Abstract
In 2007 a paper in Nature Medicine sparked a new wave of interest in the use of molecular hydrogen (H2) for medical treatments. Since then there has been a flurry of papers looking at a range of medical aspects, from neurodegenerative disease to sports injuries. Several methods of application have been developed, including breathing the gas, or making a hydrogen-rich solution that can be ingested, or administered as saline. H2 treatments are deemed to be safe and can be used in agricultural practice as well as in the biomedical arena. However, the first studies to investigate the use of H2 in medicine were carried out by those interested in the use of a range of gases and how this may affect respiration in humans. Beddoes was published in 1793 and Cavallo in 1798, with Davy following shortly after in 1800. With so many papers now appearing on H2 in biological systems, it seems timely, and interesting, to revisit the early papers, to humbly remind us of what these pioneers of respiratory research did.
Keywords:
Beddoes; Davy; Cavallo; hydrogen gas; molecular hydrogen; nitrous oxide; oxygen; respiration; Watt 1. Introduction
The use of molecular hydrogen (H2) in medicine [1,2] and agriculture [3] is now thought to be worthy of investigation and future investment. It has been suggested to be beneficial for neurogenerative disease [4], and even COVID-19 [5], for example.
Hydrogen can be administered to animals, including humans, as a gas or as an enriched solution. Gas can be inhaled, whilst hydrogen rich-water (HRW) can be ingested. It is now advocated as a sports supplement for example [6]. With plants, HRW can be used in feedwater or sprayed onto foliage. It has also been suggested as a post-harvest treatment [7]. Now nanotechnology in hydrogen medicine is being suggested [8].
Hydrogen was discovered by Henry Cavendish in 1766 [9]. However, the presence of an inflammable gas was known before this. It has been suggested that Paracelsus added sulfuric acid to iron filings and noted a flammable gas in the early 1500s. However, others have disputed this. Leonard Dobbin states, after a look at the original papers and translations, that “it appears to be desirable that the assertion that Paracelsus observed the evolution of hydrogen during the interaction of sulfuric acid with iron or other metal should be permitted to disappear from current literature” [10]. However, others were doing similar work. Robert Boyle observed the same as Paracelsus in 1671. However, Cavendish receives credit. He showed that when hydrogen burns, water was formed. This gave hydrogen its name, coined from hydro-gen, as in generating water, as suggested by Antoine-Laurent Lavoisier, a person instrumental in the early work on oxygen, which he also named. Despite his contributions, he was guillotined during the French revolution on trumped-up charges [11].
In the late 18th century, there was a lot of interest in the gases which were being discovered, including oxygen (discovered in the early to mid-1770s [11]) and hydrogen.
In that vein, two instrumental papers were published at the end of the 18th Century and beginning of the 19th. The first was by Tiberius Cavallo (1798) [12] and the second was by Humphry Davy (1800) [13].
Tiberius Cavallo (30 March 1749–21 December 1809) was born in Naples but went to London in 1779, and in 1781 was admitted to the Royal Society [14]. He wrote The History and Practice of Aerostation (1785)—the history of ballooning—but of pertinence here, he suggested hydrogen as a gas to be used in balloons for flight. However, he was best known for his work on electricity, magnetism, and pneumatics.
Humphry Davy (17 December 1778–29 May 1829) [15] was born in Penzance, in the county of Cornwall, in the most southwest part of England. This was a major mining region, with several minerals being extracted, including tin, copper, and arsenic. On 2 October 1798, Davy moved to Bristol and became the superintendent at the Medical Pneumatic Institution, which was in the Hotwells area of the city, known for its spa waters, thought to have medical benefits. It was here that he did his work discussed below, under the leadership of Dr. Thomas Beddoes. However, Davy went on to have a momentous career. In addition to characterizing nitrous oxide (and naming it laughing gas), he isolated several elements, including potassium, sodium, calcium, magnesium, strontium, and barium. In 1803 he was elected to be a Fellow of the Royal Society, and in 1805 he became Director of the Royal Institution. His lectures were amazingly popular. In 1812, he was knighted, and in the year 1815, he invented the safety lamp (the Davy Lamp). He went on to become President of the Royal Society, and co-founder of the London Zoological Society, amongst a host of other achievements. He was also a poet and even edited the works of Wordsworth and Coleridge. However, it was in the year 1800, at an age of only 21, that he wrote about his characterization of nitrous oxide, a book which is the focus of the discussion below.
Here, we will look more closely at the two papers (books) by Cavallo and Davy [12,13]. These were written about their work on respiration and nitrous oxide, respectively. It is the work that they described which is relevant to molecular hydrogen research that is the focus of the discussion below. A significant amount of their work is ignored here, but that does not belittle the relevance of the other aspects of their investigations.
However, before continuing, it is worth pointing out a few features of these papers. Firstly, they can be quite rambling and jumbled, so we have gone through the works in the order in which they were written, rather than trying to put back a more logical train of thought, and thereby we have kept them as original as possible. Secondly, it is not always entirely clear what was meant, so we have used numerous quotes so that the original meaning is not lost. Thirdly, these papers were written in “old English”, and many “s” letters are written as “∫”, known as a “long s” [16], and we have endeavoured to quote them in the original. Although this makes it harder to read, it is also more authentic.
The authors often use archaic terms in referring to these gases, which makes it difficult for the modern reader to follow. Table 1 lists some of the commonly used terms and their identification with a comment about them. Additional confusion arises because sometimes the authors seem to liberally interchange terms, perhaps due to a lack of standardized nomenclature [17].

Table 1.
Arachic terms of various gases with their associated identity.
2. The Work of Cavallo (1798): An Essay on the Medicinal Properties of Factitious Airs: With an Appendix, on the Nature of Blood
Cavallo starts his discussion [12] with the statement: “It is not quite forty year ∫ince the artificial aerial fluids began to be admini∫tered as remedies to the human body”. This is interesting for two reasons. Firstly, it shows the pace of progress that was expected in the late 18th century. Secondly, oxygen was discovered in the early 1770s (probably by Carl Wilhelm Scheele (1772), but arguably by Joseph Priestley in 1774 or Antoine-Laurent Lavoisier, as discussed previously [11]) so within the forty years that Cavallo quotes. Cavallo goes on to complain that the progress was slow and doubtful, so obviously decided to do something about it.
He talks about his concerns regarding the purity of air, its composition, and ventilation, e.g., in hospitals and sick rooms. He says, “By the admixture of oxygen gas, a quantity of common air may be improved to almost any degree”. He also knew that plants produced oxygen which mixed with the air. He discusses how a hundred leaves of Indian cress (spelt cre∫s: na∫turtium Indicum. Additionally, note the capital letters in the Latin naming here) in a gallon of spring water, if exposed to the sun for three hours, will produce approximately ten cubic inches of oxygen. He says it will not be pure but will be better than common air. He goes on to say that no plant he knows of does this better.
Of pertinence here, Cavallo explains that work to this point has identified various “ela∫tic fluids, analagous to common air”. As well as oxygen, one of these is “dephogi∫licated air, or vital air, or oxygen air” (his italics). Of relevance here, he also lists “the inflammable gas, or hydrogen gas”. He goes on to say: “However, as of all the different airs five only appear to be applicable to the human body, viz. the common, the oxygen, the azotic, the carbonic acid, and the hydrogen airs”, and then he dismisses the rest. Therefore, it appears that this is one of the earliest claims that hydrogen gas, which is one of the hydrogen airs, may be applicable to human physiology, and this was in 1798.
So, what else did he go on to say, which was relevant to hydrogen research?
Cavallo says that inflammable air is the lightest of the “ela∫tic fluids”, and that it is combustible, but will only burn if ignited and in contact with air or oxygen. Interestingly, he says: “Though this fort of ela∫tic fluid be ab∫olutely unfit for re∫piration, it is not, however, ∫o noxious as carbonic acid”. He also says that it is not affected by mixing with nitrous air, and then describes how hydrogen expands with temperature—by one 400th of volume for each 1° F. He describes its production during the deterioration of both animal and vegetable matter, as it arises from ponds, burial grounds, and other places where biological material decays. Although he states that this gas is inflammable air and calls it hydrogen, his description seems to resemble more of hydrogen sulfide (H2S), which is also inflammable and could be considered a “hydrogen air”. It may also have been a mixture of hydrogen sulfide with hydrogen gas. He continues writing about this inflammable hydrogen air, saying that it “frequently come out of the earth” where there are other inflammable materials, such as at coal mines or “mines of ∫ulphureous metallic ores”. Mining was a big business in that period, as discussed with Davy, who invented the mining safety lamp. Interestingly, Cavallo says that the hydrogen will rise quickly into the upper reaches of the atmosphere. This is a concern today, as many treatments are transient as the hydrogen re-enters the gas phase and disperses rapidly into the atmosphere. Cavallo says “and leaves the air, adjacent to the ground, very little, if at all, infected, excepting in vaulted ∫ubterranean places …”, such as mines, it would be assumed he was thinking. Such thoughts are pertinent today for anyone spraying crops with H2 solutions.
For hydrogen production, Cavallo suggests the treatment of iron or zinc with vitriolic acid (sulfuric acid), or passing steam over a red-hot iron. He also suggests using passing steam over charcoal. However, this produces a different gas mixture, which he refers to as hydrocarbonate. This is a mixture mainly of H2 and carbon monoxide (CO), and some carbon dioxide (CO2). The mechanism of the production of this gas mix was explained by Long and Sykes in 1948 [18]. It is worth considering this, as later in the paper Cavallo describes using this gas mixture as a treatment for a range of patients.
Cavallo explored what happened to hydrogen during reactions and lists a few compounds that could be produced, such as “pho∫phoric hydrogen gas” (probably phosphine gas), and points out that “hydrogen gas” may give different specific gravities and smells. Such language confuses the discussion, as it was unclear if Cavallo knew what was being produced and tended to group these “hydrogen gases” together. This reflects the early methods used and the lack of knowing exactly what was present. This makes using such gas mixtures as treatments problematic and makes drawing modern conclusions from the data very difficult.
Of course, the paper Cavallo wrote was about respiration, and as such the mention of hydrogen comes and goes (we will see the same pattern in Davy’s work). Cavallo goes on to discuss respiration and the effects of both oxygen and carbon dioxide, and even discusses how long a person can breathe the gases he made, with reference to divers. Throughout the paper, there is little reference to the work of others, but interestingly he says that, as far as he knew, Priestley was the first person to work on the breathing of oxygen air. He quotes Priestley in saying that the breathing of oxygen was not much different from breathing common air. On the other hand, Dr. Beddoes was quoted as saying that breathing oxygen was extremely hurtful, stating that “it feels like ardent ∫pirit applied to the palate …”. Cavallo does say that oxygen air can be kept in the lungs longer than common air. This is interesting for several reasons. Firstly, it shows that even though these people knew that the gases were impure, and in fact had little idea of their true composition, they were willing to breathe them in and note the effects. Secondly, they had some useful insights into the effects of gases such as oxygen. Thirdly, they knew of each other’s work. For relevance here, Dr. Beddoes was the head of the institute where Davy worked; he was Davy’s boss.
Cavallo goes on to describe work on oxygen which would be frowned upon these days. Rabbits were held underwater until they were nearly drowned and then an attempt to revive them was made using various gases. Even dogs and cats were given the same treatment; only oxygen, but not air, could revive these nearly dead animals. He points out that such experiments were “repeated with equal ∫ucce∫s by different per∫ons …”, so it was not an uncommon experiment to perform.
In Chapter 3 of the paper, Cavallo returns to hydrogen. He states that “It has been repeatedly affected and denied, that pure and unmixed hydrogen, or inflammable gas, may be re∫pired with impunity for a con∫iderable time …”. Therefore, there was some debate as to whether breathing hydrogen was harmful. However, he says that this is because of the variable nature of the gases used, and the variable amount of common air that was still in the lungs. He complains that the hydrogen gas used is seldom very pure. He also suggests a way that a person can expire as much air as possible before breathing in the hydrogen. Clearly, having some oxygen still in the lungs was not a priority here. He suggests that making H2 by passing steam over hot iron is “lea∫t offen∫ive”. However, he goes on to say that if the experiment is carried out properly, with expiration in a bent posture, breathing in from the vessel with the nose closed, then “about three or four in∫pirations, that the florid colour of his face is vani∫hed, and his ∫trength is ∫o far dimini∫hed as to prevent the pro∫ecution of the experiment”. He says that his own observations were that only two inspirations were required to see the effects and that the gas was obtained from iron and diluted vitriolic acid (sulfuric acid). However, he goes on to point out that inflammable gas may be less noxious if agitated in water. Of course, the lack of oxygen here is probably the reason for such observations.
Having carried out these crude experiments, he goes on to say that if the hydrogen is mixed with air, at equal proportions or smaller, it can be breathed safely for a considerable time. He points out that the lungs have a “peculiar ∫en∫ation of levity”. It says that it is beneficial for inflammation of the lungs, convulsive coughs, etc, where “the object is to dimini∫h the irritability of the parts affected”. He says that the face would grow dark or livid, but its natural colour will soon be restored once air is breathed.
The gas he calls hydrocarbonate (i.e., H2/CO mixture) he says is more “pernicious” and that animals will die much sooner if this is used. Two or three breaths are all that is needed for death to occur, which is odd considering some of the case studies he relates—as discussed below. Even when diluted, 20–30 times, this gas is still dangerous. A person breathing this diluted gas for about fifteen minutes will be “made ∫ick”, they will feel cold and their lips will become blue, their face livid, and their pulse feeble. However, their lungs become less sensitive, and it can reduce lung pain. He says that the use of this gas cannot be used with too much care. He points out the danger of carbonic gas and says that carbonic gas and hydrocarbonate will kill more quickly than not breathing at all. The size and method of respiration of animals make a difference, with those with the largest lungs being most affected, and amphibians and insects being least affected. Clearly, he was using a range of animals in his experiments here, just as Davy did.
Cavallo then discusses the mixing of gases and worries that their specific gravities will lead to gases separating, especially inflammable gases which are the lightest. He encourages gas mixtures to be mixed so that they do not separate but worries that separation may take place in the lungs. He suggests that this may account for the dangerous effects of carbonic gas and hydrocarbonate, which may settle in the lungs.
By making incisions in the skin and then closing them after the injections of gases, Cavallo looked at the effects on the surrounding tissues. Air had a short-term effect, which resulted in swelling and unease for about three weeks. In contrast, the effect of oxygen was shorter, but the animals were unusually lively. Hydrogen “produced heavine∫s and ∫hiverings” but the animals recovered more quickly than when air was used. However, inflammable gas did not increase or decrease the putrefaction of animal material, although he noted that fruit and meat lasted longer in carbonic acid air. As hydrogen has been mooted to be beneficial for post-harvest treatment of fruit, vegetables, and flowers [19], this is an interesting observation.
Cavallo’s discussion moves on to the dispersal of heat and why we feel cold. He discusses the effects of gases, including oxygen, which consists of ∫ui generis, “which is called oxygen, combined with caloric, and, in all probability, with the matter of light al∫o”. Caloric here would be their interpretation of heat. This highlights the lack of clarity about what these gases actually were, and yet they were happy to breathe them in. Hydrogen is described as consisting of a “substance, called hydrogen, and caloric”. Other components, such as phosphorus, particles of iron, etc., were dismissed as contaminants. Interestingly, water is described as consisting of hydrogen gas and oxygen gas, in the “proportion of three of the former to ∫eventeen of the latter”. He says water is formed by the combustion of these gases, and importantly, that the gases can be recreated from water “by placing it, under certain circum∫tances, in contact with bodies that attract one of its components, or by the action of electricity”, which anyone who electrolyzes water to produce H2 can attest, such as in the machines that make hydrogen gas or hydro-oxygen mixtures for medical use. For a reference to electrical hydrolysis of water Cavallo cites, “Fourcroy’s Chemi∫try, the third volume of my Electricity, and the Phil Tran∫. for 1797, P.I.”.
Cavallo then turns to the uses of respiration, dismissing the voice and coughing, but concentrating on the delivery of oxygen. He describes a surprisingly accurate function of the lungs and blood and the delivery of gases to the tissues, including the blood absorbing oxygen through the pores of the “thin membrane, which ∫eparated it from the air in the cels of the lungs”. He even hints about the action of haemoglobin, as “oxygen is attracted by the ferruginous particles of the blood, and that the redne∫s of the blood is to be attributed to the red colour of the oxyde of iron”. However, he questions whether iron is the only way blood binds to oxygen, and doubts that hydrogen and oxygen combine in the lungs to form water, suggesting that the lungs separate out the water, so it can be expired.
The experiments which Cavallo referred to needed equipment, and for this he turned to “Mr. James Watt, engineer, of Birmingham”. We shall see that Davy also acquired his apparatus from Watt, who himself became a world-renowned engineer. Cavallo discussed the various aspects of the bags he could use for gas storage, such as oil-silk bags and bladders. In Lamont-Brown’s biography of Davy, he says that “Watt devised for Beddoes an apparatus to supply him with gases such as oxygen and hydrogen” (page 33) [15].
Returning to the uses of the gases themselves, we find Cavallo says that the use of azotic gas (i.e., containing nitrogen) and various species of hydrogen gas (again, note that he does not see hydrogen as being a single entity), diminishes the irritability of animal fibres and is therefore useful for a variety of disorders, such as inflammation, coughs, and spasms. He called these gases “reduced atmospheres”, as they contain “a ∫maller quantity of re∫pirable fluid”. Although he claims such gases are useful, he also says that some of them can produce “alarming ∫ymptoms”. He says that a mix of azotic air and common air should never be more than 25% of the former, and suggests that the same is true for the “mild ∫ort of hydrogen gas”. He points out that using hydrocarbonate air is more dangerous, which should be mixed with 20–30 times the amount of air, and breathed only about five minutes a day, but he does say that it may be useful for “∫ome di∫orders that have hitherto eluded all medical applications”; and goes on to suggest it could be used for treating hydrophobia or madness.
The effects of ether are also explored, which can be breathed in from the spout of a common teapot!
However, Cavallo’s paper then explores several disorders and ends with several case studies. Under asthma, he suggests using an air hydrogen mixture (15:4 v/v), and if that does not work, using hydrocarbonate, although care and stopping occasionally seem to be sensible. For cancer, he says that he knows no one who had been cured, but the use of gases certainly helped with pain relief, but he does not say which gas should be used. For catarrh (mucus build-up) he suggests hydrogen and air (4:20 v/v), and points out that you may only need to do this for three days. Under consumption (tuberculosis) he suggests hydrocarbonate, but with the caveats mentioned above, i.e., it reduces the patient’s strength and needs to be used with caution.
All this discussion leads to Chapter VIII and the case studies. These he had gathered from other people, and several involve “hydrogen gases”. The first of these was from a “Dr. J. Lind of Wind∫or”, and addressed the case of an “officer of the Exci∫e”, or excise collector. He had fallen ill during the severe weather of January 1797. He was prescribed hydrocarbonate and by 20 February was back to full health and returned to work. In the second case, hydrocarbonate was also used on a patient with inflammation of the lungs. The third case also used hydrocarbonate, this time being treated by Dr. Carmichael, in Birmingham, in 1795. The gas was used as a 1:19 proportion mix, but with no effects, the mixture was strengthened. Eventually, this worker, who made molds in a cast-iron factory, returned to work, albeit with indoor duties. Carmichael also related the treatment of another patient, using hydrocarbonate. Considering that this was a dangerous form of the “inflammable gas”, it is surprising in many respects that these patients were not killed, but in each case, they seemed to improve.
Hydrogen gas was said to be the treatment for case VII, which was a girl of 16 who seems to have the symptoms of phthisis (e.g., tuberculosis). The patient had nauseous and vomiting, but eventually “the whole train of phthi∫ical ∫ymptoms left her”.
It is clear that different forms of “hydrogen gas” were being used by a range of physicians, each relating to Cavallo their success. This is rather amazing, firstly because Cavallo could garner these views and stories, and secondly because they appeared to be so successful. However, no accounts of people being killed were relayed, so it is hard to be sure what the success rate was.
Cavallo’s paper did not end there either. He then wrote a critique of the equipment available if anyone wished to venture into these types of treatments. This included the production of heavy inflammable gas or hydrocarbonate. One piece of sensible advice was “It is likewi∫e advi∫ible for the very obvious rea∫on, not to conduct this prece∫s by candle light”. However, he also suggested that the gases degenerate and suggested that some, such as hydrocarbonate, should be made fresh each time, rather than keeping it for 2–3 days.
The paper ends with an Appendix, and a long discussion on the composition of blood and what we now know are red blood cells. After 256 pages (although each page is relatively short by modern standards) the work finished with an index and a list of the author’s other books, including treatises on Electricity, Magnetism, and Aerostation, as mentioned above. The final page lists where materials and equipment can be purchased, which would have been useful to anyone venturing into this type of medicine at the time.
Looking back at this paper it is very wide-ranging and insightful. It may have given an overly positive and unbalanced view, but it is impossible to say. It certainly shows that the thought at the time was that certain gases, including hydrogen, could be of use for the treatment of a variety of disorders, including inflammation, which is a focus of much of hydrogen medical use today [5].
However, this type of work appeared to not be widely adopted, although in Bristol, UK, there was a big push to carry out similar work and to make it popular. This was the work of Beddoes and Davy, which we will explore next.
3. The Work of Humphry Davy (1800): Research, Chemical, and Philosophical; Chiefly Concerning Nitrous Oxide, or Dephlogisticated Nitrous Air, and Its Respiration
Davy starts his paper [13] by recognising that his studies were based on the work of Joseph Priestley, published in 1799, and acknowledges the assistance of Priestley’s eldest son, also called Joseph. He also acknowledges the work of Lavoisier and Cavendish, amongst many others, including Scheele, Bergman, Kirwan, Higgins, and Berthollet. However, there is no mention of Cavallo, which seems surprising as Cavallo mentions Davy’s head of the institute, Beddoes, such as mentioned on page 252 when Davy says he followed Beddoes’ suggestion. So, one would assume there was a connection. It is also worth pointing out that Davy, like Cavallo, used James Watt as a supplier of much of his equipment, as mentioned above. It appears that Davy knew of Cavallo, at least by 1806, as Davy mentioned him in a Bakerian Lecture [20]. On the other hand, it is no surprise that Cavallo did not mention Davy. Cavallo’s paper was published in 1798, Davy only moved to Bristol and started this work in October of that year. Interestingly, a portrait at the National Portrait Gallery in London thought to be of Davy was later reassigned and the sitter is now thought to be Tiberius Cavallo [21].
Davy wished to investigate further the effects of nitrous oxide on biological systems, and the first part of his book is devoted to making the gas, and then characterising it, in comparison to what others had found. This quite long section can probably be summed up by this statement: “When compact nitrate of ammoniac is ∫lowly decompo∫ed, the nitrous oxide produced is almo∫t immediately fit for re∫piration”, although he then goes on to a long set of details of how to do this. This section ends with his thoughts: “Thus, if the plea∫urable effects, or medical properties of the nitrous oxide, ∫hould ever make it an article of general reque∫t, it may be procured with much le∫s time, labor, and expence, * than mo∫t of the luxuries, or even nece∫∫aries, of life” (* leads to a footnote in the original).
In the next section of the book (Division IV, page 122), Davy looks at the absorption of nitrous oxide by “different bodies”—not biological but chemical—which included several combustion studies. Interestingly, when Davy mixed equal quantities of hydrogen and nitrous oxide, no combustion was seen even in the presence of a strong electric shock (p136), suggesting that they did not react. This assumption was repeated on page 251, when Davy mixed (at 57° [F]) nitrous oxide with other gases including oxygen, air, hydrocarbonate, hydrogen, and nitrogen, all with no effect. This is reiterated on p313 when he says that nitrous oxide and hydrocarbonate have no effect on each other, except at high temperatures. The low reactivity of hydrogen with other molecules has been confirmed by other studies that indicate that H2 can only react with hydroxyl radicals and perhaps to a lesser extent peroxynitrite [22,23].
Davy also observes the relative solubility of gases in water, saying the nitrous oxide will drive hydrogen out of the solution.
The book continues as Davy explores a range of possible chemical reactions of nitrous oxide, and on more than one occasion says that he is only reporting the most “conclusive and accurate” of his experiments. Additionally, then on page 330, Davy starts a new “Research [from the title]” on “Relating to the respiration of nitrous oxide and other gases”. The first of these he says is focused on animals. On page 334 he pointed out that nitrogen and hydrogen have no effects on venous blood, and that animals immersed in such gases die as though they were deprived of air, or submerged in water. Hydrocarbonate, he notes, has a positive effect on blood, but kills as it stops the supply of “principles e∫∫ential to ∫en∫ibility and irratability”.
Davy described numerous experiments where animals including dogs, cats, rodents, a hen, and fish were put in nitrous oxide and observed. He concluded that nitrous oxide was destructive to warm-blooded animals, but they can recover if not left too long. Interesting here, he compares his data to Beddoes, his superior. Additionally, then on page 343, he starts to compare these effects with that of hydrogen and water. His title here says immersion in hydrogen and water, although phrasing such as a rabbit being introduced into hydrogen “through water” makes an exact understanding of what he had done difficult. However, on page 345 it is clear that animals were held underwater for a period of time. He had decided that nitrous oxide had a positive effect, but eventually caused death. On page 344 he took two rabbits, placed one in nitrous oxide and one in hydrogen; both died. In the next experiment, a shorter period of immersion in the gases was used. A rabbit in hydrogen recovered when removed after about thirty seconds, taking a few minutes to regain its composure. Next, he drowned a kitten, whilst a second kitten was placed in nitrous oxide. He concluded that animals live longer in nitrous oxide than in hydrogen or underwater. Clearly, the presence of oxygen was not deemed to be important here and the effects seen would have probably been due to hypoxia, rather than the effects of nitrous oxide or hydrogen. His colleague, Dr. Kinglake, undertook dissections for him, which were described. In the next experiment, the effects of nitrous oxide were compared to a rabbit bashed on the head, as a control. He was interested in the internal effects of the gas, as compared to an animal killed in air. Rabbits were then killed by nitrous oxide or hydrogen immersion and the blood, brain, and lungs were compared. Such experiments and dissections continued with guinea pigs, mice, and birds. This work was aided by two surgeons, Mr. Smith, and Mr. King. No difference in the appearance of nerves was seen whether rabbits were killed in nitrous oxide or hydrogen. Blood from pulmonary veins from animals killed in nitrous oxide did not differ from arterial blood from hydrogen-killed rabbits. Observing that he often received blood from people, he said that venous blood agitated in nitrous oxide was darker and more purple than that agitated in air, but brighter and “more florid” than that treated with hydrogen or nitrogen. Having treated a rabbit with nitrous oxide and then drowned it, on page 358 he started to mix gases and observe the effects. Nitrous oxide and hydrogen (50:50) killed a rabbit in four and half minutes, and the dissection showed the effects appear to be due to the nitrous oxide. A mouse died next in nitrous oxide and hydrogen (1:3 v/v). Nitrous oxide and oxygen (3:1) did not kill a guinea pig, and a mouse survived nitrous oxide and air (3:1), whilst a cat and a fish survived similar treatment. On page 362, his attention moved to cold-blooded animals: amphibians. He hypothesised that they would be less affected. Water lizards (we assume newts) were his first focus, but hydrogen was used as a control gas. After two to three minutes, the animal in nitrous oxide was “very uneas∫y” but the one in hydrogen was apparently unaffected. The animal in nitrous oxide was removed and eventually was found to survive. The one in hydrogen suffered “very little” after about 45 min and after an hour was removed and was fine. The poor animals were then, after “some hours”, swapped over into the other gases and unfortunately, both died, and then were dissected. Davy concluded that nitrous oxide killed amphibians faster than either hydrogen or immersion in water (they were fully submerged in previously boiled water), and concluded that it was not just because they were deprived of air. He then laments that, because of the season, he cannot obtain frogs and toads, and says, “This I regret very much”.
Section VIII of this research focused on fish. He used what he called thornbacks, which, in a footnote, he says is a common fish in England, similar to a minnow. Thornback C was introduced to hydrogen in boiled water, whilst another (D) was treated with nitrous oxide. Fish C died after thirteen minutes. D survived fifteen minutes of treatment and recovered in normal water. A repeat of this experiment with smaller thornbacks gave a similar result. He concluded that the fish did not die even though there was a lack of air, but because of the positive effect of nitrous oxide. Having done his fish experiments, he turned to insects (section XI). Butterflies died in nitrous oxide and hydrogen, whilst a large fly was made senseless in about 15 s in hydrogen, one assumes in the absence of oxygen. Hydrocarbonate rendered a fly senseless immediately, but it did recover once removed. Davy suggested that air was trapped in the hairs near the respiratory holes of the insects, sufficient for them to survive for a short period of time. Nitrous oxide was then shown to be more harmful to snails and earthworms than water or hydrogen.
In the next Division of his work (II), Davy wanted to explore the effects of gases on blood. He collected blood by making “a large orifice” in the vein of “a tolerably healthy man…”. Using his apparatus, he could estimate how much gas was absorbed by the blood. He concluded that nitrous oxide was absorbed but that a minute amount of carbonic acid, and probably nitrogen, was produced. A little later in the book he repeats the experiments with a range of gases, including oxygen, nitrogen, carbonic acid gas, and hydrocarbonate, but interestingly, hydrogen is not listed here (page 380). Additionally, then on page 384, he states:
“Venous blood, after agitation with hydrogene or nitrogene, oxygenates when expo∫ed to the atmo∫phere in the ∫ame manner as ∫imple venous blood. I had the curio∫ity to try whether venous blood expo∫ed to hydrogene, would retain its power of being oxygenated longer than blood ∫aturated with nitrous oxide…”
He was curious to understand some of the same questions we are still asking today, such as what hydrogen gas may do and how it may work [23]. However, Davy left his blood samples at room temperature (56° to 63° [F]) for three days before inspecting them, so it is hard to glean any useful information here. To look at putrefaction of the blood it was left for two weeks, either with hydrocarbonate, hydrogen, air, or nitrous oxide. The blood treated with hydrogen or air were “both black, and ∫tunk very much”. The sample in hydrocarbonate was “perfectly ∫weet” whilst that in nitrous oxide had “no di∫agreeable ∫mell”. In a subsequent experiment, hydrocarbonate kept the blood sweet for three weeks, and he noted that the ability of hydrocarbonate to preserve blood had previously been noted by Mr. Watt.
In the next set of experiments, Davy was exploring what would happen when gases were breathed in. This work was mainly focused on the effects of nitrous oxide, being the focus of the book, but there are occasions where hydrogen was used too. For example, on page 398:
“I attempted to in∫pire nitrous oxide, after having made two in∫pirations and a complete expiration of hydrogene; but in this experiment the effects of the hydrogene were ∫o debilitating, and the con∫equent ∫timulation by the nitrous oxide ∫o great, as to deprive me of ∫en∫e. After the ∫ir∫t three in∫pirations, I lo∫t all power of standing,, and fell on my back, carrying in my lips the mouth-piece ∫eparated from the cylinder, to the great alarm of Mr. Patrick Dwyer, who was noting the periods of in∫piration”.
It is a wonder he did not kill himself with such self-experimentation. He was only 50 when he died, so one cannot help but wonder if these experiments did have an effect. He carried on and used hydrogen inspiration to try to work out the source of carbonic acid (carbon dioxide, which, when dissolved in water, forms carbonic acid) in his breath. He said that he could not solve this problem, and that breathing hydrogen did not alter his breath capacity, that on breathing pure hydrogen “little or no alteration in volume took place”. However, the expired gas was mixed with nitrogen, oxygen, and carbonic acid. This would have been from the residual gas in his lungs. He said: “… hydrogene was not ab∫orbed or altered when re∫pired; but only mingled with the re∫idual ga∫es of the lungs”.
The next section (IV) on page 400 was devoted to the “Re∫piration of Hydrogene”. He obtained the hydrogen from the decomposition of water, using clean iron filings and diluted sulphuric acid. He then used his mercurial airholder to breathe in the gas he had created, which was the same apparatus that he had used in the previous nitrous oxide experiments. However, after the expiration of his lungs, he found it difficult to breathe in the hydrogen for more than about 30 s, as it “produced unea∫y feelings in the che∫t, momentary lo∫s of mu∫cular power, and ∫ometimes a tran∫ient giddine∫s”.
He says that the giddiness he felt stopped him from making conclusive deductions but did explain how he ensured the purity of the hydrogen before he breathed it in. This would exclude the presence of oxygen, and no doubt the effects seen were oxygen deficiency, rather the effects of the hydrogen per se. His ill effects did not put him off. On page 402 he breathed in 102 cubic inches of what he thought was pure hydrogen, at a temperature of 59°, in “∫even quick respirations”. He then examined the expired gases. Interestingly, he suggested that his calculations showed that “24 cubic inches of hydrogen remained in the “organs of re∫piration”. In the next experiment, he breathed in 182 cubic inches of hydrogen (at 61°) over about 30 s, but this time in six long inspirations. This time he calculated that 28.4 cubic inches of hydrogen remained in the lungs. He suggested an improvement to these experiments and then dismissed the idea. Instead, he breathed in two deep inspirations of 141 cubic inches of hydrogen. From this series of experiments, Davy calculated the exhausted capacity of his lungs (about 41 cubic inches), the total capacity, and the composition of expired air. As nitrous oxide was the focus of his paper, he then repeated the breathing experiments with this gas, comparing the data he obtained from his hydrogen experiments. He was particularly interested in whether or not the nitrous oxide was disintegrated by the blood, and could account for changes in nitrogen and oxygen concentrations seen, and where the carbonic acid came from. On page 424, he writes that he mixed nitrous oxide with hydrogen (4:3), but concluded very little. However, on the next page, he stated that “Nitrous oxide can be re∫pired without danger by the human animal for a much longer time that required for the death of the ∫maller quadrupeds in it”. Hence, he was able to convince many people that it was safe to breathe in. On page 426, he gives some excellent advice for those wishing to use it. “I am inclined from two or three experiments to believe that nitrous oxide is a∫sorbed more rapidly after hearty meals or during ∫timulation from wine or ∫pirits, than at other times”. As nitrous oxide is commonly used at parties in the 21st century [24], this sounds similar to apposite advice.
Section VI on page 429 looks at the respiration of air. Here, he acknowledges Lavoisier, Goodwyn, and Priestley. Section VII concentrated on the respiration of oxygen. He concluded this section by pondering why pure oxygen is incapable of supporting life. In the next section he talks about Mr. Cigna, and some of the data he obtains is compared to what he had earlier found using hydrogen. However, in these sections, there appears to be no mention of Cavallo, even though Cavallo’s paper was two years earlier, as mentioned above.
Research IV looked at the effects of nitrous oxide on individuals. Here, he starts by citing a little of the history of the topic. He says that Dr. Mitchill’s Theory of Contagion (old germ theory) pointed him to Priestley’s work, in March 1798. This was the year of Cavallo’s paper. Davy complains about not being able to produce nitrous oxide in sufficient quantities, and how he then communicated this to Beddoes. He talks about being at the Medical Pneumatic Institution in 1799, but in March he prepared large amounts of impure nitrous oxide. This did not stop him from breathing it in and complaining of the effects, such as a tendency to faint. Others tried it too, with “∫imilar effects”. By April he had obtained nitrous oxide in a “∫tate of purity”. He then breathed this in, as he could not use animals since they died. He says: “I was aware of the danger of this experiment”. Even so, he then outlined a series of self-experiments he carried out using nitrous oxide. From May to July, he was using the gas three or four times a day for weeks! As he continued to use nitrous oxide, he observed that he became irritable, and “felt more acutely from tri∫ling circum∫tances”. However, he also said that it gave him pain relief, mentioning a headache and tooth issues, for example. He said: “The power of … the gas in removing inten∫e phy∫ical pain”. Here, was evidence that nitrous oxide could be used as an anaesthetic.
Returning to hydrogen, on page 465, in a footnote, Davy points this out: “Pure hydrogene has been often re∫pired by different Philo∫ophers, particularly by Scheele, Fontana, and the adventurous and unfortunate Rofier”. Scheele was the first person to isolate oxygen [11]. Abbé Gasparo Ferdinando Felice Fontana (1730–1805) was well known for his work on venoms, and was thought to be the founder of toxinology [25], but he also played with gases including hydrogen. In 1780 he noted that, if hot coal was quenched with water, an ignitable gas was created [26]. Rofier was probably Jean-François Pilâtre de Rozier. He studied pharmacy in Paris, had an interest in gases, and even invented a breathing apparatus [27]. He became famous because Rozier was one of the people, along with the Marquis d’Arlandes, who was the first to take flight in a hot-air balloon, on 21 November 1783. On 15 June 1785, Pilâtre de Rozier took flight in an odd flying machine, which was a hydrogen balloon above a hot-air balloon, which does not seem the wisest of inventions considering a naked flame is used to heat the air in the lower balloon. It crashed and Rozier was killed, hence Davy describing him as “unfortunate” one assumes. Rozier’s death, along with the other aeronaut, the artist Romains, was even depicted in a famous drawing, as was his original flight. Rozier was famous throughout Europe, and hence Davy would have known about these adventures.
In the next section of his work, Davy describes his experiments breathing in hydrogen. Firstly, he respires four quarts of what he describes as “nearly pure” hydrogen, produced from zinc and muriatic acid (hydrochloric acid). He emptied his lungs and closed his nose, and then breathed it in for nearly a minute. Firstly, there were no effects, but eventually, he felt as though he was suffocating, his pulse quickened and was feeble, and he was told his cheeks became purple. He then repeated this experiment using gas from iron and diluted sulphuric acid. The effects were much larger, he felt giddy, his pulse was affected, and he had to stop before he got to a minute. These symptoms appear to be at odds with the safety of pure H2, which suggest either his H2 was unknowingly contaminated and/or a symptom of hypoxia. These observations should be considered interesting due to the fact that they were carried out, and not used to obtain novel molecular insights into the biological effects of H2.
Next, he turned to other gases, with the third experiment using nitrogen and some carbonic acid (CO2). Amazingly, he then says that Watt and Beddoes had told him of the destructive power of hydrocarbonate, and how it killed animals, but Davy says that he was keen to compare the effects of hydrocarbonate to nitrous oxide, so he breathed the former in, knowing it was dangerous. He did dilute it 3:2 with air, and in a footnote, it says he believed no one had tried such a dilute mixture before. He was giddy, had a headache, and also had a loss of voluntary movement. However, “emboldened by this trial” he turned to pure hydrocarbonate. He nearly lost consciousness and says he recalled articulating “I do not think I ∫hall die”—his italics. He managed to leave the building but collapsed on the grass. As a recovery, he then breathed in a mixture of nitrous oxide and oxygen. He says it was early afternoon, but still complained of weakness as he went to bed that evening, and the next day woke up feeble and hungry. In his explanation of what happened, he says that he took two or three doses of nitric acid on Beddoes’ advice.
The breathing of gases continued, and Davy took carbonic acid, stating he was not aware of the work of Ro∫ier at the time. This continued by the use of nitrous oxide. However, all this breathing of gases started to take its toll, and he returned to Cornwall for a rest, and what he describes as “common excerci∫e, a pure atmos∫pere, luxurious diet and moderate indulgence in wine”. After a month he felt better. However, on his return to Bristol, he immediately started to breathe in nitrous oxide which made him recollect his previous work, so much so that “I called out “what an amazing concatenation of ideas!””—his italics. He continued as he worked and travelled to use nitrous oxide and at one point says that no one else had experimented with it over such a length of time.
Davy, who says that he rarely drank alcohol, then drank a whole bottle of wine and tried to see if nitrous oxide could be used as a hangover cure, but it seems to have had little effect. He then took the experiments a stage further and enclosed himself in an air-tight breathing box, into which gases could be added. This was a design of Watt.
The book continues (Division II of this Research) with the recollection of other people who breathed in nitrous oxide at the Medical Pneumatic Institution, and many of these observations had been previously published by Beddoes. People involved here included Robert Kinglake (his assistant), S.T. Coleridge (assumed to be Samuel Taylor, English poet, as they were friends [28]), Mr Wedgewood (assumed to be Tom Wedgewood who worked with Davy on light-sensitive materials [29]), Henry Wansey (a wool manufacturer) and many others [30]. In his conclusions (Division II) Davy also lists the effects seen by different people. Mentioned, as trying the gas, are Josiah Wedgewood and Joseph Priestley. Lamont-Brown lists many famous “breathers” in his biography of Davy (page 44) [15]. Clearly, Davy had a wide range of friends and famous people already willing to try his new gas, nitrous oxide.
The paper ends with a conclusion section, where he suggests that adding hydrocarbonate or hydrogen to pure nitrous oxide may be beneficial (p557), but then says that his experiments do not support this idea. His conclusion says:
“Pneumatic chemi∫try in its application to medicine, is an art in infancy, weak, almost u∫ele∫s, but apparently po∫∫e∫∫ of capabilities of improvement. To be rendered ∫trong and mature, ∫he mu∫t be nouri∫hed by facts, ∫trengthened by excerci∫e, and cautiou∫ly directed in the application of her powers by rational ∫ceptici∫m”.
Now in the 21st century, 200 years later than Davy, at least for hydrogen, this is exactly what is being sought. However, back in the turn of the 18th/19th centuries, it did not stop a range of famous people from breathing in gases made at the Medical Pneumatic Institution.
However, Davy’s work does not actually finish here. The next section is on the effects of nitrous oxide on vegetation. This also included treating plants with hydrogen and hydrocarbonate. He appears to have used mint and left the roots in water and the leaves in the light. The plant in hydrogen died in less than five days. The one in hydrocarbonate was better than the one in air. Davy published this work as an Appendix and invited others to repeat such experiments, suggesting that if the work on hydrocarbonate could be confirmed it would shed light on the use of manures.
In the notes at the end of the paper, he moans that he could not procure the papers of Dr. Menzie on respiration which would have “∫aved me ∫ome labor”. He notes that there is no evidence that hydrogen is converted by respiration into nitrogen, water, carbonic acid, or oxygen. He continues to say that there is no change to hydrogen by respiration, and tries to account for some data from Scheele on this topic, which Davy says is accountable because of the mixing of gases in the lungs. He then describes yet another experiment where he breathed in hydrogen and noticed that the gas was then not as explosive as it was before.
Towards the end of the book is a description of the apparatus, and it refers to Figures, which did not seem to have been reproduced in the copy we read. However, a reproduction of the figures is available [31], and the annotation can be found in Davy’s book on page 573. Furthermore, in 1968, at the University of Leeds, a replica was made and a picture of it can be found online [32].
The book finally ends with an observation by Beddoes on accidental observations in medicine and completes a work of 580 pages.
4. What Preceded the Work of Cavallo and Davy?
Although the focus here is on the papers of Cavallo and Davy, they were not the first to publish about the medical effects of hydrogen. That honour probably should be given to Thomas Beddoes. In the summer (June-July) of 1793, he wrote a public letter to Erasmus Darwin [33], the grandfather of the founder of evolution as we know it, Charles Darwin—On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life: 1859. Beddoes was born on the 13 April 1760 in Shropshire, England, but as discussed, he set up his base in Bristol, having left Pembroke College, Oxford, in 1792. In his letter to Darwin [33], Beddoes suggests that a remedy for consumption (pulmonary tuberculosis) was to lower the amount of oxygen breathed in by the addition of hydrogen and carbon dioxide [34]. It appears that Beddoes and James Watt had much correspondence. Watt knew, as we discussed above, the danger of hydrocarbonate, so must have done his own experimenting on what he called “inflammable air”. In July 1795, Watt wrote to Beddoes with plans for an apparatus to produce and collect various gases which could be used for medical purposes, suggesting at the same time that these could be improved upon by a younger man. Therefore, this sort of work had progressed quite well before Cavallo wrote his paper, or even before Davy moved to Bristol under Beddoes’s supervision.
A closer look at the 1793 paper from Beddoes [33], on page 10 he talks about an acquaintance, Dr. Crump from Shropshire (a surgeon), and then says: “The air he in∫pired was partly hydrogene air. Additionally, it ∫eems pretty well a∫certained that a∫phyxia is le∫s ∫suddenly produced by hydrogene, than by any other ∫pecies of air, unfit for re∫piration”. He later says he wishes to use animals as his subjects and then transfer the information he finds to humans, and then ponders if divers would be better breathing air of a higher standard, or even pure oxygen. His pondering drifts to the French diet, and whether this deprives people of oxygen, and says this might account for the lower levels of consumption (tuberculosis) there. The letter continues with a description of the disease and some remedies which had been tried, and at one point says that one person tried the waters and air of Bristol “without advantage”.—the spring water there being used for medication and one assumes that is what he meant. On page 36, he has an account of a consumption case, and although it is not clear what was given to the patient, in the footnote it is noted that the hydrogen is produced either using iron or charcoal, so it was not always clear what the gas was—either hydrogen or hydrocarbonate one assumes. On page 39 he says: “
“… in which I had even ventured to ∫ugge∫t that it would be more advantageous to lower the atmo∫pheric air with hydrogene than with azotic air, because the hydrogene has be ob∫erved in experiments made upon blood out of the body “to have the power of darkening its colour” ”(Observations p. 141)
Here, Beddoes shows that in vitro experiments with blood and hydrogen had already been undertaken, and also refers to a previous publication. He goes on to say that this gas, i.e., hydrogen, is absorbed more by the blood and possesses no irritating qualities.
On page 40, Beddoes talks about needing to acquire a good apparatus as he wanted to use several gases, including oxygen and hydrogen, so he could give these in sufficient quantities to his patients. He also wanted to test this on more diseases, and also test mixtures of the gases, either together or with air. He clearly had ambitions in this direction well before Davy arrives in 1799.
Interestingly on page 44, Beddoes says: “OF THE THREE AIRS [his caps] above mentioned, I hope the mo∫t from the employment of hydrogene to reduce the air of the atmo∫phere to a lower ∫tandard”—which is what he wanted as he thought lower oxygen was the key for his consumption (tuberculosis) cure. He goes on to say that it appeared to be “totally free from all irritating and po∫itively deleterious properties …” and that it may be breathed “unmixed with impunity” for longer than other gases that could not support life. He says that “Mr Scheele” (first person to isolate oxygen [11]) reported that he could take twenty inspirations with no problems. He then says Pilatre de Rozier (discussed above) frequently repeated the same experiments. Rozier apparently breathed in the hydrogen, and then lit it so this breath was a flame. Beddoes also worked with other aeronauts too and says that he took the pulse of Mr. Sadler while he breathed in hydrogen. His pulse increased and then became weak. However, a young woman breathed hydrogen for two minutes without effect, until she had to walk down a flight of stairs, at which point she felt sick. She recovered after about two hours. These observations continued, with others having no effects, although it is noted that their noses were not closed so could still breathe in some air. Interestingly, at this point, he says that animals immersed in hydrogen are made as deleterious as in carbonic acid, and then quotes Priestley (Priestly’s Exp. New Ed. I. 229).
On page 48 Beddoes writes that now that his apparatus was working, he used hydrogen in one pulmonic case, and he was satisfied with the result. The gas he used was air which comprised about one-eighth (12.5%) hydrogen, and was breathed in for 15 min. This practice was done several times for three days, after which the woman’s symptoms disappeared. Beddoes then says that he thought that this treatment would be useful for inflammation.
On the negative side, on page 67 Beddoes reports in a P.S. about what he had been told by Dr. Wood of Newcastle. He says that typhus is produced by—and then he gives a quote which reads—“the acumulation or over-proportion of carbone and hydrogene …”. Here, it is suggested that hydrogen is causing a medical issue, but Beddoes dismisses this and says that the fever would not last long enough for this to happen, and that oxygen is the active agent.
Beddoes’ letter ends on page 72 with a list of other publications from the same publisher. The copy we read is held at the University of Bristol but is digitised here [33].
However, similar to Cavallo and Davy, Beddoes was building on the recent work of others. Probably the first, or at least one of the first, experiments on the effects of hydrogen on biological systems was carried out by Antoine-Laurent Lavoisier. In 1785, he gave a lecture to the Society of Medicine, in which he discussed his work on birds and guinea pigs which were placed in oxygen. In subsequent experiments, he replaced the nitrogen in air with hydrogen [35]. These experiments were carried out with his assistant Armand Jean François Séguin (Lavoisier used Séguin as a test subject in some of his experiments), and no adverse effects of the hydrogen were found [36], and were carried out in 1789. Certainly there was work being carried out at Laboratoire du Arsenal by the likes of Lavoisier and Seguin [37]. Lavoisier did much work on the production of hydrogen and even its use in hydrogen balloons [38]. However, it is very hard to be sure exactly who the first person was that worked with hydrogen and living organisms, as so many famous and prolific scientists were involved, including Priestley, Scheele, Watt, and Lavoisier. Taking the discovery of oxygen as an example, it has never been firmly established who should be given the credit [11].
It can clearly be seen here that neither Cavallo nor Davy were the first to do this type of experiment with hydrogen. They perhaps wrote the most extensive treatises on the topic at the time, but their work was preceded by the exploits of several others, including those that worked in oxygen, such as Lavoisier and Scheele. Others were playing too, such as Rozier, making his breath into flames. Clearly, there was a great interest in gases such as hydrogen and the time was right for Cavallo and Davy to take up the gauntlet.
5. Some Thoughts on the Work of Beddoes, Cavallo, and Davy
As mentioned above, the papers by Beddoes, Cavallo, and Davy are all in rambling and loose language and are hard to follow at times. It is not always obvious what they meant or did, and, because much of it is written chronologically, the text tends to feel rather jumbled by modern standards. However, what comes across is that they were far-reaching in their exploits, with experiments on a range of animals, humans, and plants. They were also not afraid to give these gases, often not exactly knowing what was in them, to other people, be that patients or friends. Additionally, they were quite happy to breathe the gases in themselves, even when they knew that they were dangerous. It appeared that Davy nearly killed himself on more than one occasion. However, it was a time of the adventurer, as exemplified by the balloon crash and death of Rozier [27].
In that vein, many of the inhalation experiments were carried out “∫tanding over mercury”, etc. and it is hard to know exactly what he did. Regarding the breathing apparatus, as discussed above, Davy talks about it being a “mercurial airholder” (Davy p391) with a small trough of mercury. It is known that mercury can cause madness [39]. For example, it has been suggested that the use of mercury as bearings under the lights in lighthouses might have contributed to the occasional madness of the keepers [40], and here it is tempting to suggest that the high use of mercury may have led to some laissez-faire attitudes. It is amazing that they did not become extremely ill, although the effects of mercury itself are ignored in these early works, no doubt because of an ignorance of the toxicity of this metal. It is possible that some of the effects noted were due to Hg contamination, but in hindsight, this can never be determined. It is interesting to note that Davy took a holiday back to Cornwall as he was not feeling well, which, considering what he was breathing in on a regular basis, is not a surprise.
It should be remembered what all these researchers were doing. Beddoes was promoting the Institute in Bristol, for example, and reporting on negative effects, or even deaths, may not have been in their best interests. It is impossible to say, but it may be that they were putting the best observations into these books.
Even with these caveats, it is not difficult to see that these scientists were pushing the boundaries of our understanding of the biological effects of gases. Hydrogen was not their focus but was used quite extensively. Why? Perhaps, because it was one of the gases recently discovered and of interest? Or on the off chance that hydrogen would be useful? However, what we do know, is that the whole area of exploration went rather quiet, at least for hydrogen. A look at literature databases such as PubMed or Google Scholar shows decades of little or no interest. However, we are now in a period of quite intense interest in hydrogen as a medical treatment. So, what happened?
6. The Modern Renaissance
In 1975 Malcolm M. Dole, F. Ray Wilson, and William P. Fife published a paper in Science entitled Hyperbaric Hydrogen Therapy: A Possible Treatment for Cancer [41]. They used albino mice that had squamous cell carcinomas. Using a gas mix of 2.5 oxygen and 97.5 hydrogen (at eight atmospheres for up to 2 weeks), they showed a significant reduction of the tumors. They described hydrogen as a “free radical decay catalyzer” and suggested that hyperbaric hydrogen may be considered as a cancer treatment. According to a PubMed search, this paper was not well cited until 2010, but since then there has been what appears to be an exponential increase in interest.
A very pertinent paper was published in 2007, which really sparked a new interest in this area. The paper was by Ohsawa et al. [2] and was published in Nature Medicine. They reported that hydrogen had neuroprotective effects in a rat model of cerebral infarction. They also suggested that hydrogen was acting as a selective antioxidant by reducing only hydroxyl radicals, but not other reactive oxygen species (ROS), which are known to have important effects on cells. They mooted that this was the action of hydrogen gas and as such this molecular mechanism might account for the physiological effects seen. Understandably, this paper attracted significant interest and did much to ignite further work on hydrogen and biological systems.
A search today (November 2022) sees that there is already a flurry of papers with 2023 publication dates. One calls hydrogen a “machine gun”, rather than similar to conventional drugs, described as a “rifle gun” [42]. International conferences in the biological sciences fields are now organized with hydrogen as the focus [43], and Special Issues of journals are dedicated to it [44]. What Cavallo and Davy started two hundred years ago is now a burgeoning field of research.
7. Conclusions
This is not the first paper that points out that people in the 1700s were working on a range of gases, including hydrogen [45]. However, the focus here is on hydrogen and gases which may contain hydrogen, and in particular two early papers, both published about work carried out at the end of the 18th Century. The authors, Cavallo and Davy, were brave researchers and their work was far-reaching.
A look at a simple overview (Figure 1) and at a timeline of the work on hydrogen in biological systems shows that there is a long history of people thinking about hydrogen, and a multitude of other gases and their effects on organisms including humans. The early work by Beddoes et al. appeared to go quiescent for a long time. In the 1940s, it was realised that hydrogen was useful as a gas for breathing whilst deepsea diving [46], but this was a long time after the early papers. Dole et al. (1975) [41] and then significantly Ohsawa et al. (2007) [2] resurrected the idea, and now there is a large resurgence of interest.
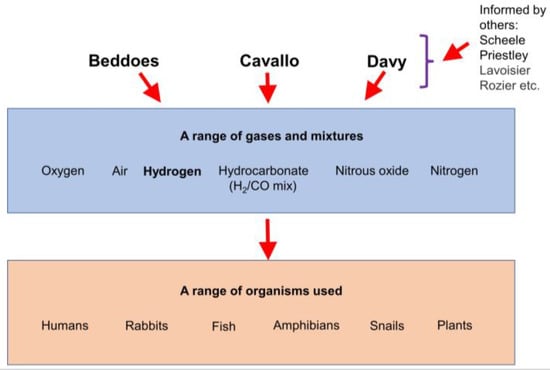
Figure 1.
A schematic giving an overview of the work that was carried out by Beddoes, Cavallo, and Davy.
We often look at modern and recent literature and believe that what we are reading is novel and inventive. However, this is often not the case. If we look at the history of hydrogen, it is not only the chemistry and physics that researchers have been investigating since its discovery, but also its possible use in medicine. Today we cite papers that say things such as “proposed the possibility of developing Hydrogen Biology and Hydrogen Medicine as new disciplines of biology and medicine” [47], but we ought to remember that over two hundred years ago scientists were having similar thoughts. Obviously, not identical ones, but searching to see if gases, including H2, have any medical use. Three of these people were Thomas Beddoes, Tiberius Cavallo, and Humphry Davy, each exploring the use of gases in medical treatments. Each was interested in how such gases interact with biological systems. Davy characterised nitrous oxide (laughing gas), which is widely used today [48]. These scientists were not single-minded either. Cavallo wrote treatises on electricity and magnetism as well as worrying about red blood cells and the function of the lungs. Davy discovered several elements and invented the mining safety lamp (the Davy Lamp), which saved thousands of lives. However, here we are reminded that these scientists also were foresighted enough to point out that several gases, including hydrogen or gas containing hydrogen, may have beneficial effects in a range of human disorders, including those that involve inflammation. Hydrogen has a bright future in medical research, and it is certain that modern thoughts and methods have been instrumental in bringing this to the attention of the medical industry, but it is humbling to remember that people such as Beddoes, Cavallo, and Davy were doing this over two hundred years ago.
Author Contributions
J.T.H. conceived this paper and wrote the first draft. T.W.L. edited the work and added his thoughts, including Table 1. All authors have read and agreed to the published version of the manuscript.
Funding
No specific funding was used for this paper. We acknowledge the support of the University of the West of England, Bristol, who supported JTH’s time and access to literature.
Acknowledgments
We would like to thank Grace Russell for her thoughts and conversations on the history of hydrogen.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A novel option in human disease treatment. Oxid. Med. Cell. Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Russell, G.; Hancock, J.T. Molecular hydrogen in agriculture. Planta 2021, 254, 56. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh Thi, T.; Akter, R.; Goh, S.H.; Kim, C.S.; Lee, K.J. Redox effects of molecular hydrogen and its therapeutic efficacy in the treatment of neurodegenerative diseases. Processes 2021, 9, 308. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Lian, N.; Wang, Y.; Zheng, W.; Xie, K. Molecular hydrogen: A promising adjunctive strategy for the treatment of the COVID-19. Front. Med. 2021, 8, 671215. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Molecular hydrogen in sports medicine: New therapeutic perspectives. Int. J. Sport. Med. 2015, 36, 273–279. [Google Scholar] [CrossRef]
- Wang, C.; Fang, H.; Gong, T.; Zhang, J.; Niu, L.; Huang, D.; Huo, J.; Liao, W. Hydrogen gas alleviates postharvest senescence of cut rose ‘Movie star’ by antagonizing ethylene. Plant Mol. Biol. 2020, 102, 271–285. [Google Scholar] [CrossRef]
- Holmes, B.; Castro, N.J.; Li, J.; Keidar, M.; Zhang, L.G. Enhanced human bone marrow mesenchymal stem cell functions in novel 3D cartilage scaffolds with hydrogen treated multi-walled carbon nanotubes. Nanotechnology 2013, 24, 365102. [Google Scholar] [CrossRef]
- Royal Society of Chemistry. Hydrogen. Available online: https://www.rsc.org/periodic-table/element/1/hydrogen (accessed on 25 November 2022).
- Dobbin, L. Paracelsus and the discovery of hydrogen. J. Chem. Educ. 1932, 9, 1122. [Google Scholar] [CrossRef]
- Hancock, J.T. A brief history of oxygen: 250 years on. Oxygen 2022, 2, 31–39. [Google Scholar] [CrossRef]
- Cavallo, T. An Essay on the Medicinal Properties of Factitious Airs. With an Appendix, on the Nature of Blood; Dilly: London, UK, 1798; pp. 1–256. Available online: https://books.google.co.uk/books?id=tr1XAAAAYAAJ&printsec=frontcover&source=gbs_ge_summary_r&redir_esc=y#v=onepage&q&f=false (accessed on 25 November 2022).
- Davy, H. Researches, Chemical and Philosophical; Chiefly Concerning Nitrous Oxide, or Dephlogisticated Nitrous Air, and Its Respiration; J. Johnson: London, UK, 1800; Available online: https://books.google.sk/books?hl=en&lr=&id=jhUAAAAAQAAJ&oi=fnd&pg=PR11&ots=KnQilzGcyW&sig=gWIizR8SIxrO-qPlGdT0tMlw8pI&redir_esc=y#v=onepage&q&f=false (accessed on 25 November 2022).
- National Air and Space Museum. Tiberius Cavallo, F.R.S. Available online: https://airandspace.si.edu/collection-objects/tiberius-cavallo-frs/nasm_A20140626000 (accessed on 25 November 2022).
- Lamont-Brown, R. Humphry Davy: Life beyond the Lamp; Sutton Publishing Limited: Gloucestershire, UK, 2004; ISBN 0750932317. [Google Scholar]
- Live Science. Available online: https://www.livescience.com/65560-long-s-old-texts.html (accessed on 20 November 2022).
- Beddoes, T. Considerations on the Medicinal Use, and on the Production of Factitious Airs: Part I. By Thomas Beddoes, MD Part II. By James Watt, Engineer; Bulgin and Rosser: Bristol, England, 1796. [Google Scholar]
- Long, F.J.; Sykes, K.W. The mechanism of the steam-carbon reaction. Proc. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1948, 193, 377–399. [Google Scholar]
- Hancock, J.T.; Russell, G.; Stratakos, A.C. Molecular hydrogen: The postharvest use in fruits, vegetables and the floriculture industry. Appl. Sci. 2022, 12, 10448. [Google Scholar] [CrossRef]
- Davy, H. The Bakerian Lecture, On Some Chemical Agencies of Electricity. In The Collected Works of Sir Humphry Davy; Smith, Elder and Co.: London, UK, 1806; Available online: http://knarf.english.upenn.edu/Davy/davy5.html#1 (accessed on 27 November 2022).
- National Portrait Gallery. Probably Cornelis Rudolphus Theodorus Krayenhoff, Formerly Known as Tiberius Cavallo. Available online: https://www.npg.org.uk/collections/search/portraitExtended/mw01153/Probably-Cornelis-Rudolphus-Theodorus-Krayenhoff-formerly-known-as-Tiberius-Cavallo (accessed on 27 November 2022).
- Penders, J.; Kissner, R.; Koppenol, W.H. ONOOH does not react with H2: Potential beneficial effects of H2 as an antioxidant by selective reaction with hydroxyl radicals and peroxynitrite. Free Radic. Biol. Med. 2014, 75, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Russell, G.; Craig, T.J.; May, J.; Morse, H.R.; Stamler, J.S. Understanding Hydrogen: Lessons to Be Learned from Physical Interactions between the Inert Gases and the Globin Superfamily. Oxygen 2022, 2, 578–590. [Google Scholar] [CrossRef]
- Guardian: ‘Everyone’s Gagging for It’—How Britain Got High on Nitrous Oxide. Available online: https://www.theguardian.com/society/2021/nov/27/how-britain-got-high-on-nitrous-oxide-laughing-gas (accessed on 13 December 2022).
- Hawgood, B.J. Abbé Felice Fontana (1730–1805): Founder of modern toxinology. Toxicon 1995, 33, 591–601. [Google Scholar] [CrossRef]
- Van Heek, K.H. Technical status and new developments of coal gasification processes. In Carbon and Coal Gasification; Springer: Dordrecht, The Netherlands, 1986; pp. 403–420. [Google Scholar]
- Lynn, M.R. Modern-Day Icarus: Jean-François Pilâtre de Rozier. The Ultimate History Project. Available online: http://ultimatehistoryproject.com/jean-francois-pilatre-de-rozier-modern-day-icarus.html (accessed on 25 November 2022).
- James, F.A.; Ruston, S. New Studies on Humphry Davy: Introduction. Ambix 2019, 66, 95–102. [Google Scholar] [CrossRef]
- Batchen, G. Tom Wedgwood and Humphry Davy: ‘An account of a method’. Hist. Photogr. 1993, 17, 172–183. [Google Scholar] [CrossRef]
- The Guardian, ‘Oh Excellent Air Bag!’ Review—Two Centuries of Laughing Gas. Available online: https://www.theguardian.com/books/2016/sep/28/oh-excellent-air-bag-review (accessed on 25 November 2022).
- PFT History. Sir Humphry Davy’s Mercurial Air Holder, 1800. Available online: https://www.pftforum.com/history/sir-humphy-davys-mercury-air-holder-1800/ (accessed on 25 November 2022).
- Science Museum Group. Replica of Clayfield’s Mercurial Holder, Leeds, England, 1968. Available online: https://collection.sciencemuseumgroup.org.uk/objects/co114802/replica-of-clayfields-mercurial-holder-leeds-england-1968-mercurial-air-holder (accessed on 25 November 2022).
- Beddoes, T. A Letter to Erasmus Darwin, M.D. On a New Method of Treating Pulmonary Consumption, and Some Other Diseases Hitherto Found Incurable. Available online: https://wellcomecollection.org/works/a43j52a9 (accessed on 2 December 2022).
- Cartwright, F.F. The association of Thomas Beddoes, MD with James Watt, FR S. Notes Rec. R. Soc. Lond. 1967, 22, 131–143. [Google Scholar]
- Hartley, H.B. Antoine Laurent Lavoisier 26 August 1743–8 May 1794. Proc. Roy. Soc. 1947, 189A, 427–456. [Google Scholar]
- Sun, Q.; Han, W.; Nakao, A. Biological Safety of Hydrogen. In Hydrogen Molecular Biology and Medicine; Springer: Dordrecht, The Netherlands, 2015; pp. 35–48. [Google Scholar]
- Beretta, M.; Brenni, P. Lavoisier’s Sites of Experimental Practice: From the Field to the Laboratory (1764–1794). In The Arsenal of Eighteenth-Century Chemistry; Brill: Leiden, The Netherlands, 2022; pp. 50–72. [Google Scholar]
- Gyung Kim, M. ‘Public’Science: Hydrogen balloons and Lavoisier’s decomposition of water. Ann. Sci. 2006, 63, 291–318. [Google Scholar] [CrossRef]
- McNutt, M. Mercury and health. Science 2013, 341, 1430. [Google Scholar] [CrossRef] [PubMed]
- Van Netten, C.; Teschke, K.E. Assessment of mercury presence and exposure in a lighthouse with a mercury drive system. Environ. Res. 1988, 45, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.I.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Conventional drug acts as a “rifle gun” while hydrogen as a “machine gun”. Med. Gas Res. 2023, 13, 89. [Google Scholar] [CrossRef]
- European Academy for Molecular Hydrogen Research in Biomedicine. Available online: https://euh2academy.org/1st-international-conference/general-information/ (accessed on 25 November 2022).
- Hancock, J.T. (Ed.) Production and role of molecular hydrogen in plants. In Plant; MDPI: Beijing, China, 2022; ISSN 2223-7747. [Google Scholar]
- Hopper, C.P.; Zambrana, P.N.; Goebel, U.; Wollborn, J. A brief history of carbon monoxide and its therapeutic origins. Nitric Oxide 2021, 111, 45–63. [Google Scholar] [CrossRef]
- ZetterstrÖm, A. Deep-sea diving with synthetic gas mixtures. Mil. Surg. 1948, 103, 104–106. [Google Scholar] [CrossRef]
- Du, D.; Zhao, L.; Shen, M.; Noda, M.; Qin, S.; Long, J.; Sun, X.; Liu, J. Hydrogen medicine: A rising star in gas medicine. Tradit. Med. Mod. Med. 2020, 3, 153–161. [Google Scholar] [CrossRef]
- Buhre, W.; Disma, N.; Hendrickx, J.; DeHert, S.; Hollmann, M.W.; Huhn, R.; Jakobsson, J.; Nagele, P.; Peyton, P.; Vutskits, L. European Society of Anaesthesiology Task Force on Nitrous Oxide: A narrative review of its role in clinical practice. Br. J. Anaesth. 2019, 122, 587–604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).