A Mechanistic Insight on Phytoconstituents Delivering Hypoglycemic Activity: A Comprehensive Overview
Abstract
1. Introduction
2. Classification of Diabetes mellitus
- T1DM is recognized by autoimmune destruction of β-cell responsible for production of insulin. T2DM leads to the development of insulin resistance [9].
- Gestational diabetes mellitus (GDM) represents an increase in glucose level and is commonly seen during second or third trimesters of pregnancy.
- Monogenic diabetes caused through various genetic reasons and grouped together under the term “Other Specific Types” [10].
2.1. T1DM
2.2. T2DM
2.3. GDM
2.4. Other Specific Type of Diabetes (Monogenic Diabetes)
3. Complications of Diabetes
3.1. Diabetic Nephropathy (DN)
3.2. Diabetic Retinopathy (DR)
3.3. Diabetic Peripheral Neuropathy (DPN)
3.4. Sexual Dysfunction
3.5. Heart Stroke and Heart Diseases
3.6. Diabetic Foot Ulcer
4. Molecular Mechanism of Phytoconstituents Exhibiting Antihyperglycemic Action Might Be Useful for Prevention and Treatment of Diabetes and Its Associated Complications
4.1. Flavonoids
4.1.1. Diosmin
4.1.2. Morin
4.1.3. Fisetin
4.1.4. Hesperidin
4.1.5. Eriodictyol
4.1.6. Naringenin
4.1.7. Apigenin
4.1.8. Kaempferol
4.1.9. Chrysin
4.1.10. Baicalein
4.1.11. Luteolin
4.1.12. Tangeretin
4.1.13. Isorhamnetin
4.1.14. Wogonin
4.1.15. Rutin
4.1.16. Quercetin
4.1.17. Genistein
4.1.18. Daidzein
4.1.19. Delphinidin
4.1.20. Cyanidin
4.1.21. Pelargonidin
4.2. Saponins
4.2.1. Diosgenin
4.2.2. Arjunolic Acid
4.2.3. Platyconic Acid
4.3. Alkaloids
- True alkaloids (Atropine, morphine and, nicotine etc.)
- Protoalkaloids (Adrenaline, ephedrine and, mescaline etc.)
- Polyamine alkaloids: Example; Spermidine, epinephrine, putrescine and spermine.
- Pseudoalkaloids: Example; Caffeine, theophylline and, theobromine, etc.
4.3.1. Koenidine
4.3.2. Canthin-6-One Derivatives
4.3.3. Vindoline I, Vindolidine II, Vindocine III and Vindocine IV
4.4. Tannins
- Hydrolysable tannins (gallic acid),
- Non-hydrolysable or Condensed tannins (flavones), and
- Phlorotannins (phloroglucinol).
4.4.1. Catechin
4.4.2. Epigallocatechin
4.5. Terpenes
4.5.1. Linalool
4.5.2. α-Pinene
4.6. Glycosides
5. Nutrivigilance (Compounds’ Adverse Effects)—As a Possible Limitation of Their Use
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA Carboxylase |
| AFABP | Adipocyte-specific Fatty Acid Binding Protein |
| AGEs | Advanced Glycation Endproducts |
| ALT | Alanine Aminotransferase |
| AMPK | AMP-activated Protein Kinase |
| ATF-4 | Activating Transcription Factor-4 |
| BDNF | Brain-Derived Neurotrophic Factor |
| BMI | Body Mass Index |
| Bw | Body weight |
| cAMP | Cyclic Adenosine Monophosphate |
| CDKN1B | Cyclin-Dependent Kinase Inhibitor 1B |
| CHOP | Cyclophosphamide, Hydroxyl daunorubicin, Oncovin and Prednisone |
| CKD | Chronic Kidney Disease |
| CVD | Cardio Vascular Diseases |
| DFU | Diabetic Foot Ulcer |
| DKA | Diabetic Keto Acidosis |
| DME | Diabetic Macular Edema |
| DN | Diabetic Nephropathy |
| DNMT1 | DNA (cytosine-5)-methyl transferase 1 |
| DPN | Diabetic Peripheral Neuropathy |
| DR | Diabetic Retinopathy |
| DRN | Diabetic Retinal Neurodegeneration |
| ED | Erectile Dysfunction |
| EGCG | Epigallocatechin |
| eNOS | Endothelial Nitric Oxide Synthase |
| ERK | Extracellular signal-regulated kinase |
| FFA | Free Fatty Acids |
| Fox | Forkhead Box |
| FPG | Fasting Plasma Glucose |
| FSD | Female Sexual Dysfunction |
| GDM | Gestational Diabetes Mellitus |
| GFR | Glomerular Filtration Rate |
| GLUT4 | Glucose Transporter 4 IL-1beta |
| GSH | Glutathione |
| GWAS | Genome-Wide Association Studies |
| HbA1 | Hemoglobin A1 |
| HbA1c | Hemoglobin A1c |
| HDL | High Density Lipoprotein |
| HG | High Glucose |
| HIF-1 | Hypoxia-Inducible Factors-1 |
| HO-1 | Heme Oxygenase |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| ICAM-1 | Intercellular Adhesion Molecules |
| IDDM | Insulin Dependent Diabetes Mellitus |
| IDF | International Diabetes Federation |
| IL-1beta | Interleukin 1-beta |
| IL-6 | Interleukin-6 |
| iNOS | Inducible Nitric Oxide Synthase |
| INS | Insulin |
| INSR | Insulin Receptor |
| IRMA | Intra Retinal Microvascular Abnormalities. |
| IRSs | Insulin Receptor Substrate |
| JNK | c-Jun N-terminal kinase |
| LDH | Lactate Dehydrogenase |
| LDL | Low-Density Lipoprotein |
| LPS | Lipopolysaccharides |
| LXR | Liver X Receptor |
| MAPK | Mitogen Activated Protein Kinase |
| MCP-1 | Monocyte Chemotactic Protein-1 |
| MDA | Melondialdehyde |
| MG | Methyl Glyoxal |
| MODY | Maturity Onset Diabetes of the Young |
| NAD | Nicotinamide Adenine Dinucleotide |
| NADH | Nicotinamide Adenine Dinucleotide Hydrogen |
| NF-kB | Nuclear Factor Kappa B |
| NGF | Nerve Growth Factor |
| NIDDM | Non-Insulin Dependent Diabetes Mellitus |
| NLRP3 | NLR family pyrin domain containing 3 |
| NPDR | Nonproliferative Diabetic Retinopathy |
| Nrf2 | Nuclear factor erythroid 2 related factor |
| OGTT | Oral Glucose Tolerance Test |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PDR | Proliferative Diabetic Retinopathy |
| PI3K-PKB/Akt | Phosphoinositide-3-kinase–protein kinase B/Akt |
| PKA | Protein kinase |
| PKC | Protein kinase C |
| PKR | Protein kinase R |
| PPARγ | Peroxisome proliferation-activated receptor gamma |
| PTP-1B | Protein tyrosinase phosphatase-1B |
| PWT | Paw withdrawal threshold |
| QE | Quercetin |
| RAGE | Receptor for advanced glycation endproducts |
| RGC-5 | Retinal ganglion cell-5 |
| RhoA | Ras Homolog Family Member A |
| ROS | Reactive Oxygen Species |
| RPTEC | Renal proximal tubule epithelial cells |
| SOD | Superoxide dismutase |
| SREBP-1c | Sterol Regulatory Element Binding Protein 1c |
| STZ | Streptozotocin |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TBARS | Thio-barbituric acid reactive substances |
| TFG-1 | Transforming growth factor |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor Necrosis Factor alpha |
| mTOR | Mammalian target of rapamycin |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
References
- WHO Diabetes Programme. WHO. 2019. Available online: https://www.who.int/diabetes/en/ (accessed on 18 May 2022).
- Canivell, S.; Gomis, R. Diagnosis and classification of autoimmune diabetes mellitus. Autoimmun. Rev. 2014, 13, 403–407. [Google Scholar] [CrossRef] [PubMed]
- IDF Website. International Diabetes Federation. 2022. Available online: https://idf.org/ (accessed on 18 May 2022).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; Available online: https://diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf (accessed on 18 May 2022).
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J.A. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet Lond Engl. 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Baynest, H.W. Classification, Pathophysiology, Diagnosis and Management of Diabetes Mellitus. J. Diabetes Metab. 2015, 6, 541. [Google Scholar] [CrossRef]
- Saisho, Y. Importance of Beta Cell Function for the Treatment of Type 2 Diabetes. J. Clin. Med. 2014, 3, 923–943. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Molina, C.E.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Roberts, M.S.; Burbelo, P.D.; Spichtig, D.E.; Perwad, F.; Romero, C.J.; Ichikawa, S.; Farrow, E.; Econs, M.J.; Guthrie, L.C.; Collins, M.T.; et al. Autoimmune hyperphosphatemic tumoral calcinosis in a patient with FGF23 autoantibodies. J. Clin. Investig. 2018, 128, 5368–5373. [Google Scholar] [CrossRef]
- Pozzilli, P.; Pieralice, S. Latent Autoimmune Diabetes in Adults: Current Status and New Horizons. Endocrinol. Metab. 2018, 33, 147–159. [Google Scholar] [CrossRef]
- Lucier, J.; Weinstock, R.S. Diabetes Mellitus Type 1. In StatPearls; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Esmaili, S.; Hemmati, M.; Karamian, M. Physiological role of adiponectin in different tissues: A review. Arch. Physiol. Biochem. 2020, 126, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Verges, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2017, 14, 88–98. [Google Scholar] [CrossRef]
- Lim, A.K.H. Diabetic nephropathy—Complications and treatment. Int. J. Nephrol. Renov. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef]
- Vallon, V.; Komers, R. Pathophysiology of the Diabetic Kidney. Compr. Physiol. 2011, 1, 1175–1232. [Google Scholar] [CrossRef]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef]
- Himasa, F.I.; Singhal, M.; Ojha, A.; Kumar, B. Prospective for Diagnosis and Treatment of Diabetic Retinopathy. Curr. Pharm. Des. 2022, 28, 560–569. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Peng, W.; Zhou, J.; Liu, Z. Acupuncture for postherpetic neuralgia: Systematic review and meta-analysis. Medicine 2018, 97, e11986. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Banu, R.; Chee, M.L. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol. 2019, 7, 140–149. [Google Scholar] [CrossRef]
- Sloan, G.; Shillo, P.; Selvarajah, D.; Wu, J.; Wilkinson, L.D.; Tracey, I.; Anand, P.; Tesfaye, S. A new look at painful diabetic neuropathy. Diabetes Res. Clin. Pract. 2018, 144, 177–191. [Google Scholar] [CrossRef]
- Edwards, J.L.; Vincent, A.; Cheng, T.; Feldman, E.L. Diabetic Neuropathy: Mechanisms to Management. Pharmacol Ther. 2008, 120, 1–34. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Impotence, Impotence—NIH Consensus Conference. JAMA 1993, 270, 83–90. Available online: https://pubmed.ncbi.nlm.nih.gov/8510302/ (accessed on 18 May 2022). [CrossRef]
- Imprialos, K.P.; Stavropoulos, K.; Doumas, M.; Tziomalos, K.; Karagiannis, A.; Athyros, V.G. Sexual dysfunction, cardiovascular risk and effects of pharmacotherapy. Curr. Vasc. Pharmacol. 2018, 16, 130–142. [Google Scholar] [CrossRef]
- Corona, G.C.; Giorda, B.; Cucinotta, D.; Guida, P.; Nada, E. Sexual dysfunction at the onset of type 2 diabetes: The interplay of depression, hormonal and cardiovascular factors. J. Sex. Med. 2014, 11, 2065–2073. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Cortelazzi, D.; Morabito, A. Female sexual dysfunction and diabetes: A systematic review and meta-analysis. J. Sex. Med. 2013, 10, 1044–1045. [Google Scholar] [CrossRef]
- Maseroli, E.; Scavello, I.; Vignozzi, L. Cardiometabolic risk and female sexuality-part I. risk factors and potential pathophysiological underpinnings for female vasculogenic sexual dysfunction syndromes. Sex. Med. Rev. 2018, 6, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mittal, S.; Aggarwal, R.; Chauhan, M.K. Diabetes and cardiovascular disease: Inter-relation of risk factors and treatment. Future J. Pharm. Sci. 2020, 6, 130. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Keulenaer, G.W.D. Pathophysiology of diastolic dysfunction in chronic heart failure. Future Cardiol. 2013, 9, 711–720. [Google Scholar] [CrossRef]
- Coffey, L.; Mahon, C.; Gallagher, P. Perceptions and experiences of diabetic foot ulceration and foot care in people with diabetes: A qualitative meta-synthesis. Int. Wound J. 2019, 16, 183–210. [Google Scholar] [CrossRef]
- Acosta, J.B.; Montequin, J.F.; Perez, C.V.; Gutierrez, W.S.; Marí, Y.M.; Ojalvo, A.G.; Cama, V.F.; Herrera, D.G.D.B.; Mayola, M.F.; Vázquez, H.P.S.E.P.; et al. Diabetic Foot Ulcers and Epidermal Growth Factor: Revisiting the Local Delivery Route for a Successful Outcome. BioMed Res. Int. 2017, 2017, 2923759. [Google Scholar] [CrossRef]
- Amin, N.; Doupis, J. Diabetic foot disease: From the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World J. Diabetes 2016, 7, 153–164. [Google Scholar] [CrossRef]
- Ahmad, J. The diabetic foot. Diabetes Metab. Syndr. 2016, 10, 48–60. [Google Scholar] [CrossRef]
- Prabhakar, P.; Banerjee, M. Antidiabetic Phytochemicals: A comprehensive Review on Opportunities and Challenges in Targeted Therapy for Herbal Drug Development. Int. J. Pharm. Res. 2020, 14, 1673–1696. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Dendi, V.S.R.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Baska, A.; Leis, K.; Gałązka, P. Berberine in the Treatment of Diabetes Mellitus: A Review. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, M.U.K.; Sadiq, A.; Faidah, H.S.; Khurram, M.; Amin, M.U.; Haseeb, A.; Kakar, M. Berberine nanoparticles with enhanced in vitro bioavailability: Characterization and antimicrobial activity. Drug Des. Dev. Ther. 2018, 12, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine improves glucose metabolism through induction of glycolysis. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E148–E156. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Tartagni, E. Antidiabetic properties of berberine: From cellular pharmacology to clinical effects. Hosp. Pract. 2012, 40, 56–63. [Google Scholar] [CrossRef]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in the treatment of type 2 diabetes mellitus: A systemic review and meta-analysis. Evid. Based Complement. Altern. Med. 2012, 2012, 591654. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, L. Berberine in type 2 diabetes therapy: A new perspective for an old antidiarrheal drug? Acta Pharm. Sin. B 2012, 2, 379–386. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Khan, H. Berberine: As a therapeutic target for treating obese diabetes. SciForschen J. Diabetes Res. Ther. 2016, 2, 1–2. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abdel-Rahman, M.M.; Bastawy, N.A.; Eissa, H.M. Modulatory effect of berberine on adipose tissue PPARγ, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci. 2017, 7, 1–10. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.; Cang, Z.; Sun, H.; Nie, X.; Wang, N.; Lu, Y. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. BMC Endocr. Disord. 2017, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, H.; Zhang, X.; Lou, W.; Zhang, P.; Qiu, Y.; Zhang, C.; Wang, Y.; Jing, W. The Effect of Berberine on Metabolic Profiles in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Oxidative Med. Cell. Longev. 2021, 2021, 2074610. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, D.; Wei, G.; Ge, H. Berberine and Metformin in the Treatment of Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis of Randomized Clinical Trials. Health 2021, 13, 1314–1329. [Google Scholar] [CrossRef]
- Yoshinari, O.; Igarashi, K. Chapter 85—Antidiabetic Effects of Trigonelline: Comparison with Nicotinic Acid. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 765–775. [Google Scholar] [CrossRef]
- Mohammed, A.; Islam, M.S. Spice-Derived Bioactive Ingredients: Potential Agents or Food Adjuvant in the Management of Diabetes Mellitus. Front. Pharm. 2018, 9, 893. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Wu, J.J.; Liu, Z.Q.; Lin, N. Development of a hydrophilic interaction chromatography-UPLC assay to determine trigonelline in rat plasma and its application in a pharmacokinetic study. Chin. J. Nat. Med. 2013, 11, 164–170. [Google Scholar] [CrossRef]
- Ghule, A.E.; Jadhav, S.S.; Bodhankar, S.L. Trigonelline ameliorates diabetic hypertensive nephropathy by suppression of oxidative stress in kidney and reduction in renal cell apoptosis and fibrosis in streptozotocin induced neonatal diabetic (nSTZ) rats. Int. Immunopharmacol. 2012, 14, 740–748. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S. Protection of Trigonelline on Experimental Diabetic Peripheral Neuropathy. Evid. Based Complement. Altern. Med. 2012, 2012, 164219. [Google Scholar] [CrossRef]
- Hamden, K.; Bengara, A.; Amri, Z.; Elfeki, A. Experimental diabetes treated with trigonelline: Effect on key enzymes related to diabetes and hypertension, β-cell and liver function. Mol. Cell. Biochem. 2013, 381, 85–94. [Google Scholar] [CrossRef]
- Subramanian, S.P.; Prasath, G.S. Antidiabetic and antidyslipidemic nature of trigonelline, a major alkaloid of fenugreek seeds studied in high-fat-fed and low-dose streptozotocin-induced experimental diabetic rats. Biomed. Prev. Nutr. 2014, 4, 475–480. [Google Scholar] [CrossRef]
- Zhou, J.; Du, X.; Zhang, Z.; Qian, G. Trigonelline Inhibits Inflammation and Protects β Cells to Prevent Fetal Growth Restriction during Pregnancy in a Mouse Model of Diabetes. Pharmacology 2017, 100, 209–217. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, C.; Lou, Z.; Li, Q. Trigonelline reduced diabetic nephropathy and insulin resistance in type 2 diabetic rats through peroxisome proliferator-activated receptor-γ. Exp. Ther. Med. 2019, 18, 1331–1337. [Google Scholar] [CrossRef]
- Singh, S.; Mazumder, A.; Chakaraborthy, G.S. Potential herbs against diabetes mellitus—An update. Int. J. Pharm. Sci. Res. 2019, 10, 3619–3626. [Google Scholar] [CrossRef]
- Yang, S.; Mi, J.; Liu, Z.; Wang, B.; Xia, X.; Wang, R.; Liu, Y.; Li, Y. Pharmacokinetics, Tissue Distribution, and Elimination of Three Active Alkaloids in Rats after Oral Administration of the Effective Fraction of Alkaloids from Ramulus Mori, an Innovative Hypoglycemic Agent. Molecules 2017, 22, 1616. [Google Scholar] [CrossRef]
- Gómez, L.; Molinar-Toribio, E.; Calvo-Torras, M.A.; Adelantado, C.; Juan, M.E.; Planas, J.; Cañas, X.; Lozano, C.; Pumarola, S.; Clapés, P.; et al. D-Fagomine lowers postprandial blood glucose and modulates bacterial adhesion. Br. J. Nutr. 2012, 107, 1739–1746. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, B.; Han, L.; Gao, C.; Wang, M. d-Fagomine Attenuates High Glucose-Induced Endothelial Cell Oxidative Damage by Upregulating the Expression of PGC-1α. J. Agric. Food Chem. 2018, 66, 2758–2764. [Google Scholar] [CrossRef]
- Molinar-Toribio, E.; Pérez-Jiménez, J.; Ramos-Romero, S.; Gómez, L.; Taltavull, N.; Nogués, M.R.; Adeva, A.; Jáuregui, O.; Joglar, J.; Clapés, P.; et al. D-Fagomine attenuates metabolic alterations induced by a high-energy-dense diet in rats. Food Funct. 2015, 6, 2614–2619. [Google Scholar] [CrossRef]
- Parida, I.S.; Takasu, S.; Nakagawa, K.A. Comprehensive review on the production, pharmacokinetics and health benefits of mulberry leaf iminosugars: Main focus on 1-deoxynojirimycin, d-fagomine, and 2-O-ɑ-d-galactopyranosyl-DNJ. Crit. Rev. Food Sci. Nutr. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Chen, F.; Nakashima, N.; Kimura, I.; Kimura, M.; Asano, N.; Koya, S. Potentiating effects on pilocarpine-induced saliva secretion, by extracts and N-containing sugars derived from mulberry leaves, in streptozocin-diabetic mice. Biol. Pharm. Bull. 1995, 18, 1676–1680. [Google Scholar] [CrossRef]
- Tambe, V.S.; Waichal, D.D.; Chanshetty, R.R. Bioactivity enhanced isolated carpaine from Carica papaya leaves for platelet stimulating activity. Indian J. Pharm. Sci. 2021, 83, 723–731. [Google Scholar] [CrossRef]
- Hameda, A.N.E.; Abouelela, M.E.; Zowalaty, A.E.E.; Badrf, M.M.; Abdelkader, M.S.A. Chemical constituents from Carica papaya Linn. leaves as potential cytotoxic, EGFRwt and aromatase (CYP19A) inhibitors; a study supported by molecular docking. RSC Adv. 2022, 12, 9154–9162. [Google Scholar] [CrossRef]
- Bharti, S.K.; Krishnan, S.; Kumar, A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. [Google Scholar] [CrossRef]
- Fazal, J.; Naz, L.; Sohail, S.; Yasmeen, G.; Khan, N.I.; Zehra, N. Anti-diabetic activity of Carica Papaya Linn in Alloxan-Induced diabetic rats. Int. J. Endorsing Health Sci. Res. 2022, 1, 42–48. [Google Scholar] [CrossRef]
- Dey, P.; Singh, J.; Suluvoy, J.K.; Dilip, K.J.; Nayak, J. Utilization of Swertia chirayita Plant Extracts for Management of Diabetes and Associated Disorders: Present Status, Future Prospects and Limitations. Nat. Prod. Bioprospect. 2020, 10, 431–443. [Google Scholar] [CrossRef]
- Vaidya, H.; Goyal, R.K.; Cheema, S.K. Anti-diabetic activity of swertiamarin is due to an active metabolite, gentianine, that upregulates PPAR-γ gene expression in 3T3-L1 cells. Phytother. Res. 2013, 27, 624–627. [Google Scholar] [CrossRef]
- Tiong, S.H.; Looi, C.Y.; Arya, A.; Wong, W.F.; Hazni, H.; Mustafa, M.R.; Awang, K. Vindogentianine, a hypoglycemic alkaloid from Catharanthus roseus (L.) G. Don (Apocynaceae). Fitoterapia 2015, 102, 182–188. [Google Scholar] [CrossRef]
- Yan, X.; Huang, N. Effects of Three Different Types of Aloin on Optical, Mechanical, and Antibacterial Properties of Waterborne Coating on Tilia europaea Surface. Coating 2021, 11, 1537. [Google Scholar] [CrossRef]
- Park, M.; Kwon, H.; Sung, M. Intestinal absorption of aloin, aloe-emodin, and aloesin; A comparative study using two in vitro absorption models. Nutr Res Pr. 2009, 3, 9–14. [Google Scholar] [CrossRef]
- Zhong, R.; Chen., L.; Liu, Y.; Xie, S.; Li, S.; Liu, B.; Zhao, C. Anti-diabetic effect of aloin via JNK-IRS1/PI3K pathways and regulation of gut microbiota. Food Sci. Hum. Wellness 2022, 11, 189–198. [Google Scholar] [CrossRef]
- Araya, T.Y.; Karim, A.; Hailu, G.S.; Periasamy, G.; Kahsay, G. Antihyperglycemic Activity of TLC Isolates from the Leaves of Aloe megalacantha Baker in Streptozotocin-Induced Diabetic Mice. Diabetes Metab. Syndr. Obes. 2021, 14, 1153–1166. [Google Scholar] [CrossRef]
- Froldi, G.; Baronchelli, F.; Marin, E.; Grison, M. Antiglycation Activity and HT-29 Cellular Uptake of Aloe-Emodin, Aloin, and Aloe arborescens Leaf Extracts. Molecules 2019, 24, 2128. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Narasimhaiah, A.L.; Kundu, S.; Anand, S. Anti-hyperglycemic study of natural inhibitors for Insulin receptor. Bioinformation 2012, 8, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Beppu, H.; Shimpo, K.; Chihara, T.; Tamai, I.; Nomoto-Yamaji, S.; Ozaki, S.; Ito, S.; Kuzuya, H. Inhibitory effects of aloe carboxypeptidase fraction on streptozotocin-induced enhancement of vascular permeability in the pancreatic islets. Phytomedicine 2006, 13, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Pérez, Y.Y.; Jiménez-Ferrer, E.; Zamilpa, A.; Hernández-Valencia, M.; Alarcón-Aguilar, F.J.; Tortoriello, J.; Román-Ramos, R. Effect of a polyphenol-rich extract from Aloe vera gel on experimentally induced insulin resistance in mice. Am. J. Chin. Med. 2007, 35, 1037–1046. [Google Scholar] [CrossRef]
- Eid, S.A.; Adams, M.; Scherer, T.; Torres-Gómez, H.; Hackl, M.T.; Kaplanian, M.; Riedl, R.; Luger, A.; Fürnsinn, C. Emodin, a compound with putative antidiabetic potential, deteriorates glucose tolerance in rodents. Eur. J. Pharmacol. 2017, 798, 77–84. [Google Scholar] [CrossRef]
- Wang, S.; Chen, T.; Chen, R.; Hu, Y.; Chen, M.; Wang, Y. Emodin loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 238–246. [Google Scholar] [CrossRef]
- Cao, X. A systematic evaluation of biopharmaceutics classification system of main efficacy and toxic ingredients in Polygoni Multiflori Radix Praeparata. Chin. Tradit. Herb. Drugs 2020, 24, 3451–3456. [Google Scholar]
- Duan, H.G.; Wei, Y.H.; Li, B.X.; Qin, H.; Wu, X. Improving the dissolution and oral bioavailability of the poorly water-soluble drug aloe-emodin by solid dispersion with polyethylene glycol 6000. Drug Dev. Res. 2009, 70, 363–369. [Google Scholar] [CrossRef]
- Jangra, S.; Sharma, B.; Singh, S. Aloe-emodin-loaded SBA-15 and its in vitro release properties and cytotoxicity to cervical cancer cells. Mater. Res. Innov. 2021, 25, 264–275. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Ni, H.J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Liu, Y.; You, L.; Yin, X.; Fu, J.; Ni, J. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 2020, 34, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Martorell, M.; Castro, N.; Victoriano, M.; Capó, X.; Tejada, S.; Vitalini, S.; Pezzani, R.; Sureda, A. An Update of Anthraquinone Derivatives Emodin, Diacerein, and Catenarin in Diabetes. Evid. Based Complement. Altern. Med. 2021, 2021, 3313419. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Hu, Y.; Fu, Y.; Wang, R.; Zhang, H.; Li, M.; Li, Z.; Zhang, Y.; Xuan, L.; Li, X.; et al. Emodin improves glucose metabolism by targeting microRNA-20b in insulin-resistant skeletal muscle. Phytomedicine 2019, 59, 152758. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Li, S.; Li, X.; Liu, R. Advances in the study of emodin: An update on pharmacological properties and mechanistic basis. Chin. Med. 2021, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, H.; Li, X.; Yang, G.; Ru, J.; Liu, T. Emodin protects hyperglycemia-induced injury in PC-12 cells by up-regulation of miR-9. Mol. Cell. Endocrinol. 2018, 474, 194–200. [Google Scholar] [CrossRef]
- Chang, K.; Li, L.; Sanborn, T.M.; Shieh, B.; Lenhart, P.; Ammar, D.; LaBarbera, D.V.; Petrash, J.M. Characterization of Emodin as a Therapeutic Agent for Diabetic Cataract. J. Nat. Prod. 2016, 79, 1439–1444. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Shi, G.; Yang, W.; Zhang, Y.; Chen, W.; Wan, S.; Xiong, F.; Wang, Z. Emodin Ameliorates Renal Damage and Podocyte Injury in a Rat Model of Diabetic Nephropathy via Regulating AMPK/mTOR-Mediated Autophagy Signaling Pathway. Diabetes Metab Syndr Obes. 2021, 14, 1253–1266. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.; Lee, D.; Lee, T.; Bae, J. Emodin-6-O-β-D--glucoside inhibits high-glucose-induced vascular inflammation. Inflammation 2014, 37, 306–313. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Lv., P.; Yang, J.; Deng, Y.; Xu, J.; Zhu, R.; Zhang, D.; Yang, Y. Emodin up-regulates glucose metabolism, decreases lipolysis, and attenuates inflammation in vitro. J. Diabetes 2015, 7, 360–368. [Google Scholar] [CrossRef]
- Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Aliyu, A.B.; Isah, M.B. Antidiabetic potential of anthraquinones: A review. Phytother. Res. 2020, 34, 486–504. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Wang, W.; Su, Z.; Guo, C.; Cao, S. Emodin suppresses hyperglycemia-induced proliferation and fibronectin expression in mesangial cells via inhibiting cFLIP. PLoS ONE 2014, 9, e93588. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sheng, X.; Zhao, S.; Zou, L.; Han, X.; Gong, Y.; Yuan, H.; Shi, L.; Guo, L.; Jia, T.; et al. Nanoparticle-encapsulated emodin decreases diabetic neuropathic pain probably via a mechanism involving P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2017, 13, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, J.; Li, G.; Xu, H.; Yi, Y.; Wu, Q.; Song, M.; Bee, Y.M.; Huang, L.; Tan, M.; et al. Protection of vascular endothelial cells from high glucose-induced cytotoxicity by emodin. Biochem. Pharmacol. 2015, 94, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, S.; Dou, W.; Zhang, S.; Chen, J.; Shen, Y.; Shen, J.; Leng, Y. Emodin, a natural product, selectively inhibits 11beta-hydroxysteroid dehydrogenase type 1 and ameliorates metabolic disorder in diet-induced obese mice. Br. J. Pharmacol. 2010, 161, 113–126. [Google Scholar] [CrossRef]
- Cao, Y.; Chang, S.; Dong, J.; Zhu, S.; Zheng, X.; Li, J.; Long, R.; Zhou, Y.; Cui, J.; Zhang, Y. Emodin ameliorates high-fat-diet induced insulin resistance in rats by reducing lipid accumulation in skeletal muscle. Eur. J. Pharmacol. 2016, 780, 194–201. [Google Scholar] [CrossRef]
- Lu, L.; Li, Y. Aloe-Emodin Ameliorates Diabetic Nephropathy by Targeting Interferon Regulatory Factor 4. Evid. Based Complement. Altern. Med. 2022, 2022, 2421624. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, L.Y.; Xiao, W.; Gui, D.; Wang, X.; Wang, N. Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in-vitro and in-vivo. Cell. Physiol. Biochem. 2015, 35, 1425–1436. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Q.; Ke, D.; Li, G.; Deng, W. Emodin protects against diabetic cardiomyopathy by regulating the AKT/GSK-3β signaling pathway in the rat model. Molecules 2014, 19, 14782–14793. [Google Scholar] [CrossRef]
- Xue, J.; Ding, W.; Liu, Y. Anti-diabetic effects of emodin involved in the activation of PPAR gamma on high-fat diet-fed and low dose of streptozotocin-induced diabetic mice. Fitoterapia 2010, 81, 173–177. [Google Scholar] [CrossRef]
- Jing, D.; Bai, H.; Yin, S. Renoprotective effects of emodin against diabetic nephropathy in rat models are mediated via PI3K/Akt/GSK-3β and Bax/caspase-3 signaling pathways. Exp. Ther. Med. 2017, 14, 5163–5169. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Wang, Q.; Liu, P.; Deng, Y.; Lan, T.; Zhang, X.; Qiu, B.; Ning, H.; Huang, H. Emodin suppresses cell proliferation and fibronectin expression via p38MAPK pathway in rat mesangial cells cultured under high glucose. Mol. Cell. Endocrinol. 2009, 307, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, H.; Liu, P.; Tang, F.; Qin, J.; Huang, W.; Chen, F.; Guo, F.; Liu, W.; Yang, B. Inhibition of phosphorylation of p38 MAPK involved in the protection of nephropathy by emodin in diabetic rats. Eur. J. Pharmacol. 2006, 553, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qiao, G.; Li, B.; Chi, L.; Li, Z.; Lu, Y.; Yang, B. Hypoglycaemic and hypolipidemic effects of emodin and its effect on L-type calcium channels in dyslipidaemia-diabetic rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 29–34. [Google Scholar] [CrossRef]
- Arvindekar, A.; More, T.; Payghan, P.V.; Laddha, K.; Ghoshal, N. Evaluation of anti-diabetic and alpha glucosidase inhibitory action of anthraquinones from Rheum emodi. Food Funct. 2015, 6, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, Q.; Jiang, W.; Pei, X.; Sun, Y.; Hao, H.; Hao, K. Pharmacokinetics and pharmacodynamics of rhubarb anthraquinones extract in normal and disease rats. Biomed. Pharmacother. 2017, 91, 425–435. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.S.; Jo, K.; Kim, J.S. Extract of Rhizoma Polygonum cuspidatum reduces early renal podocyte injury in streptozotocin-induced diabetic rats and its active compound emodin inhibits methylglyoxal-mediated glycation of proteins. Mol. Med. Rep. 2015, 12, 5837–5845. [Google Scholar] [CrossRef]
- Hu, H.C.; Zheng, L.T.; Yin, H.Y.; Tao, Y.; Luo, X.Q.; Wei, K.S.; Yin, L.P. A Significant Association between Rhein and Diabetic Nephropathy in Animals: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Wang, J.; Qian, Y.; Qian, C.; Shi, F.; Yao, J.; Bi, X.; Chen, Z. A novel β-cyclodextrin-rhein conjugate for improving the water solubility and bioavailability of rhein. Carbohydr Res 2020, 490, 107958. [Google Scholar] [CrossRef]
- Li, G.; Chen, J.; Zhang, H.; Cao, X.; Sun, C.; Peng, F.; Yin, Y.; Lin, Z.; Yu, L.; Chen, Y.; et al. Update on Pharmacological Activities, Security, and Pharmacokinetics of Rhein. Evid. Based Complement. Alternat. Med. 2021, 2021, 4582412. [Google Scholar] [CrossRef]
- Pei, R.; Jiang, Y.; Lei, G.; Chen, J.; Liu, M.; Liu, S. Rhein Derivatives, A Promising Pivot? Mini Rev. Med. Chem. 2021, 21, 554–575. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Q.; Pi, R.; Chen, J. A research update on the therapeutic potential of rhein and its derivatives. Eur. J. Pharmacol. 2021, 899, 173908. [Google Scholar] [CrossRef]
- Waldmann, S.; Almukainzi, M.; Chacra, N.A.B.; Amidon, G.L.; Lee, B.J.; Feng, J.; Kanfer, I.; Zuo, J.Z.; Wei, H.; Bolger, M.B.; et al. Provisional Biopharmaceutical Classification of Some Common Herbs Used in Western Medicine. Mol. Pharm. 2012, 2, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Cavallito, C.J.; Bailey, J.H. Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Subramanian, M.S.; Ms, G.N.; Nordin, S.A.; Thilakavathy, K.; Joseph, N. Prevailing Knowledge on the Bioavailability and Biological Activities of Sulphur Compounds from Alliums: A Potential Drug Candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef]
- Lawson, L.D.; Hunsaker, S.M. Allicin Bioavailability and Bioequivalence from Garlic Supplements and Garlic Foods. Nutrients 2018, 10, 812. [Google Scholar] [CrossRef]
- Arellano-Buendía, A.S.; Castañeda-Lara, L.G.; Loredo-Mendoza, M.L.; Fernando, E.; García-Arroyo, F.E.; Rojas-Morales, P.; Raúl Argüello-García, R.; Juárez-Rojas, J.G.; Tapia, E.; José Pedraza-Chaverri, J.; et al. Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes. Antioxidants 2020, 9, 1134. [Google Scholar] [CrossRef]
- Zhai, B.; Zhang, C.; Sheng, Y.; Zhao, C.; He, X.; Xu, W.; Huang, K.; Luo, Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci. Rep. 2018, 8, 3527. [Google Scholar] [CrossRef]
- Nasim, S.A.; Dhir, B.; Kapoor, R.; Fatima, S.; Mahmooduzzafar; Mujib, A. Alliin obtained from leaf extract of garlic grown under in situ conditions possess higher therapeutic potency as analyzed in alloxan-induced diabetic rats. Pharm. Biol. 2011, 49, 416–421. [Google Scholar] [CrossRef] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed). Scientific Opinion on the safety and efficacy of betaine (betaine anhydrous and betaine hydrochloride) as a feed additive for all animal species based on a dossier submitted by VITAC EEIG. EFSA J. 2013, 11, 3210. [Google Scholar] [CrossRef]
- Schwahn, B.C.; Hafner, D.; Hohlfeld, T.; Balkenhol, N.; Laryea, M.D.; Wendel, U. Pharmacokinetics of oral betaine in healthy subjects and patients with homocystinuria. Br. J. Clin. Pharmacol. 2003, 55, 6–13. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. The anti-diabetic potential of betaine. Mechanisms of action in rodent models of type 2 diabetes. Biomed. Pharmacother. 2022, 150, 112946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Wehsling, M.; Mignon, M.L.; Wille, G.; Rey, Y.; Schnellbaecher, A.; Zabezhinsky, D.; Fischer, M.; Zimmer, A. Lactoyl leucine and isoleucine are bioavailable alternatives for canonical amino acids in cell culture media. Biotechnol. Bioeng. 2021, 118, 3395–3408. [Google Scholar] [CrossRef]
- Elovaris, R.A.; Bitarafan, V.; Agah, S.; Ullrich, S.S.; Lange, K.; Horowitz, M.; Feinle-Bisset, C.F. Comparative Effects of the Branched-Chain Amino Acids, Leucine, Isoleucine and Valine, on Gastric Emptying, Plasma Glucose, C-Peptide and Glucagon in Healthy Men. Nutrients 2021, 13, 1613. [Google Scholar] [CrossRef] [PubMed]
- Okun, J.G.; Rusu, P.M.; Chan, A.Y.; Wu, Y.; Yap, Y.W.; Sharkie, T.; Schumacher, J.; Schmidt, K.V.; Thomson, K.M.R.; Russell, R.D.; et al. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat. Metab. 2021, 3, 394–409. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Qu, W.; Wang, H.; Liu, Y.; Borjigdai, A.; Cui, J.; Dong, Z. Biopharmaceutics classification and absorption mechanisms primary study on four kinds of flavonoids. Zhongguo Zhong Yao Za Zhi 2016, 41, 1198–1203. [Google Scholar] [CrossRef]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.; Kong, A. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D’Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G. A Critical Appraisal of Solubility Enhancement Techniques of Polyphenols. J. Pharm. 2014, 2014, 180845. [Google Scholar] [CrossRef]
- Kaşıkcı, M.B.; Bağdatlıoğlu, N. Bioavailability of Quercetin. Curr. Res. Nutr. Food Sci. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- ALTamimi, J.Z.; BinMowyna, M.N.; AlFaris, N.A.; Alagal, R.I.; El-Kott, A.F.; Al-Farga, A.M. Fisetin protects against streptozotocin-induced diabetic cardiomyopathy in rats by suppressing fatty acid oxidation and inhibiting protein kinase R. Saudi Pharm. J. 2021, 29, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.; Gasmi, H.; Milon, N.; Bounoure, F.; Malika, L.S. Water Solubility and Dissolution Enhancement of Fisetin by Spherical Amorphous Solid Dispersion in Polymer of Cyclodextrin. J. Adv. Biotechnol. Bioeng. 2021, 8, 1106. [Google Scholar] [CrossRef]
- Li, R.Z.; Ding, X.W.; Geetha, T.; Al-Nakkash, L.; Broderick, T.L.; Babu, J.R. Beneficial Effect of Genistein on Diabetes-Induced Brain Damage in the ob/ob Mouse Model. Drug Des. Dev. Ther. 2020, 14, 3325–3336. [Google Scholar] [CrossRef]
- Chen, F.; Peng, J.; Lei, D.; Liu, J.; Zhao, G. Optimization of genistein solubilization by κ-carrageenan hydrogel using response surface methodology. Food Sci. Hum. Wellness 2013, 2, 124–131. [Google Scholar] [CrossRef]
- Laddha, A.; Kulkarni, Y.A. Daidzein ameliorates diabetic retinopathy in experimental animals. Life Sci. 2020, 265, 118779. [Google Scholar] [CrossRef] [PubMed]
- Panizzon, G.P.; Bueno, F.G.; Nakamura, T.U.; Nakamura, C.V.; Filho, B.P.D. Manufacturing Different Types of Solid Dispersions of BCS Class IV Polyphenol (Daidzein) by Spray Drying: Formulation and Bioavailability. Pharmaceutics 2019, 11, 492. [Google Scholar] [CrossRef]
- Miura, A.; Sugiyama, C.; Sakakibara, H.; Simoi, K.; Goda, T. Bioavailability of isoflavones from soy products in equol producers and non-producers in Japanese women. J. Nutr. Intermed. Metab. 2016, 6, 41–47. [Google Scholar] [CrossRef]
- Khalid, A.; Naseem, I. 2022. Antidiabetic and antiglycating potential of chrysin is enhanced after nano formulation: An in vitro approach. J. Mol. Struct. 2022, 1261, 13906. [Google Scholar] [CrossRef]
- Baidya, D.; Kushwaha, J.; Mahadik, K.; Patil, S. Chrysin-loaded folate conjugated PF127-F68 mixed micelles with enhanced oral bioavailability and anticancer activity against human breast cancer cells. Drug Dev. Ind. Pharm. 2018, 45, 852–860. [Google Scholar] [CrossRef]
- Mohammadian, F.; Abhari, A.; Dariushnejad, H.; Nikanfar, A.; Soltanahmadi, Y.P.; Zarghami, N. Effects of Chrysin-PLGA-PEG Nanoparticles on Proliferation and Gene Expression of miRNAs in Gastric Cancer Cell Line. Iran. J. Cancer Prev. 2016, 9, e4190. [Google Scholar] [CrossRef] [PubMed]
- Crescenti, A.; Caimari, A.; Alcaide-Hidalgo, J.M.; Mariné-Casadó, R.; Valls, R.M.; Companys, J.; Salamanca, P.; Calderón-Pérez, L.; Pla-Pagà, L.; Pedret, A.; et al. Hesperidin Bioavailability Is Increased by the Presence of 2S-Diastereoisomer and Micronization-A Randomized, Crossover and Double-Blind Clinical Trial. Nutrients 2022, 14, 2481. [Google Scholar] [CrossRef]
- Rekha, S.S.; Pradeepkiran, J.A.; Bhaskar, M. Bioflavonoid hesperidin possesses the anti-hyperglycemic and hypolipidemic property in STZ induced diabetic myocardial infarction (DMI) in male Wister rats. J. Nutr. Intermed. Metab. 2019, 15, 58–64. [Google Scholar] [CrossRef]
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. J. Cell. Biochem. 2019, 120, 5644–5651. [Google Scholar] [CrossRef]
- Rajan, V.K.; Muraleedharan, K.; Hussan, K.P.S. Structural Evaluation and Toxicological Study of a Bitter Masking Bioactive Flavanone, ‘Eriodictyol’. In Polyphenols: Prevention and Treatment of Human Disease; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, P.; Arya, S.K. Naringin: A potential natural product in the field of biomedical applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100068. [Google Scholar] [CrossRef]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Devel. Ther. 2016, 10, 911–925. [Google Scholar] [CrossRef]
- Khan, M.F.; Mathur, A.; Pandey, V.K.; Kakkar, P. Naringenin alleviates hyperglycemia-induced renal toxicity by regulating activating transcription factor 4-C/EBP homologous protein mediated apoptosis. J. Cell Commun. Signal. 2022, 16, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41. [Google Scholar] [CrossRef]
- Maggioli, A. Chronic venous disorders: Pharmacological and clinical aspects of micronized purified flavonoid fraction. Phlebolymphology 2016, 23, 57–120. [Google Scholar]
- Russo, R.; Chandradhara, D.; Tommasi, N.D. Comparative Bioavailability of Two Diosmin Formulations after Oral Administration to Healthy Volunteers. Molecules 2018, 23, 2174. [Google Scholar] [CrossRef]
- Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Gunasekaran, P.; Sivasubramanian, S.; Ramkumar, K.M. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and β-cell function in streptozotocin-induced diabetic rats. Environ. Toxicol. Pharmacol. 2014, 37, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Ju, Y.; Fu, Y.; Gong, T.; Zhang, Z. Mechanism of enhanced oral absorption of morin by phospholipid complex based self-nanoemulsifying drug delivery system. Mol. Pharm. 2015, 12, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.A.; Wang, X.; Yan, H. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Ning, E.; Peng, Y.; Zhang, J. Mechanisms of poor oral bioavailability of flavonoid Morin in rats: From physicochemical to biopharmaceutical evaluations. Eur. J. Pharm. Sci. 2019, 128, 290–298. [Google Scholar] [CrossRef]
- Abuohashish, H.M.; AlAsmari, A.F.; Mohany, M.; Ahmed, M.M.; Al-Rejaie, S.S. Supplementation of Morin Restores the Altered Bone Histomorphometry in Hyperglycemic Rodents via Regulation of Insulin/IGF-1 Signaling. Nutrients 2021, 13, 2365. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, W.; Yu, N.; Sun, J.; Yu, X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.; Xiu, Z.; et al. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]
- Jakab, G.; Bogdan, D.; Mazak, K.; Deme, R.; Mucsi, Z.; Mandity, I.M.; Noszal, B.; Szabo, N.K.; Antal, I. Physicochemical Profiling of Baicalin Along with the Development and Characterization of Cyclodextrin Inclusion Complexes. AAPS PharmSciTech 2019, 20, 314. [Google Scholar] [CrossRef]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Tangeretin, a polymethoxylated flavone, modulates lipid homeostasis and decreases oxidative stress by inhibiting NF-κB activation and proinflammatory cytokines in cardiac tissue of streptozotocin-induced diabetic rats. J. Funct. Foods 2015, 16, 315–333. [Google Scholar] [CrossRef]
- Elhennawy, M.G.; Lin, H. Determination of Tangeretin in Rat Plasma: Assessment of Its Clearance and Absolute Oral Bioavailability. Pharmaceutics 2018, 10, 3. [Google Scholar] [CrossRef]
- Hung, W.L.; Chang, W.S.; Lu, W.C.; Wei, G.J.; Wang, Y.; Ho, C.T.; Hwang, L.S. Pharmacokinetics, bioavailability, tissue distribution and excretion of tangeretin in rat. J. Food Drug Anal. 2018, 26, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Kalai, F.Z.; Boulaaba, M.; Ferdousi, F.; Isoda, H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of in vitro and in vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int. J. Mol. Sci. 2022, 23, 704. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Luo, H.; Duan, J.; Hong, C.; Ma, P.; Li, G.; Zhang, T.; Wu, T.; Ji, G. Phytic acid enhances the oral absorption of isorhamnetin, quercetin, and kaempferol in total flavones of Hippophae rhamnoides L. Fitoterapia 2014, 93, 216–225. [Google Scholar] [CrossRef]
- Zhao, G.; Duan, J.; Xie, Y.; Lin, G.; Luo, H.; Li, G.; Yuan, X. Effects of solid dispersion and self-emulsifying formulations on the solubility, dissolution, permeability and pharmacokinetics of isorhamnetin, quercetin and kaempferol in total flavones of Hippophae rhamnoides L. Drug Dev. Ind. Pharm. 2013, 39, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Zhang, D.; Zhang, Y.; Li, M.; Wang, C. Wogonin attenuates diabetic cardiomyopathy through its anti-inflammatory and anti-oxidative properties. Mol. Cell. Endocrinol. 2016, 428, 101–108. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yan, Y.; Sun, S. Study of the inclusion complex and antioxidating activity of Wogonin with b-cyclodextrin and hydroxypropyl-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 115–120. [Google Scholar] [CrossRef]
- Zhu, N.; Li, J.C.; Zhu, J.X.; Wang, X.; Zhang, J. Characterization and Bioavailability of Wogonin by Different Administration Routes in Beagles. Med. Sci. Monit. 2016, 22, 3737–3745. [Google Scholar] [CrossRef]
- Hasanein, P.; Emamjomeh, A.; Chenarani, N.; Bohlooli, M. Beneficial effects of rutin in diabetes-induced deficits in acquisition learning, retention memory and pain perception in rats. Nutr. Neurosci. 2018, 23, 563–574. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Bhalkar, K.G. Development and Evaluation of Rutin- HPβCD Inclusion Complex Based Mouth Dissolving Tablets. Int. J. Pharm. Sci. Dev. Res. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Das, S.; Roy, P.; Pal, R.; Auddy, R.G.; Chakraborti, A.S.; Mukherjee, A. Engineered Silybin Nanoparticles Educe Efficient Control in Experimental Diabetes. PLoS ONE 2014, 9, e101818. [Google Scholar] [CrossRef]
- Sahibzada, M.U.K.; Sadiq, A.; Khan, H.S.; Naseemullah, F.; Khurram, M.; Amin, M.U.; Haseeb, A. Fabrication, characterization and in vitro evaluation of silibinin nanoparticles: An attempt to enhance its oral bioavailability. Drug Des. Dev. Ther. 2017, 15, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2019, 2019, 5147468. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, A.M.; Malik, U.R.; Shahzad, Y.; Mahmood, T.; Hussain, T. Silymarin-laden PVP-PEG polymeric composite for enhanced aqueous solubility and dissolution rate: Preparation and in vitro characterization. J. Pharm. Anal. 2019, 9, 34–39. [Google Scholar] [CrossRef]
- Jose, M.A.; Abraham, A.; Narmadha, M.P. Effect of silymarin in diabetes mellitus patients with liver diseases. J. Pharmacol. Pharmacother. 2011, 2, 287–289. [Google Scholar] [CrossRef]
- Gharib, A.; Faezizadeh, Z.; Godarzee, M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Med. 2013, 79, 1599–1604. [Google Scholar] [CrossRef]
- Matsumoto, H.; Chiyanagi, T.I.; Iida, H.; Ito, K.; Tsuda, T.; Hirayama, M.; Konishi, T. Ingested delphinidin-3-rutinoside is primarily excreted to urine as the intact form and to bile as the methylated form in rats. J. Agric. Food Chem. 2000, 54, 578–582. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Hu, L.; Shi, Y.; Li, J.H.; Gao, N.; Ji, J.; Niu, F.; Chen, Q.; Yang, X.; Wang, S. Enhancement of Oral Bioavailability of Curcumin by a Novel Solid Dispersion System. AAPS PharmSciTech 2015, 16, 6. [Google Scholar] [CrossRef]
- Zhang, D.W.; Fu, M.; Gao, S.H.; Liu, J.L. Curcumin and Diabetes: A Systematic Review. Evid. Based Complement. Altern. Med. 2013, 2013, 636053. [Google Scholar] [CrossRef]
- Bacanli, M. Limonene and ursolic acid in the treatment of diabetes: Citrus phenolic limonene, triterpenoid ursolic acid, antioxidants and diabetes. In Diabetes (Second Edition) Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Eloy, J.O.; Saraiva, J.; Albuquerque, S.D.; Marchetti, J.M. Preparation, characterization and evaluation of the in vivo trypanocidal activity of ursolic acid-loaded solid dispersion with poloxamer 407 and sodium caprate. Braz. J. Pharm. Sci. 2015, 51, 101–109. [Google Scholar] [CrossRef]
- Yu, D.; Kan, Z.; Shan, F.; Zang, J.; Zhou, J. Triple Strategies to Improve Oral Bioavailability by Fabricating Co-amorphous Forms of Ursolic Acid with Piperine: Enhancing Water-Solubility, Permeability and Inhibiting Cytochrome P450 Isozymes. Mol. Pharm. 2020, 17, 12. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Andrie, M.; Warditiani, N.K.; Siswanto, E.; Pramono, S.; Lukitaningsih, E. Antidiabetic and antihyperlipidemic effect of Andrographis paniculata (Burm. f.) Nees and Andrographolide in high-fructose-fat-fed rats. Indian J. Pharmacol. 2012, 44, 377–381. [Google Scholar] [CrossRef]
- Agrawal, A.; Goldfarb, A.; Teodoridis, F. Solubility enhancement of Andrographolide and formulation development of Hollow microspheres. World J. Pharm. Res. 2016, 5, 628–638. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, L.H.; Du, G.H. Andrographolide. In Natural Small Molecule Drugs from Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 357–362. [Google Scholar] [CrossRef]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L. Herbal Extracts and Phytochemicals: Plant Secondary Metabolites and the Enhancement of Human Brain Function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef]
- Kumar, S.; Shachi, K.; Prasad, N.K. Diabetes Mellitus and Allopathic Medication Increase the Risk of Cancer Malignancy, but no Side Effect Associated with the Use of Antidiabetic Herbal Medicine. Curr. Res. Diabetes Obes. J. 2020, 13, 555868. [Google Scholar] [CrossRef]
- Palhares, R.M.; Drummond, M.G.; Brasil, B.D.S.A.F.; Cosenza, G.P.; Brandao, M.D.G.L.; Oliveira, G. Medicinal Plants Recommended by the World Health Organization: DNA Barcode Identification Associated with Chemical Analyses Guarantees Their Quality. Safety for the Consumers of Medicinal. Plants 2015, 10, e0127866. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Varshney, M.; Kumar, B.; Rana, V.S.; Sethiya, N.K. An overview on therapeutic and medicinal potential of poly-hydroxy flavone viz. Heptamethoxyflavone, Kaempferitrin, Vitexin and Amentoflavone for management of Alzheimer’s and Parkinson’s diseases: A critical analysis on mechanistic insight. Crit. Rev. Food Sci. Nutr. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as alpha glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Sarian, M.N.; Ahmed, Q.U.; So’ad, S.Z.M.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Mohamad, S.N.A.S.; Khatib, A.; Latip, J. Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. BioMed Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Jain, N.; Mohan, S.C.; Sumathi, S. Mechanisms of Action of Flavonoids in the Management of Diabetes mellitus. J. Drug Deliv. Ther. 2021, 11, 194–202. [Google Scholar] [CrossRef]
- Serra, R.; Ielapi, N.; Bitonti, A.; Candido, S.; Fregola, S.; Gallo, A.; Loria, A.; Muraca, L.; Raimondo, L.; Velcean, L.; et al. Efficacy of a Low-Dose Diosmin Therapy on Improving Symptoms and Quality of Life in Patients with Chronic Venous Disease: Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 999. [Google Scholar] [CrossRef]
- Sendrayaperumal, V.; Pillai, S.I.; Subramanian, S. Design, synthesis and characterization of zinc-morin, a metal flavonol complex and evaluation of its anti-diabetic potential in HFD-STZ induced type 2 diabetes in rats. Chem.-Biol. Interact. 2014, 219, 9–17. [Google Scholar] [CrossRef]
- Abuohashish, H.M.; Al-Rejaie1, S.S.; Al-Hosaini, K.A.; Parmar, M.Y.; Ahmed, M.M. Alleviating effects of morin against experimentally-induced diabetic osteopenia. Diabetol. Metab. Syndr. 2013, 5, 5. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.M.; Gu, T.T.; Ding, X.Q.; Fan, C.Y.; Zhu, Q.; Shi, Y.W.; Hong, Y.; Kong, L.D. Morin reduces hepatic inflammation-associated lipid accumulation in high fructose-fed rats via inhibiting sphingosine kinase 1/sphingosine 1-phosphate signaling pathway. Biochem. Pharmacol. 2013, 86, 791–1804. [Google Scholar] [CrossRef]
- Prasath, G.S.; Pillai, S.I.; Subramanian, S.P. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2014, 740, 248–254. [Google Scholar] [CrossRef]
- Dong, W.; Jia, C.; Li, J.; Zhou, Y.; Luo, Y.; Liu, J.; Zhao, Z.; Zhang, J.; Lin, S.; Chen, Y. Fisetin Attenuates Diabetic Nephropathy-Induced Podocyte Injury by Inhibiting NLRP3 Inflammasome. Front. Pharmacol. 2022, 13, 783706. [Google Scholar] [CrossRef] [PubMed]
- Visnagri, A.; Kandhare, A.D.; Chakravarty, S.; Ghosh, P.; Bodhankar, S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 2014, 52, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.O.; Sharma, P.K.; Shrivastava, B.; Ojha, S.; Upadhya, H.M.; Arya, D.S.; Goyal, S.N. Hesperidin produces cardioprotective activity via PPARγ pathway in ischemic heart disease model in diabetic rats. PLoS ONE 2014, 9, e111212. [Google Scholar] [CrossRef]
- Tian, M.; Han, Y.B.; Zhao, C.C.; Liu, L.; Zhang, F.L. Hesperidin alleviates insulin resistance by improving HG-induced oxidative stress and mitochondrial dysfunction by restoring miR-149. Diabetol. Metab. Syndr. 2021, 13, 50. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, K.; Du, P.; Wang, S.S.; Ren, B.; Ren, Y.L.; Yan, H.S.; Liang, Y.; Wu, F.H. Phenolic compounds from Clinopodium chinense (Benth.) O. Kuntze and their inhibitory effects on alpha-Glucosidase and vascular Endothelial cells injury. Chem. Biodivers. 2016, 13, 596–601. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-kappaB in Male C57BL/6J Mice and BV2 Microglial Cells. J. Agric. Food Chem. 2018, 66, 10205–10214. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Choi, M.S. Dietary eriodictyol alleviates adiposity, hepatic steatosis, insulin resistance, and inflammation in diet-induced obese mice. Int. J. Mol. Sci. 2019, 20, 1227. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Du, Q.; Li, X.; Zheng, X.; Lv, F.; Xi, X.; Huang, G.; Yang, J.; Liu, S. Eriodictyol Inhibits Proliferation, Metastasis and Induces Apoptosis of Glioma Cells via PI3K/Akt/NF-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Hafizur, R.M.; Hussain, N.; Raza, S.A.; Rehman, M.; Ashraf, S.; Ul-Haq, Z.; Khan, F.; Abbas, G.; Choudhary, M.I. Eriodictyol stimulates insulin secretion through cAMP/PKA signaling pathway in mice islets. Eur. J. Pharmacol. 2018, 820, 245–255. [Google Scholar] [CrossRef]
- Wen, S.; Hu, M.; Xiong, Y. Effect of Eriodictyol on Retinoblastoma via the PI3K/Akt Pathway. J. Healthc. Eng. 2021, 2021, 6091585. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Jayakumar, M.; Thirumurugan, K. Flavanone naringenin: An effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J. Funct. Foods 2015, 14, 363–373. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF- β1-MAPK-fibronectin pathways. Am. J. Physiol. Renal Physiol. 2017, 313, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Gondaliya, P.; Tiwari, V.; Kalia, K. Kaempferol attenuates diabetic nephropathy by inhibiting RhoA/Rho-kinase mediated inflammatory signaling. Biomed. Pharmacother. 2019, 109, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Aba, P.E.; Asuzu, I.U. Mechanisms of actions of some bioactive anti-diabetic principles from phytochemicals of medicinal plants: A review. Indian J. Nat. Prod. Resour. 2018, 9, 85–96. [Google Scholar]
- Sood, A.; Kumar, B.; Singh, S.K.; Parashar, P.; Gautam, A.; Gulati, M.; Pandey, N.K.; Melkani, I.; Awasthi, A.; Saraf, S.A.; et al. Flavonoids as Potential Therapeutic Agents for the Management of Diabetic Neuropathy. Curr. Pharm. Des. 2020, 26, 5468–5487. [Google Scholar] [CrossRef]
- Espinosa, J.J.R.; Rios, J.S.; Jimenez, S.G.; Molina, R.V.; Villarreal, G.A.; Ocampo, A.N.R.; Fernández, G.B.; Soto, S.E. Chrysin induces antidiabetic, Antidyslipidemic and anti-inflammatory effects in athymic nude diabetic mice. Molecules 2018, 23, 67. [Google Scholar] [CrossRef]
- Ahad, A.; Mujeeb, M.; Ahsan, H.; Siddiqui, W.A. Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie. 2014, 106, 101–110. [Google Scholar] [CrossRef]
- Ma, L.; Wu, F.; Shao, Q.; Chen, G.; Xu, L.; Lu, F. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des. Dev. Ther. 2021, 15, 3207–3221. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, X.; Shuai, X.; Xu, Y.; Liu, Y.; Liang, X.; Wei, D.; Su, D. Luteolin prevents uric acid-induced pancreatic β-cell dysfunction. J. Biomed. Res. 2014, 28, 292–298. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Hur, H.J.; Kwon, D.Y.; Hwang, J. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol. Cell Endocrinol. 2012, 358, 127–134. [Google Scholar] [CrossRef]
- Qin, D.; Jiang, Y.R. Tangeretin Inhibition of High-Glucose-Induced IL-1β, IL-6, TGF-β1, and VEGF Expression in Human RPE Cells. J. Diabetes Res. 2020, 2020, 9490642. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.R.; Torres, N.; Uribe, J.A.G.; Noriega, L.G.; Villalvazo, I.T.; Diaz, A.M.L.; Ricardo, M.A.; Mota, C.M.; Ordaz, G.; Santoscoy, R.A.C.; et al. The effect of isorhamnetin glycosides extracted from Opuntia ficus indica in a mouse model of diet induced obesity. Food Funct. 2015, 6, 805–815. [Google Scholar] [CrossRef]
- Matboli, M.; Saad, M.; Hasanin, A.H.; Saleh, L.A.; Baher, W.; Bekhet, M.M.; Eissa, S. New insight into the role of isorhamnetin as a regulator of insulin signaling pathway in type 2 diabetes mellitus rat model: Molecular and computational approach. Biomed. Pharmacother. 2021, 135, 111176. [Google Scholar] [CrossRef] [PubMed]
- Eun-Jung Bak, E.J.; Kim, J.; Choi, Y.H.; Kim, J.H.; Lee, D.E.; Woo, G.H.; Cha, J.H.; Yoo, Y.J. Wogonin ameliorates hyperglycemia and dyslipidemia via PPARα activation in db/db mice. Clin. Nutr. 2014, 33, 156–163. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jiang, L.; Li, Y.Y.; Huang, Y.B.; Hu, X.R.; Zhu, W.; Wang, X.; Wu, Y.G.; Meng, X.M.; Qi, X.M. Wogonin protects glomerular podocytes by targeting Bcl-2- mediated autophagy and apoptosis in diabetic kidney disease. Acta Pharmacol. Sin. 2022, 43, 96–110. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin Ahmad Ghorbani. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Guimaraes, J.F.C.; Muzio, B.P.; Rosa, C.M.; Nascimento, A.F.; Sugizaki, M.M.; Fernandes, A.A.H.; Cicogna, A.C.; Padovani, C.R.; Okoshi, M.P.; Okoshi, K. Rutin administration attenuates myocardial dysfunction in diabetic rats. Cardiovasc. Diabetol. 2015, 14, 90. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.S. Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, F.; Wang, R.; Ma, W.; Song, Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem. Biol. Interact. 2019, 1, 108665. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in type II diabetic model mice. J. Nutr. Biochem. 2014, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ge, Q.; Chen, L.; Chen, K. Studies of the Anti-Diabetic Mechanism of Pueraria lobata Based on Metabolomics and Network Pharmacology. Processes 2021, 9, 1245. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Busselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Song, G. Inhibitory effects of delphinidin on the proliferation of ovarian cancer cells via PI3K/AKT and ERK 1/2 MAPK signal transduction. Oncol. Lett. 2017, 14, 810–818. [Google Scholar] [CrossRef]
- Hidalgo, J.; Teuber, S.; Morera, F.J.; Ojeda, C.; Flores, C.A.; Hidalgo, M.A.; Nunez, L.; Villalobos, C.; Burgos, R.A. Delphinidin Reduces Glucose Uptake in Mice Jejunal Tissue and Human Intestinal Cells Lines through FFA1/GPR40. Int. J. Mol. Sci. 2017, 18, 750. [Google Scholar] [CrossRef]
- Nasri, S.; Roghani, M.; Baluchnejadmojarad, T.; Rabani, T.; Balvardi, M.M. Vascular mechanisms of cyanidin-3-glucoside response in streptozotocin-diabetic rats. Pathophysiology 2011, 18, 273–278. [Google Scholar] [CrossRef]
- Su, H.; Bao, T.; Xie, L.; Xu, Y.; Chen, W. Transcriptome profiling reveals the antihyperglycemic mechanism of pelargonidin-3-O-glucoside extracted from wild raspberry. J. Funct. Foods 2020, 64, 103657. [Google Scholar] [CrossRef]
- Zheng, T.; Shu, G.; Yang, Z.; Mo, S.; Zhao, Y.; Mei, Z. Antidiabetic effect of total saponins from Entada phaseoloides (L.) Merr.in type 2 diabetic rats. J. Ethnopharmacol. 2012, 139, 814–821. [Google Scholar] [CrossRef]
- Smith, Y.R.A.; Adanlawo, I.G.; Oni, O.S. Hypoglycaemic effect of saponin from the root of Garcinia kola on alloxan- induced diabetic rats. J. Drug Deliv. Ther. 2012, 2, 9–12. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.I.; Hsin, I.T.; Wen, L.Y.; Hung, Y.C.; Min, C.T.; Chen, L.C.; Ling, C.H. In vivo and in vitro studies to identify the hypoglycemic constituents of Momordica charantia wild variant WB24. Food Chem. 2011, 125, 521–528. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Kamdem, J.P.; Kade, I.J.; Rocha, J.B.T.; Adanlawo, I.G. Hypoglycemic anti-per oxidative and antihyperlipidemic effects of saponins from Solanum anguivi Lam. fruits in alloxan-induced diabetic rats. South Afr. J. Bot. 2013, 88, 56–61. [Google Scholar] [CrossRef]
- Nakitto, A.M.S.; Muyonga, J.H.; Byaruhanga, Y.B.; Wagner, A.E. Solanum anguivi Lam. Fruits: Their Potential Effects on Type 2 Diabetes Mellitus. Molecules 2021, 26, 2044. [Google Scholar] [CrossRef]
- Lee, C.E.; Hur, H.J.; Hwang, J.T.; Sung, M.J.; Yang, H.J.; Kim, H.J.; Park, J.H.; Kwon, D.Y.; Kim, M.S. Long-Term Consumption of Platycodi Radix Ameliorates Obesity and Insulin Resistance via the Activation of AMPK Pathways. Evid. Based Complement. Altern. Med. 2012, 2012, 759143. [Google Scholar] [CrossRef]
- Deng, Y.; He, K.; Ye, X.; Chen, X.; Huang, J.; Li, X.; Yuan, L.; Jin, Y.; Jin, Q.; Li, P. Saponin rich fractions from Polygonatu modoratum (Mill.) druce with more potential hypoglycemic effects. J. Ethnopharmacol. 2012, 141, 228–233. [Google Scholar] [CrossRef]
- Cheng, S.; Liang, S.; Liu, Q.; Deng, Z.; Zhang, Y.; Du, J.; Zhang, Y.N.; Li, S.; Cheng, B.; Ling, C. Diosgenin prevents high-fat diet-induced rat non-alcoholic fatty liver disease through the AMPK and LXR signaling pathways. Int. J. Mol. Med. 2018, 41, 1089–1095. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ji, Y.L.; Zhu, L.P.; Wang, Z.H.; Fang, C.Q.; Jiang, C.H.; Pan, K.; Zhang, J.; Yin, Z.Q. Arjunolic acid from Cyclocarya paliurus ameliorates diabetic retinopathy through AMPK/mTOR/HO-1 regulated autophagy pathway. J. Ethnopharmacol. 2022, 284, 114772. [Google Scholar] [CrossRef]
- Aamir, K.; Khan, H.U.; Hossain, C.F.; Afrin, M.R.; Jusuf, P.R.; Waheed, I.; Sethi, G.; Arya, A. Arjunolic acid downregulates elevated blood sugar and pro-inflammatory cytokines in streptozotocin (STZ)-nicotinamide induced type 2 diabetic rats. Life Sci. 2022, 289, 120232. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, S.; Ryu, S.K.; Choi, Y.H.; Cha, M.R.; Yang, H.J.; Park, S. Platyconic acid, a saponin from Platycodi radix, improves glucose homeostasis by enhancing insulin sensitivity in vitro and in vivo. Eur. J. Nutr. 2012, 51, 529–540. [Google Scholar] [CrossRef]
- Roy, A. A Review on the Alkaloids an Important Therapeutic Compound from Plants. Int. J. Plant Biotechnol. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Abou El-Soud Khalil, M.Y.; Hussein, F.S.H.; Hussein Farray, A.R. Antidiabetic effects of Fenugreek alkaloid extract in streptozotocin induced hyperglycemic rats. J. App. Sci. Res. 2007, 3, 1073–1083. [Google Scholar]
- Patel, O.P.S.; Mishra, A.; Maurya, R.; Saini, D.; Pandey, J.; Taneja, I.; Raju, K.S.R.; Kanojiya, S.; Shukla, S.K.; Srivastava, M.N.; et al. Naturally occurring Carbazole alkaloids from Murraya koenigii as potential anti-diabetic agents. J. Nat. Prod. 2015, 79, 1276–1284. [Google Scholar] [CrossRef]
- Agrawal, R.; Sethiya, N.K.; Mishra, S.H. Antidiabetic activity of alkaloids of Aerva lanata roots on streptozotocin-nicotinamide induced type II diabetes in rats. Pharm. Biol. 2013, 51, 635–642. [Google Scholar] [CrossRef]
- Tiong, S.H.; Looi, C.Y.; Hazni, H.; Arya, A.; Paydar, M.; Wong, W.F.; Cheah, S.C.; Mustafa, M.R.; Awang, K. Antidiabetic and antioxidant properties of alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013, 18, 9770–9784. [Google Scholar] [CrossRef]
- Ajebli, M.; Eddouks, M. The Promising Role of Plant Tannins as Bioactive Antidiabetic Agents. Curr. Med. Chem. 2018, 26, 4852–4884. [Google Scholar] [CrossRef]
- Omar, N.; Ismail, C.A.N.; Long, I. Tannins in the Treatment of Diabetic Neuropathic Pain: Research Progress and Future Challenges. Front. Pharmacol. 2022, 12, 805854. [Google Scholar] [CrossRef]
- Meng, J.M.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; Wang, Y.F.; Cai, S.X.; Xu, X.Y.; Zhang, P.Z.; Li, H.B. Effects and Mechanisms of tea for the Prevention and Management of Diabetes Mellitus and Diabetic Complications: An Updated Review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef]
- Addepalli, V.; Suryavanshi, S.V. Catechin Attenuates Diabetic Autonomic Neuropathy in Streptozotocin Induced Diabetic Rats. Biomed. Pharmacother. 2018, 108, 1517–1523. [Google Scholar] [CrossRef]
- Abo-Salem, O.M.; Ali, T.M.; Harisa, G.I.; Mehanna, O.M.; Younos, I.H.; Almalki, W.H. Beneficial Effects of (-)-Epigallocatechin-3-O-Gallate on Diabetic Peripheral Neuropathy in the Rat Model. J. Biochem. Mol. Toxicol. 2020, 34, e22508. [Google Scholar] [CrossRef]
- Raposo, D.; Morgado, C.; Terra, P.P.; Tavares, I. Nociceptive Spinal Cord Neurons of Laminae I-III Exhibit Oxidative Stress Damage during Diabetic Neuropathy Which Is Prevented by Early Antioxidant Treatment with Epigallocatechin-Gallate (EGCG). Brain Res. Bull. 2015, 110, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, M.B.; Suaifan, G.A.R.Y.; Abu-Odeh, A.M. Plants Secondary Metabolites as Blood Glucose Lowering Molecules. Molecules 2021, 26, 4333. [Google Scholar] [CrossRef] [PubMed]
- Suchitra, K.; Panigrahy, S.K.; Bhatt, R.; Kumar, A. Targeting type II diabetes with plant terpenes: The new and promising antidiabetic therapeutics. Biologia 2021, 76, 241–254. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Chi, G.; Wei, M.; Xie, X.; Soromou, L.W.; Liu, F.; Zhao, S. Suppression of MAPK and NF-Κb Pathways by Limonene Contributes to Attenuation of Lipopolysaccharide-Induced Inflammatory Responses in Acute Lung Injury. Inflammation 2013, 36, 501–511. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Ong, W.Y.; Farooqui, T.; Koh, H.L.; Farooqui, A.A.; Ling, E.A. Protective Effects of Ginseng on Neurological Disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef]
- Quintans, J.S.S.; Sanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.G.D.S.; Picot, L.; Júnior, L.Q. Monoterpenes Modulating Cytokines-A Review. Food Chem. Toxicol. 2019, 123, 233–257. [Google Scholar] [CrossRef]
- Audelo, M.L.D.P.; Cortes, H.; Floran, I.H.C.; Torres, M.G.; Guadarrama, L.E.; Chavez, S.A.B.; Gomez, D.M.G.; Magana, J.J.; Gomez, G.L. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Srithar, G. Alpha Pinene Modulates UVA-Induced Oxidative Stress, DNA Damage and Apoptosis in Human Skin Epidermal Keratinocytes. Life Sci. 2018, 212, 150–158. [Google Scholar] [CrossRef]
- Fernandes, J. Antitumor Monoterpenes in Bioactive Essential Oils and Cancer; de Sousa, D.P., Ed.; Springer: Cham, Switzerland, 2015; pp. 175–200. [Google Scholar] [CrossRef]
- Baz, F.E.; Aly, H.; Abd-Alla, H.I.; Saad, S.A. Bioactive flavonoid glycosides and anti-diabetic activity of Jatropha curcas on streptozotocin-induced diabetic rats. Int. J. Pharm. Rev. Res. 2014, 29, 143–156. [Google Scholar]
- Tofighi, Z.; Afrapoli, F.M.; Ebrahimi, S.N.; Goodarzi, S.; Hadjiakhoondi, A.; Neuburger, M.; Hamburger, M.; Abdollahi, M.; Yassa, N. Securigenin glycosides as hypoglycemic principles of Securigera securidaca seeds. J. Nat. Med. 2016, 71, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Admassu, S. Potential Health Benefits and Problems Associated with Phytochemicals in Food Legumes. East Afr. J. Sci. 2009, 3, 116–133. [Google Scholar] [CrossRef]
- Anonymous. Aloeaceae. Meyler’s Side Effects of Drugs, The International Encyclopedia of Adverse Drug Reactions and Interactions, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 160–161. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Khaodhiar, L.; Ricciotti, H.A.; Li, L.; Pan, W.; Schickel, M.; Zhou, J.; Blackburn, G.L. Daidzein-rich isoflavone aglycones are potentially effective in reducing hot flashes in menopausal women. Menopause 2008, 15, 125–132. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Kirjavainen, S.; Forestieri, A.M.; Trovato, A.; Tripodo, M.M. Biological effects of hesperidin, a citrus flavonoid. (Note I): Antiinflammatory and analgesic activity. Farmaco 1994, 40, 709–712. [Google Scholar]
- Alam. M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Varsha, K.; Sharma, A.; Kaur, A.; Madan, J.; Pandey, R.S.; Jain, U.K.; Chandra, R. Chapter 28—Natural plant-derived anticancer drugs nanotherapeutics: A review on preclinical to clinical success. In Micro and Nano Technologies, Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 775–809. [Google Scholar] [CrossRef]
- Loguercio, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef]
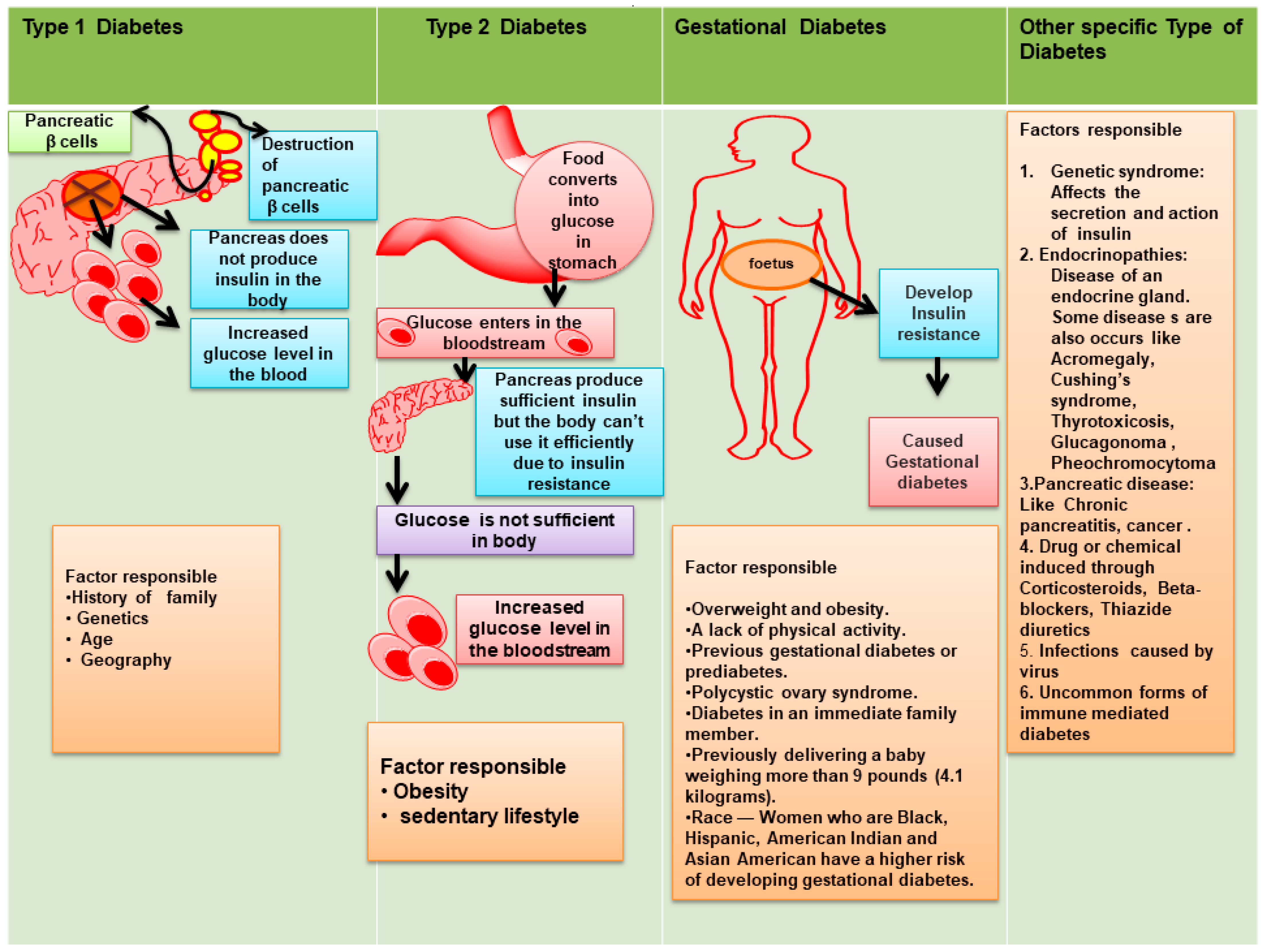
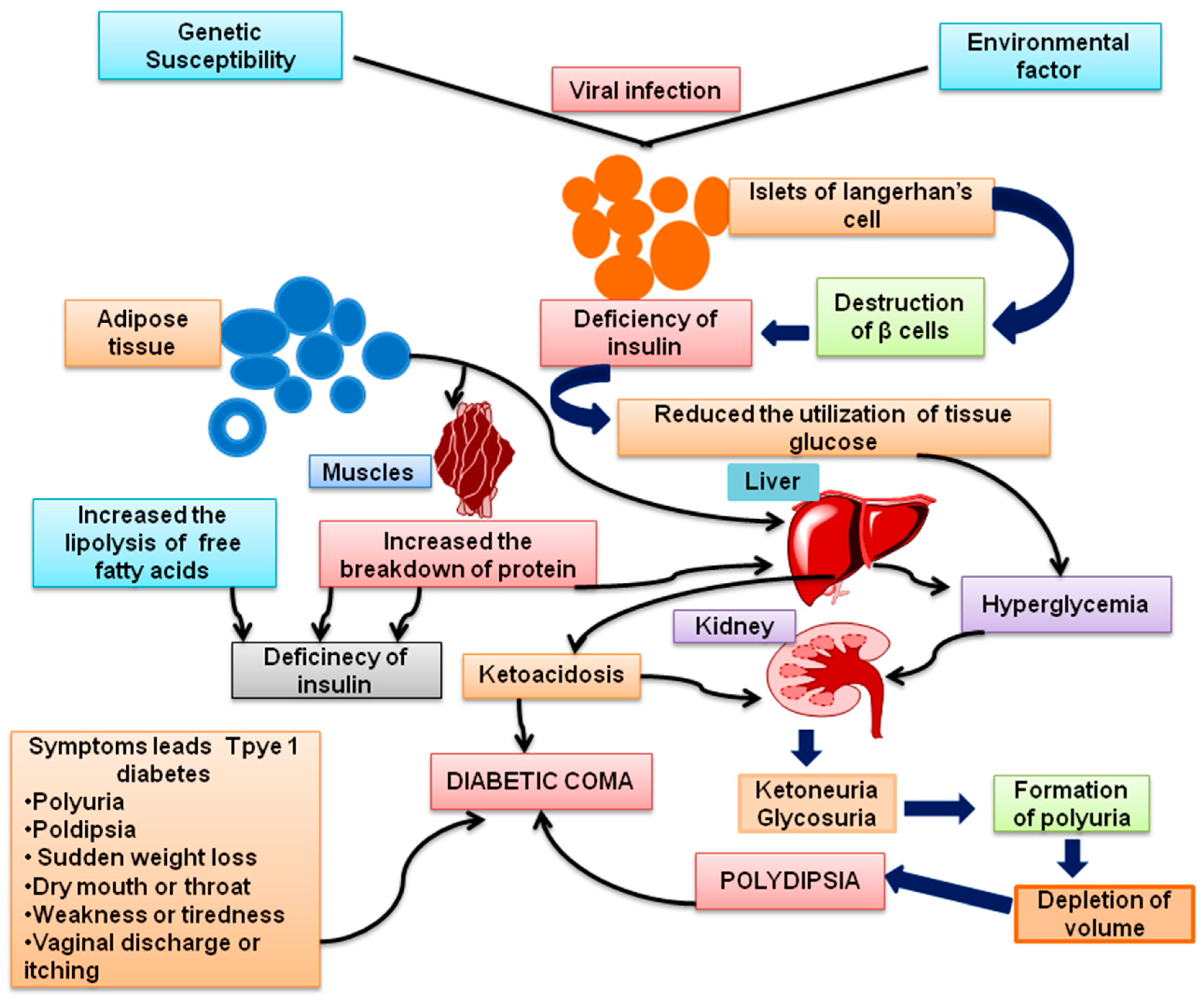
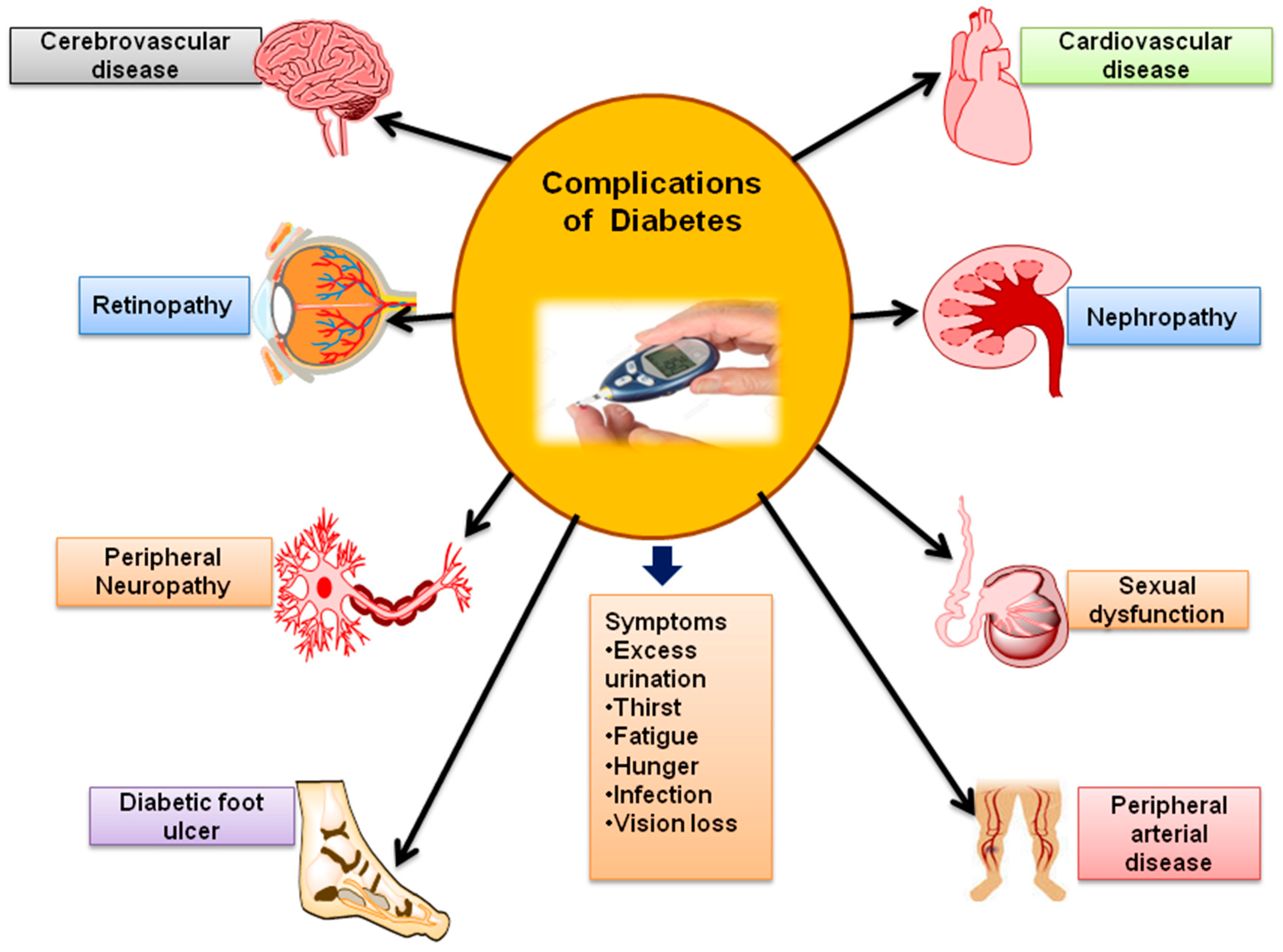
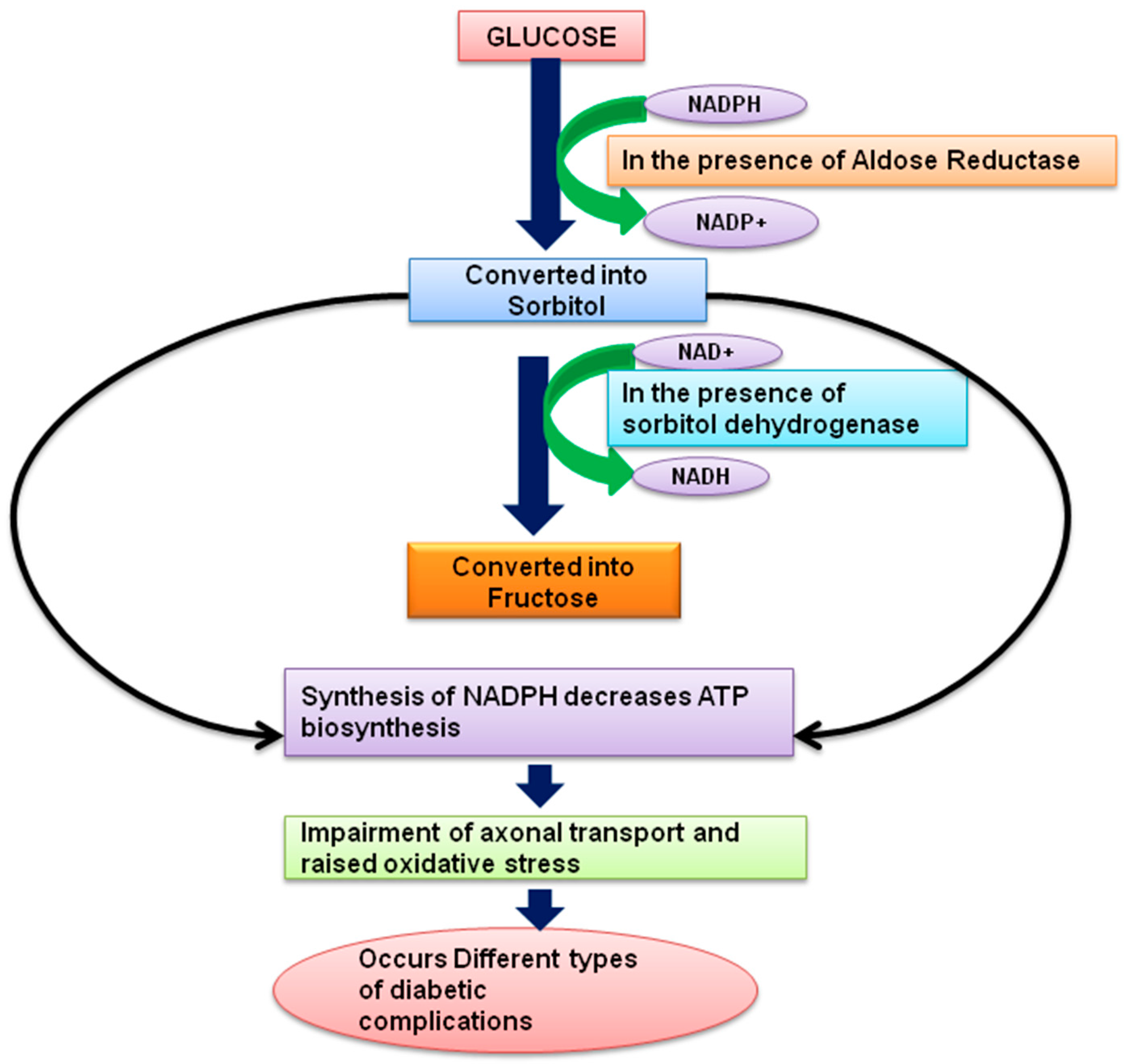
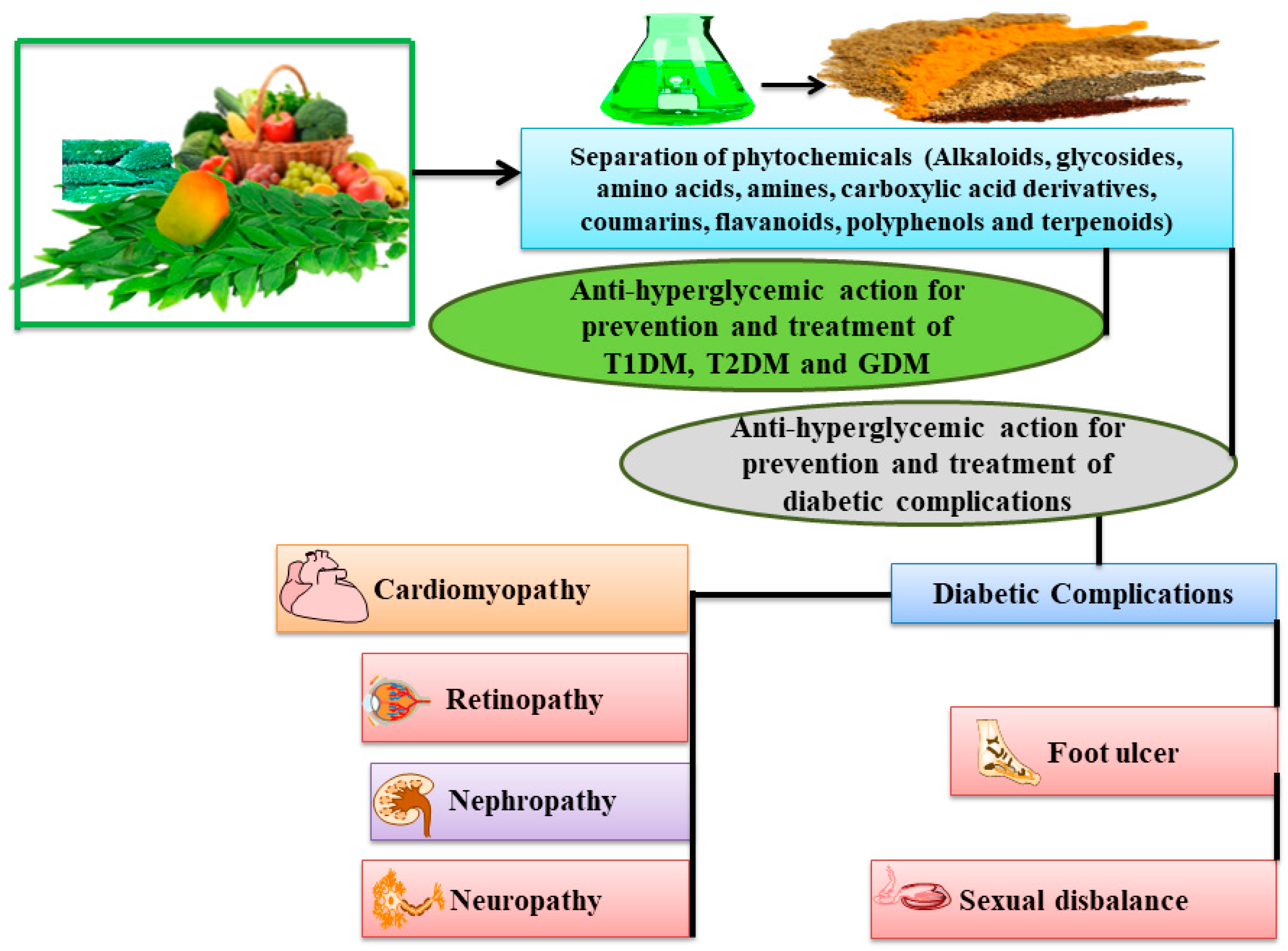
| Sub-Class | Phytochemicals | BCS * Class | Solubility (Aqueous) | Bioavailability (Oral) | Molecular Weight and Melting Point (°C) | Molecular Mechanism |
|---|---|---|---|---|---|---|
| Alkaloids | ||||||
| Isoquinoline | Berberine [46] | III [47] | Poor [47] | Low (0.68%) [48] | 336.4 and 145 °C | Improves metabolism of glucose by glycolysis stimulation via reduction in the mitochondrial oxidation of glucose. Additionally caused AMPK activation as a result of mitochondrial inhibition, which improves the AMP/ATP ratio [49,50,51,52,53,54,55,56,57,58]. |
| Pyridine | Trigonelline [59] | NF | Good [60] | 57.37% [61] | 137.14 and 218 °C | Cell regeneration, insulin secretion, glucose metabolism, reactive oxygen species, axonal extension, and neuron excitability are some of key targets mechanism. Reported to play significant role in alleviating kidney damage in n-STZ-diabetic rats [62,63,64,65,66,67]. |
| Piperidine | Fagomine [68] | NF | NF | 77.50% [69] | 147.17 and 188 °C | Reduced levels of lipids and blood sugar by improving insulin sensitivity and antioxidant enzyme activity followed by decreased in lipid peroxidation. Further, improves oxidative damage by high glucose in HUVECs via the AMPK/SIRT1/PGC-1 pathway [70,71,72,73,74]. |
| Carpaine [75] | NF | 48.12 μg/mL [75] | 0.55 score [76] | 478.7 and 119–120 °C | Glucose transport, carbohydrate digestion and absorption [77,78]. | |
| Terpenoid | Gentianine [68] | NF | Soluble [79] | NF | 175 and 84–86 °C | Significantly improve adipogenesis, which was correlated with a considerable rise in PPAR-, GLUT-4, and adiponectin mRNA expression [80,81]. |
| Glycosides | ||||||
| Anthranoids | Aloin [77] | NF | Soluble [82] | 5.51 to 6.60% [83] | 418.4 and 148 °C | Decreased serum glucose and significantly increased serum insulin levels. It was shown that serum levels of MDA and SOD were significantly decreased, while blood GSH was significantly increased as compared to diabetic rats. Produce an inhibitory effect on the vascular acute inflammatory response caused by the STZ-induced pancreatic islet lesions responsible to increases the vascular permeability [84,85,86,87,88,89]. |
| Emodin [90] | II or IV [91,92] | Poor [93,94] | Low [95] | 270.24 and 266–268 °C | Have significant glucose lowering effect and mixed type inhibition and non-toxic in the case of prolonged use in treating diabetes. Increase insulin sensitivity and glucose tolerance by activating PPAR and modifying genes involved in metabolism. Further, prevent cataract in diabetic patients by hydrogen bonding with Ser302 in the specificity pocket of aldose reductase [96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121]. | |
| Rhein [122] | NF | Poor [123,124] | >50% (Low) [124] | 284.22 and >300 °C | Ameliorate levels of TGF-β1, renal fibrosis, metabolism, and oxidative stress status [125,126]. | |
| Amino acids, amines and carboxylic acid derivatives | ||||||
| Organosulfur | Allicin [68] | I [127] | 2.5% or more oil soluble [128,129] | >65% [130] | 162.3 and 25 °C | Blood sugar reduction, insulin levels improvement, and regain in GLUT4 and IRSs expression induced by diabetes and also improve of diabetic nephropathy [131]. |
| Alliin [68] | III [127] | Soluble [129] | 16.5% [132] | 177.22 and 163 °C | Lowered the amount of a serum enzyme (alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase). Protection against glucose or MG induced glycation of SOD to prevent glycation-mediated diabetic complications [133]. | |
| Amino acids | Betaine [68] | NF | 157 g/100 mL [134] | 100% [135] | 117.15 and 310 °C | Improves glucose tolerance, insulin action, multiple genes function expression (dysregulated during diabetes). AMP-activated protein kinase involvement, alleviates endoplasmic reticulum stress, anti-inflammatory and anti-oxidant effects for improved insulin sensitivity and better blood glucose clearance [136,137]. |
| Isoleucine [68] | NF | Poor [138] | Low [138] | 131.17 and 285.5 °C | Lower postprandial blood glucose level [139]. | |
| Alanin [68] | NF | NF | NF | 89 and 300 °C | Prolonged glucocorticoid and glucagon signalling, liver alanine catabolism, promotes hyperglycemia and skeletal muscle atrophy [140]. | |
| Coumarins derivatives | ||||||
| Furocoumarin | Marmesin [77] | NF | NF | NF | 246.26 and 189–191 °C | Improve regeneration of pancreatic β cells and insulin secretion [77]. |
| Flavanoids | ||||||
| Flavonols | Kaempferol [68] | II [141] | Low [141] | Low to moderate [142] | 286.24 and 277 °C | In obese mice, increased lipid metabolism through PPAR- and SREBP-1c downregulation reduced adipose tissue development, hyperlipidemia, and diabetes. Additionally, restore to almost normal plasma glucose, insulin, lipid peroxidation products, and enzymatic and non-enzymatic antioxidants [143]. |
| Quercetin [143] | I, II or IV [144] | 0.3 μg/mL [145] | 16% [146] | 302.23 and 316.5 °C | Decreases lipid peroxidation, increases in antioxidant enzymes (like SOD, GPX, and CAT) activities, inhibition of insulin-dependent activation of PI3K, and reduction in intestinal glucose absorption by inhibiting GLUT2 [143] | |
| Fisetin [147] | II [148] | <1 mg/mL [148] | Poor [148] | 286.24 and 330 °C | Act as a dose-dependent manner for high glucose’s activation of PKR and decrease of p67 [147,148]. | |
| Isoflavones | Genistein [149] | II [141] | 0.81 μg/mL [145] | Poor [145] | 270.24 and 301.5 °C | Improve insulin sensitivity and the expression levels of the NGF, BDNF, decreased Aβ deposition and hyperphosphorylated Tau protein concentration [149,150]. |
| Diadzein [151] | IV [152] | 8.215 μg/mL [145] | 66.9%, 45.2%, 65.7%, and 57.9% [153] | 254.24 and 323 °C | Significantly reduced the level of aldose reductase and sorbitol dehydrogenase. Prevent the change in ‘a’ and ‘b’ wave amplitude and latency by electroretinography [151]. | |
| Chrysin [154] | II [155] | Low [155] | 0.003–0.02% [155] | 254.24 and 285.5 °C | Inhibit dose dependently both ɑ-amylase and ɑ-glycosidase enzyme, also reduce glycation, possess greater antioxidant, antidiabetic and antiglycating activities [156]. | |
| Flavanones | Hesperidin [68] | II [141] | 1.4 μg/mL [145] | Poor [157] | 610.6 and 250–255 °C | Improvement in plasma insulin levels and total blood lipid profiles [158]. |
| Eriodictyol [159] | NF | −2.34 [160] | NF | 288.25 and 269–270 °C | Activation of the Nrf2/HO-1 pathway, protects RGC-5 cells from high glucose-induced oxidative damage, inflammation, and cell death [159]. | |
| Naringenin [68] | II [161] | 45 μg/mL [145] | 5.8% [162] | 272.25 and 251 °C | Prevent ATF4 and CHOP nuclear translocation in diabetic kidneys and hyperglycemic renal cells. Also prevent apoptosis by down regulating expression of apoptotic marker proteins. Further, lowered serum glucose level, enhanced glucose tolerance, and restored insulin levels in diabetic rats [163]. | |
| Flavones | Diosmin [164] | IV [165] | Poor [165] | Poor [166] | 608.5 and 277–278 °C | Significantly improved the lipid profiles, increased blood sugar, reduced body weight, walking ability, thermal hyperalgesia, and cold allodynia It restores MDA, NO, superoxide dismutase activity and glutathione levels fell in diabetic rats [164]. |
| Morin [167] | IV [168] | 0.25 mg/mL [169] | 0.45% [170] | 302.23 and 303.5 °C | Attenuates serum glucose, aminotransferases, urea and creatinine. Also enhances insulin/IGF-1 signalling activation and skeletal deficits brought on by hyperglycemia [171]. | |
| Apigenin [68] | II [141,172] | 2.16 μg/mL [145,172] | 2.0% [145] | 270.24 and 347.5 °C | Improve glycogen synthesis, insulin secretion, cholesterol synthesis and glycogen synthesis [143]. | |
| Baicalein [173] | IV [174] | 0.057 mg/mL [174] | 2.2% [174] | 270.24 and 256–271 °C | Significantly reduced blood glucose and LPS levels, as well as inflammation, lipid profile, and insulin resistance. Exhibit prebiotic-like effects to alter gut flora and ameliorate metabolic abnormalities connected to T2DM [173]. | |
| Tangeretin [175] | NF | Poor [176] | 27.11% [177] | 372.4 and 154 °C | Modulates through increased insulin production, hepatic enzymes are activated, and the antioxidant capability of the compound lowers blood glucose levels in STZ-induced diabetic rats [175]. | |
| Isorhamnetin [178] | II [179] | Poor [179] | Poor [180] | 316.26 and 311–314 °C | Decreased glycaemia, improved oxidative status, decreased inflammation, modulated lipid metabolism, and improved adipocyte differentiation via controlling relevant signalling pathways [178]. | |
| Wogonin [181] | NF | Poor [182] | Poor [183] | 284.26 and 203–206 °C | Improved and restored SOD1/2 and CAT activity, ROS and MDA generation and expression of inflammatory markers (IL-1, IL-6, TNF, PAI-1 and NF-B). Additionally, reducing oxidative stress and inflammation may help to lessen the damage of the cardiomyocyte caused by hyperglycemia [181]. | |
| Rutin [184] | II [185] | Poor [185] | Poor [185] | 610.5 and 125 °C | Improve cognition and exhibit antinociceptive effects in diabetic rats [184]. | |
| Flavonolignan | Silybin [186] | II [187] | Low [187] | Poor [187] | 484.4 and 164–174 °C | Improve glycated haemoglobin level, restoration of liver glycogen content and serum insulin regeneration [186]. |
| Silymarin [188] | II [189] | <100 µg/mL [189] | Poor [189] | 482.4 and 167 °C | Hypoglycemic potential was due to its antioxidant activity by reducing insulin resistance, in diabetics with liver problems to recover liver function and also demonstrated effectiveness in reducing blood glucose level [190]. | |
| Anthocyanins | Delphinidin [191] | NF | NF | 0.49% [192] | 338.69 and −17.77 °C | Have anti-glycation activity, decrease the rate of albumin and HbA1c glycation and used for treatment of diabetes complications [191]. |
| Polyphenol and its derivative | ||||||
| Diferuloylmethane | Curcumin [193] | II [194] | 11 ng/mL [194] | 1% [194] | 368.4 and 179–182 °C | Improve glycaemia and the consequences associated with diabetes, such as liver problems, adipocyte dysfunction, neuropathy, nephropathy, vascular illnesses, pancreatic problems, and others [193,195]. |
| Terpenoids | ||||||
| Pentacyclic | Ursolic acid [196] | IV [197] | <1 µg/mL [197] | Poor [197,198] | 456.7 and 284 °C | Reduce DNA damage, GR enzyme activities, and MDA levels in diabetic rats followed by improvement in GSH, CAT, SOD, and GSH-Px levels and enzyme activities [196]. |
| Diterpenoid | Andrographolide [199] | II [200] | Poor [201] | Poor [201] | 350.4 and 231–232 °C | Lowered triglyceride, LDL, and blood sugar levels in comparison to controls. Stimulate the insulin release, and inhibits the absorption of glucose through inhibition of the enzyme alpha-glucosidase and alpha-amylase [202]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raghav, S.S.; Kumar, B.; Sethiya, N.K.; Kaul, A. A Mechanistic Insight on Phytoconstituents Delivering Hypoglycemic Activity: A Comprehensive Overview. Future Pharmacol. 2022, 2, 511-546. https://doi.org/10.3390/futurepharmacol2040032
Raghav SS, Kumar B, Sethiya NK, Kaul A. A Mechanistic Insight on Phytoconstituents Delivering Hypoglycemic Activity: A Comprehensive Overview. Future Pharmacology. 2022; 2(4):511-546. https://doi.org/10.3390/futurepharmacol2040032
Chicago/Turabian StyleRaghav, Shraddha Singh, Bhavna Kumar, Neeraj Kumar Sethiya, and Ankur Kaul. 2022. "A Mechanistic Insight on Phytoconstituents Delivering Hypoglycemic Activity: A Comprehensive Overview" Future Pharmacology 2, no. 4: 511-546. https://doi.org/10.3390/futurepharmacol2040032
APA StyleRaghav, S. S., Kumar, B., Sethiya, N. K., & Kaul, A. (2022). A Mechanistic Insight on Phytoconstituents Delivering Hypoglycemic Activity: A Comprehensive Overview. Future Pharmacology, 2(4), 511-546. https://doi.org/10.3390/futurepharmacol2040032






