The Pro-Oxidant Effect of Class A CpG ODNs on Human Neutrophils Includes Both Non-Specific Stimulation of ROS Production and Structurally Determined Induction of NO Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Neutrophils Isolation
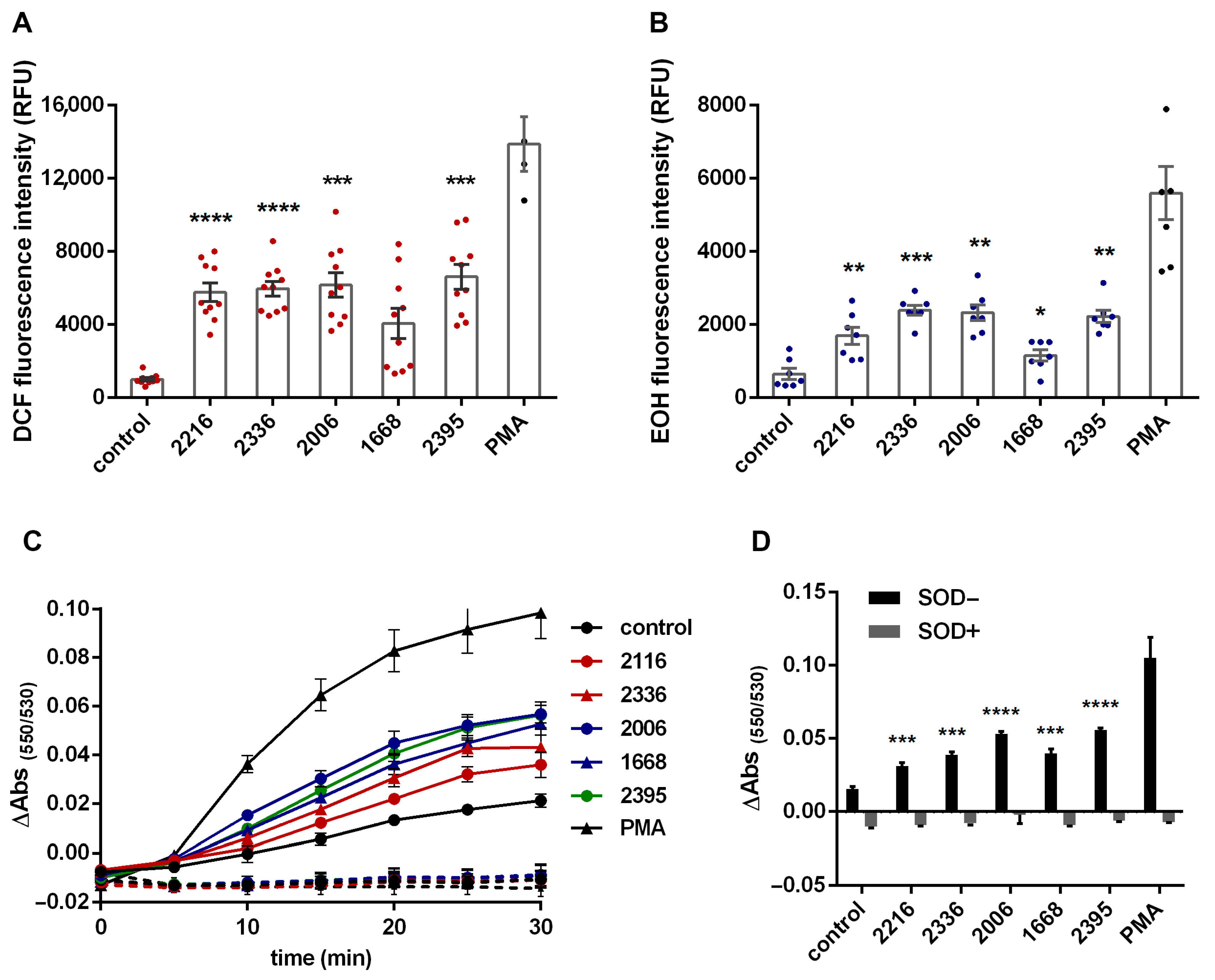
2.2. ROS Detection
2.3. Quantification of Neutrophils Adhesion
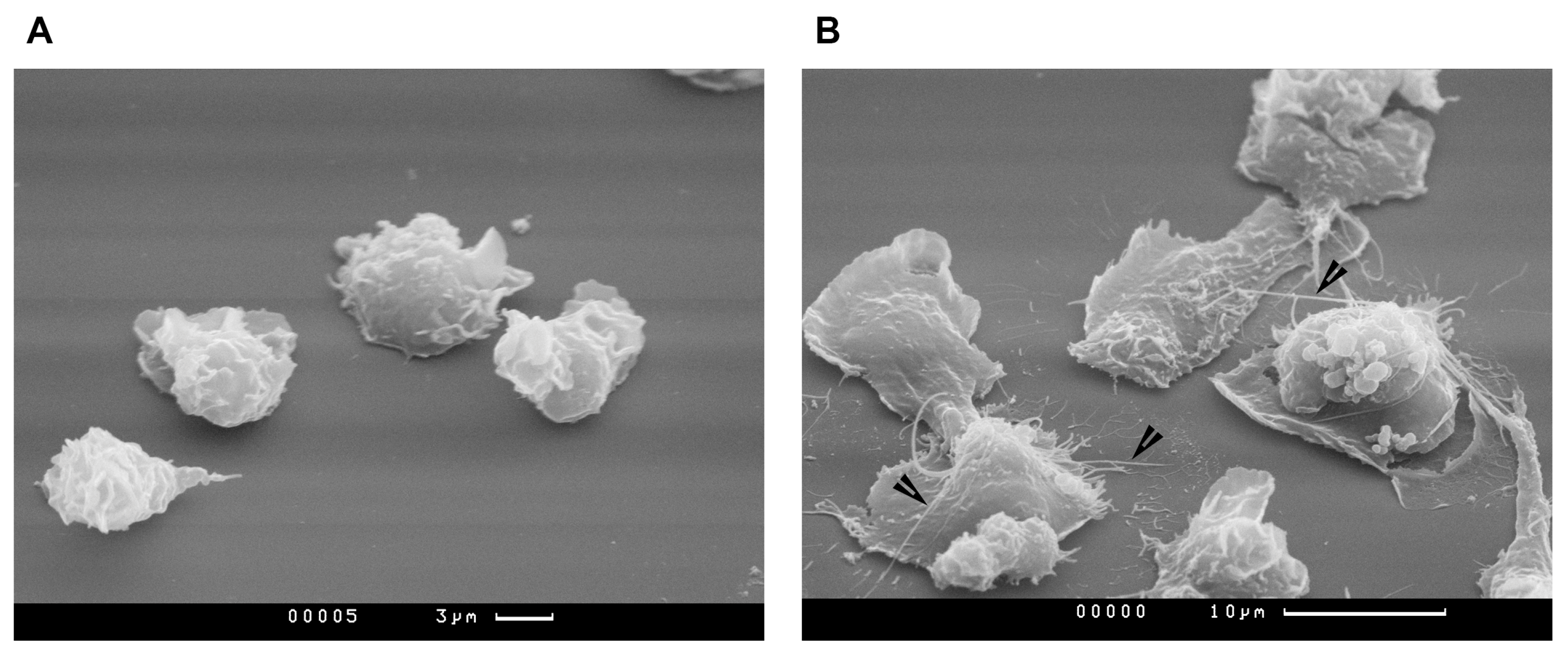
2.4. Study of PMNLs Morphology by Scanning Electron Microscopy
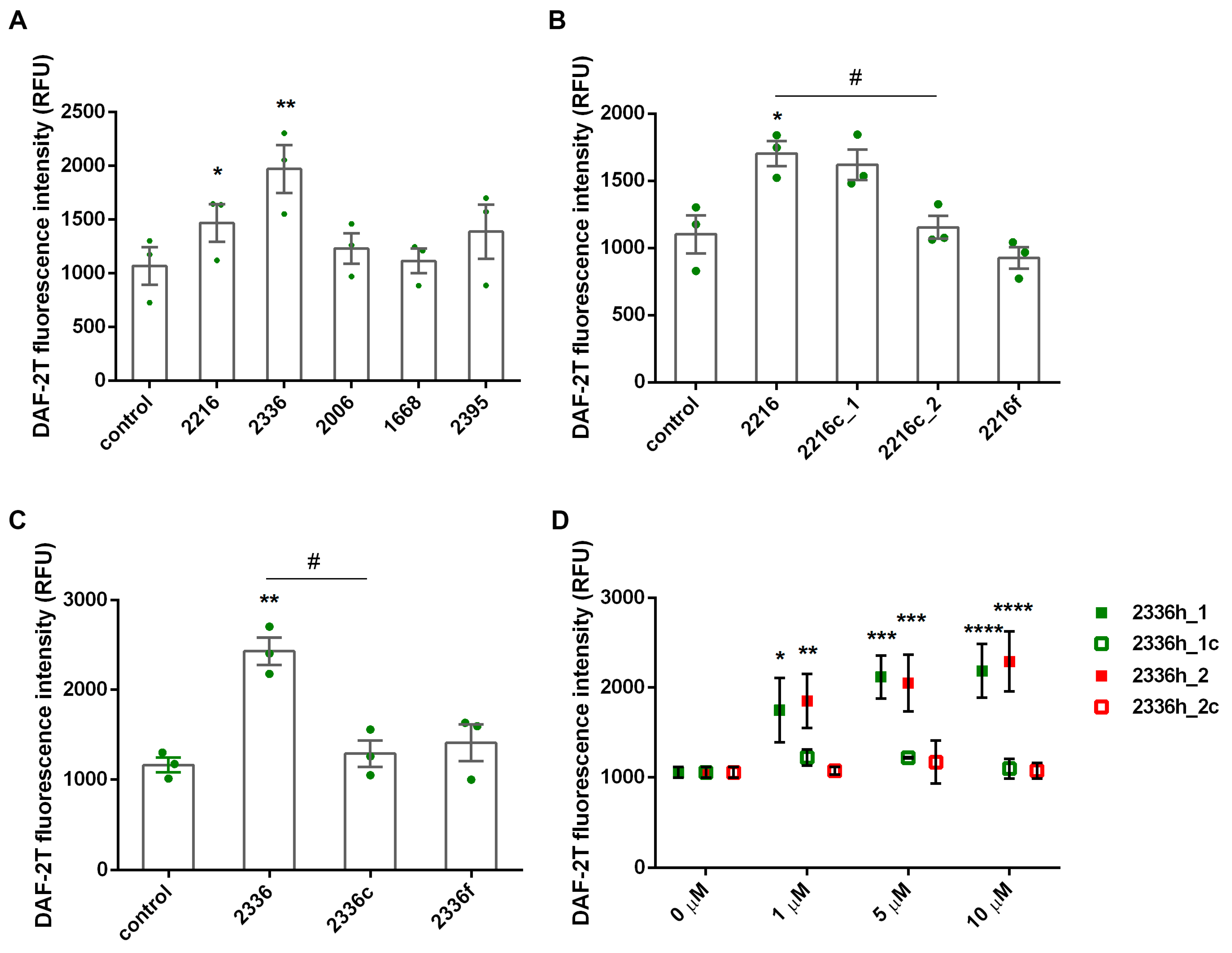
2.5. NO Detection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagner, H. Bacterial CpG DNA Activates Immune Cells to Signal Infectious Danger. Adv. Immunol. 1999, 73, 329–368. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Yamamoto, T.; Kataoka, T.; Kuramoto, E.; Yano, O.; Tokunaga, T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN [correction of INF] and augment IFN-mediated [correction of INF] natural killer activity. J. Immunol. 1992, 148, 4072–4076. [Google Scholar] [CrossRef] [PubMed]
- Krug, A.; Rothenfusser, S.; Hornung, V.; Jahrsdörfer, B.; Blackwell, S.; Ballas, Z.K.; Endres, S.; Krieg, A.M.; Hartmann, G. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur. J. Immunol. 2001, 31, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J. CpG Motifs to Modulate Innate and Adaptive Immune Responses. Int. Rev. Immunol. 2006, 25, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Heeg, K.; Wagner, H.; Lipford, G.B. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology 1999, 97, 699–705. [Google Scholar] [CrossRef]
- Vollmer, J.; Weeratna, R.; Payette, P.; Jurk, M.; Schetter, C.; Laucht, M.; Wader, T.; Tluk, S.; Liu, M.; Davis, H.L.; et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004, 34, 251–262. [Google Scholar] [CrossRef]
- Bauer, S.; Kirschning, C.J.; Häcker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar] [CrossRef]
- Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–259. [Google Scholar] [CrossRef]
- Yuen, A.R.; Sikic, B.I. Clinical studies of antisense therapy in cancer. Front. Biosci. 2000, 5, D588–D593. [Google Scholar] [CrossRef]
- DeLeo, F.; Allen, L.-A.; Apicella, M.; Nauseef, W. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 1999, 163, 6732–6740. [Google Scholar] [CrossRef]
- Roos, D. Chronic granulomatous disease. Br. Med. Bull. 2016, 118, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.P. Evidence of direct toxic effects of free radicals on the myocardium. Free Radic. Biol. Med. 1988, 4, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Görlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017, 13, 94–162. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Viryasova, G.; Golenkina, E.; Hianik, T.; Soshnikova, N.; Dolinnaya, N.; Gaponova, T.; Romanova, Y.; Sud’Ina, G. Magic Peptide: Unique Properties of the LRR11 Peptide in the Activation of Leukotriene Synthesis in Human Neutrophils. Int. J. Mol. Sci. 2021, 22, 2671. [Google Scholar] [CrossRef]
- Golenkina, E.A.; Viryasova, G.; Galkina, S.I.; Arifulin, E.A.; Gaponova, T.V.; Romanova, Y.M.; Sud’Ina, G.F. Synthetic CpG oligonucleotides as potential modulators of neutrophil survival in PAMP-associated inhibition of apoptosis. J. Leukoc. Biol. 2019, 106, 45–55. [Google Scholar] [CrossRef]
- Aleksandrov, D.A.; Zagryagskaya, A.N.; Pushkareva, M.A.; Bachschmid, M.; Peters-Golden, M.; Werz, O.; Steinhilber, D.; Sud’Ina, G.F. Cholesterol and its anionic derivatives inhibit 5-lipoxygenase activation in polymorphonuclear leukocytes and MonoMac6 cells. FEBS J. 2006, 273, 548–557. [Google Scholar] [CrossRef]
- Peshavariya, H.M.; Dusting, G.J.; Selemidis, S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic. Res. 2007, 41, 699–712. [Google Scholar] [CrossRef]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological Defense Mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef]
- Sud’Ina, G.F.; Brock, T.G.; Pushkareva, M.A.; Galkina, S.I.; Turutin, D.V.; Peters-Golden, M.; Ullrich, V. Sulphatides trigger polymorphonuclear granulocyte spreading on collagen-coated surfaces and inhibit subsequent activation of 5-lipoxygenase. Biochem. J. 2001, 359, 621–629. [Google Scholar] [CrossRef]
- Hoffstein, S.T.; Gennaro, D.E.; Manzi, R.M. Neutrophils may directly synthesize both H2O2 and O2? since surface stimuli induce their release in stimulus-specific ratios. Inflammation 1985, 9, 425–437. [Google Scholar] [CrossRef]
- Ngo, T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef]
- Sato, Y.; Roman, M.; Tighe, H.; Lee, D.; Corr, M.; Nguyen, M.-D.; Silverman, G.J.; Lotz, M.; Carson, D.A.; Raz, E. Immunostimulatory DNA Sequences Necessary for Effective Intradermal Gene Immunization. Science 1996, 273, 352–354. [Google Scholar] [CrossRef]
- Galkina, S.I.; Fedorova, N.V.; Golenkina, E.A.; Stadnichuk, V.I.; Sud’Ina, G.F. Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes. Int. J. Mol. Sci. 2020, 21, 586. [Google Scholar] [CrossRef]
- Viryasova, G.M.; Golenkina, E.A.; Galkina, S.I.; Gaponova, T.V.; Romanova, Y.M.; Sud’Ina, G.F. Effects of phosphodiester and phosphorothioate ODN2216 on leukotriene synthesis in human neutrophils and neutrophil apoptosis. Biochimie 2016, 125, 140–149. [Google Scholar] [CrossRef]
- Bonegio, R.G.; Lin, J.D.; Beaudette-Zlatanova, B.; York, M.R.; Menn-Josephy, H.; Yasuda, K. Lupus-Associated Immune Complexes Activate Human Neutrophils in an FcγRIIA-Dependent but TLR-Independent Response. J. Immunol. 2019, 202, 675–683. [Google Scholar] [CrossRef]
- Mortaz, E.; Adcock, I.M.; Ito, K.; Kraneveld, A.D.; Nijkamp, F.P.; Folkerts, G. Cigarette smoke induces CXCL8 production by human neutrophils via activation of TLR9 receptor. Eur. Respir. J. 2009, 36, 1143–1154. [Google Scholar] [CrossRef]
- Zhang, Z.; Weinschenk, T.; Schluesener, H.J. Uptake, intracellular distribution, and novel binding proteins of immunostimulatory CpG oligodeoxynucleotides in microglial cells. J. Neuroimmunol. 2005, 160, 32–40. [Google Scholar] [CrossRef]
- Ohto, U.; Shibata, T.; Tanji, H.; Ishida, H.; Krayukhina, E.; Uchiyama, S.; Miyake, K.; Shimizu, T. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 2015, 520, 702–705. [Google Scholar] [CrossRef]
- Beltinger, C.; Saragovi, H.U.; Smith, R.M.; LeSauteur, L.; Shah, N.; DeDionisio, L.; Christensen, L.; Raible, A.; Jarett, L.; Gewirtz, A.M. Binding, uptake, and intracellular trafficking of phosphorothioate-modified oligodeoxynucleotides. J. Clin. Investig. 1995, 95, 1814–1823. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Li, F.; Fan, S.; Chen, X.; Lu, Y.; Wei, Y.; Chen, Q.; Xia, L.; Tang, J.; et al. Stimulation of the class-A scavenger receptor induces neutrophil extracellular traps (NETs) by ERK dependent NOX2 and ROMO1 activation. Biochem. Biophys. Res. Commun. 2019, 511, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.; Hughes, D.; Gordon, S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature 1993, 364, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Hudik, E.; Le Bars, R.; Roux, B.; Dang, P.M.-C.; El Benna, J.; Nüsse, O.; Dupré-Crochet, S. Class I phosphoinositide 3-kinases control sustained NADPH oxidase activation in adherent neutrophils. Biochem. Pharmacol. 2020, 178, 114088. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, J.-G.; Kim, J.-R.; Baek, S.-H. Toll-like receptor 9-mediated cytosolic phospholipase A2 activation regulates expression of inducible nitric oxide synthase. Biochem. Biophys. Res. Commun. 2007, 364, 996–1001. [Google Scholar] [CrossRef]
- Pudla, M.; Srisatjaluk, R.; Utaisincharoen, P. Induction of inducible nitric oxide synthase (iNOS) in Porphyromonas gingivalis LPS-treated mouse macrophage cell line (RAW264.7) requires Toll-like receptor 9. Inflamm. Res. 2018, 67, 723–726. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta 1999, 1411, 334–350. [Google Scholar] [CrossRef]
- Galkina, S.I.; Golenkina, E.A.; Viryasova, G.; Romanova, Y.M.; Sud’Ina, G.F. Nitric Oxide in Life and Death of Neutrophils. Curr. Med. Chem. 2019, 26, 5764–5780. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef]

| Designation | Sequence (5′-3′) | Secondary Structure * | |
|---|---|---|---|
| 2216 | ggGGGACGATCGTCgggggg ** |  | A class CpG ODN, human TLR9 agonist [3] |
| 2216c_1 | ggGGGAGCATGCTGgggggg |  | |
| 2216c_2 | ggGGCAGCATGCTGgggggg |  | |
| 2216f | TCGTCgggggg |  | |
| 2336 | ggGGACGACGTCGTGgggggg |  | A class CpG ODN, human TLR9 agonist [4] |
| 2336c | ggGGAGCAGCTGCTGgggggg |  | |
| 2336f | TCGTGgggggg |  | |
| 2336h_1 | ACGACGTCGT |  | |
| 2336h_1c | AGCAGCTGCT |  | |
| 2336h_2 | GGACGACGTCGTG |  | |
| 2336h_2c | GGAGCAGCTGCTG |  | |
| 2006 | tcgtcgttttgtcgttttgtcgtt |  | B class CpG ODN, human TLR9 agonist [5] |
| 1668 | tccatgacgttcctgatgct | 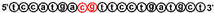 | B class CpG ODN, murine TLR9 agonist [23] |
| 2395 | tcgtcgttttcggcgcgcgccg | 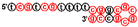 | C class CpG ODN, human TLR9 agonist [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golenkina, E.A.; Galkina, S.I.; Viryasova, G.M.; Sud’ina, G.F. The Pro-Oxidant Effect of Class A CpG ODNs on Human Neutrophils Includes Both Non-Specific Stimulation of ROS Production and Structurally Determined Induction of NO Synthesis. Oxygen 2023, 3, 20-31. https://doi.org/10.3390/oxygen3010002
Golenkina EA, Galkina SI, Viryasova GM, Sud’ina GF. The Pro-Oxidant Effect of Class A CpG ODNs on Human Neutrophils Includes Both Non-Specific Stimulation of ROS Production and Structurally Determined Induction of NO Synthesis. Oxygen. 2023; 3(1):20-31. https://doi.org/10.3390/oxygen3010002
Chicago/Turabian StyleGolenkina, Ekaterina A., Svetlana I. Galkina, Galina M. Viryasova, and Galina F. Sud’ina. 2023. "The Pro-Oxidant Effect of Class A CpG ODNs on Human Neutrophils Includes Both Non-Specific Stimulation of ROS Production and Structurally Determined Induction of NO Synthesis" Oxygen 3, no. 1: 20-31. https://doi.org/10.3390/oxygen3010002
APA StyleGolenkina, E. A., Galkina, S. I., Viryasova, G. M., & Sud’ina, G. F. (2023). The Pro-Oxidant Effect of Class A CpG ODNs on Human Neutrophils Includes Both Non-Specific Stimulation of ROS Production and Structurally Determined Induction of NO Synthesis. Oxygen, 3(1), 20-31. https://doi.org/10.3390/oxygen3010002







