jeffrey M. Witkin, and Arnold Lippa. 2024. "Preclinical Pharmacology of the Low-Impact Ampakine CX717" Future Pharmacology 4, no. 3: 494-509. https://doi.org/10.3390/futurepharmacol4030028
APA StyleRadin, D. P., Zhong, S., Cerne, R., Smith, J. L., Witkin, J. M., & Lippa, A. (2024). Preclinical Pharmacology of the Low-Impact Ampakine CX717. Future Pharmacology, 4(3), 494-509. https://doi.org/10.3390/futurepharmacol4030028






