Polyketides from Plakortis Sponges around Caribbean Coastal Regions: Collection, Isolation, Characterization, and Bioactivity
Abstract
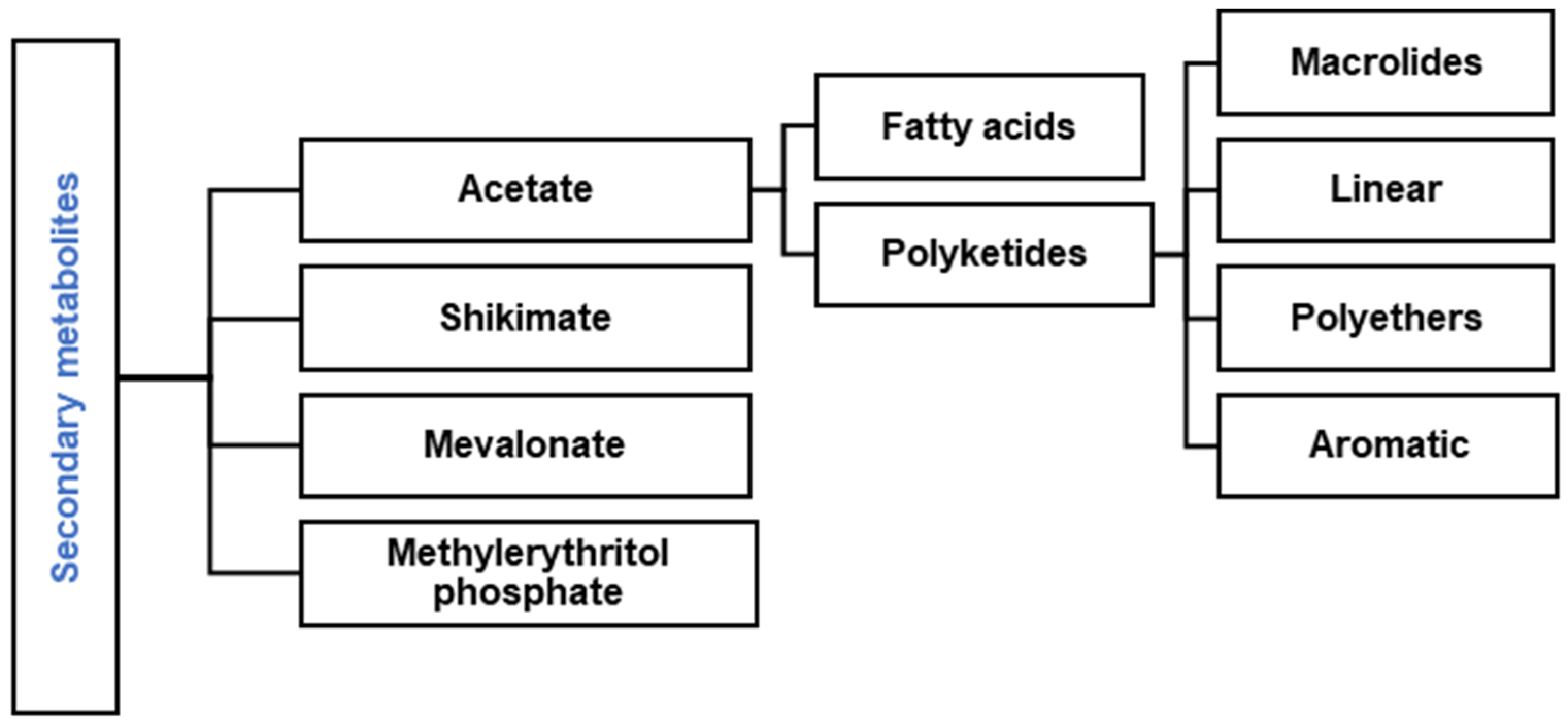
1. Introduction
2. Collection, Extraction, Isolation, Structural Characterization, and Bioactivity
2.1. Coasts of the Bahamas
2.2. Coast of Cayman Islands
2.3. Coast of Belize
2.4. Coasts of Dominica
2.5. Coasts of Jamaica
2.6. Coasts of Martinique
2.7. Coast of Panamá
2.8. Coast of Puerto Rico
2.9. Coast of Tobago Island
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd: Chichester, UK, 2009. [Google Scholar]
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Hanson, J.R. Natural Products: The Secondary Metabolites; Royal Society of Chemistry: Cambridge, UK, 2003; Volume 17, ISBN 0-85404-490-6. [Google Scholar]
- Cooper, R.J. Natural Products Chemistry: Sources, Separations and Structures; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 978-1-138-45357-9. [Google Scholar]
- Gutzeit, H.O.; Ludwig-Müller, J. Plant Natural Products: Synthesis, Biological Functions and Practical Applications; John Wiley & Sons: Weinheim, Germany, 2014; ISBN 3-527-68200-7. [Google Scholar]
- Rodríguez-Berríos, R.R.; Ríos-Delgado, A.M.; Perdomo-Lizardo, A.P.; Cardona-Rivera, A.E.; Vidal-Rosado, Á.G.; Narváez-Lozano, G.A.; Nieves-Quiñones, I.A.; Rodríguez-Vargas, J.A.; Álamo-Diverse, K.Y.; Lebrón-Acosta, N. Extraction, Isolation, Characterization, and Bioactivity of Polypropionates and Related Polyketide Metabolites from the Caribbean Region. Antibiotics 2023, 12, 1087. [Google Scholar] [CrossRef]
- Khosla, C. Structures and Mechanisms of Polyketide Synthases. J. Org. Chem. 2009, 74, 6416–6420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Zhang, W. Natural Polypropionates in 1999–2020: An Overview of Chemical and Biological Diversity. Mar. Drugs 2020, 18, 569. [Google Scholar] [CrossRef]
- Birch, A.J. Biosynthesis of Polyketides and Related Compounds. Science 1967, 156, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, C. The Biosynthetic Logic of Polyketide Diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, B.J. Biosynthesis of Polyketides. Nat. Prod. Rep. 1997, 14, 523. [Google Scholar] [CrossRef]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of Polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Lu, C.; Lin, T.; Hu, P.; Shen, Y. Seven Novel Linear Polyketides from Xylaria Sp. NCY2. Helv. Chim. Acta. 2010, 93, 925–933. [Google Scholar] [CrossRef]
- Shen, B. Polyketide Biosynthesis beyond the Type I, II and III Polyketide Synthase Paradigms. Curr. Opin. Chem. Biol. 2003, 7, 285–295. [Google Scholar] [CrossRef]
- Rohr, J. A New Role for Polyketides. Angew. Chem. Int. Ed. 2000, 39, 2847–2849. [Google Scholar] [CrossRef]
- Weissman, K.J. Chapter 1 Introduction to Polyketide Biosynthesis. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 459, pp. 3–16. ISBN 978-0-12-374591-0. [Google Scholar]
- Koskinen, A.M.; Karisalmi, K. Polyketide Stereotetrads in Natural Products. Chem. Soc. Rev. 2005, 34, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, W.; Kirst, H.A. Macrolide Antibiotics; Springer Science & Business Media: Berlin/Heidelberg, Germany; Birkhauser Verlag: Basel, Switzerland, 2002; ISBN 3-7643-6186-7. [Google Scholar]
- Birch, A.W.; Robinson, J.A. Polyethers. In Genetics and Biochemistry of Antibiotic Production; Elsevier: Amsterdam, The Netherlands, 1995; pp. 443–476. ISBN 978-0-7506-9095-9. [Google Scholar]
- Zotchev, S. Polyene Macrolide Antibiotics and Their Applications in Human Therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Perron, F.; Albizati, K.F. Chemistry of Spiroketals. Chem. Rev. 1989, 89, 1617–1661. [Google Scholar] [CrossRef]
- Han, B.-N.; Hong, L.-L.; Gu, B.-B.; Sun, Y.-T.; Wang, J.; Liu, J.-T.; Lin, H.-W. Natural Products from Sponges. In Symbiotic Microbiomes of Coral Reefs Sponges and Corals; Springer: Dordrecht, The Netherlands, 2019; pp. 329–463. [Google Scholar]
- Mbaoji, F.N.; Nweze, J.A.; Yang, L.; Huang, Y.; Huang, S.; Onwuka, A.M.; Peter, I.E.; Mbaoji, C.C.; Jiang, M.; Zhang, Y. Novel Marine Secondary Metabolites Worthy of Development as Anticancer Agents: A Review. Molecules 2021, 26, 5769. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W. Marine Microbial Natural Products: The Evolution of a New Field of Science. J. Antibiot. 2020, 73, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.-L.; Ding, Y.-F.; Zhang, W.; Lin, H.-W. Chemical and Biological Diversity of New Natural Products from Marine Sponges: A Review (2009–2018). Mar. Life Sci. Technol. 2022, 4, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Mintz, S.W. The Caribbean Region. Daedalus 1974, 103, 45–71. Available online: https://www.jstor.org/stable/20024204 (accessed on 1 July 2024).
- Demeritte, A.; Wuest, W.M. A Look around the West Indies: The Spices of Life Are Secondary Metabolites. Bioorg. Med. Chem. 2020, 28, 115792. [Google Scholar] [CrossRef]
- Toopaang, W.; Bunnak, W.; Srisuksam, C.; Wattananukit, W.; Tanticharoen, M.; Yang, Y.-L.; Amnuaykanjanasin, A. Microbial Polyketides and Their Roles in Insect Virulence: From Genomics to Biological Functions. Nat. Prod. Rep. 2022, 39, 2008–2029. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Li, G. Structural and Biological Insights into the Hot-Spot Marine Natural Products Reported from 2012 to 2021. Chin. J. Chem. 2022, 40, 1867–1889. [Google Scholar] [CrossRef]
- Norris, M.D.; Perkins, M.V. Structural Diversity and Chemical Synthesis of Peroxide and Peroxide-Derived Polyketide Metabolites from Marine Sponges. Nat. Prod. Rep. 2016, 33, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Buchan, K.C. The Bahamas. Mar. Pollut. Bull. 2000, 41, 94–111. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Taglialatela-Scafati, O.; Ianaro, A. Metabolites from the Sponge Plakortis Simplex. Determination of Absolute Stereochemistry of Plakortin. Isolation and Stereostructure of Three Plakortin Related Compounds. Tetrahedron 1999, 55, 7045–7056. [Google Scholar] [CrossRef]
- Higgs, M.D.; Faulkner, D.J. Plakortin, an Antibiotic from Plakortis Halichondrioides. J. Org. Chem. 1978, 43, 3454–3457. [Google Scholar] [CrossRef]
- Fattorusso, E.; Parapini, S.; Campagnuolo, C.; Basilico, N.; Taglialatela-Scafati, O.; Taramelli, D. Activity against Plasmodium Falciparum of Cycloperoxide Compounds Obtained from the Sponge Plakortis Simplex. J. Antimicrob. Chemother. 2002, 50, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Bean, M.F.; Carte, B.K.; Westley, J.W.; Johnson, R.K.; Lahouratate, P. The Plakortones, Novel Bicyclic Lactones from the Sponge Plakortis Halichondrioides: Activators of Cardiac SR-Ca2+-Pumping ATPase. Tetrahedron 1996, 52, 377–394. [Google Scholar] [CrossRef]
- Fattorusso, E.; Taglialatela-Scafati, O.; Di Rosa, M.; Ianaro, A. Metabolites from the Sponge Plakortis Simplex. Part 3: Isolation and Stereostructure of Novel Bioactive Cycloperoxides and Diol Analogues. Tetrahedron 2000, 56, 7959–7967. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Carte, B.; Johnson, R.K.; Lahouratate, P. Plakortides, Novel Cyclic Peroxides from the Sponge Plakortis Halichondrioides: Activators of Cardiac SR-Ca2+-Pumping ATPase. J. Nat. Prod. 1996, 59, 219–223. [Google Scholar] [CrossRef]
- Campagnuolo, C.; Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Basilico, N.; Parapini, S.; Taramelli, D. Antimalarial Polyketide Cycloperoxides from the Marine SpongePlakortis Simplex. Eur. J. Org. Chem. 2005, 2005, 5077–5083. [Google Scholar] [CrossRef]
- Stierle, D.B.; Faulkner, D.J. Metabolites of Three Marine Sponges of the Genus Plakortis. J. Org. Chem. 1980, 45, 3396–3401. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-Field FT NMR Application of Mosher’s Method. The Absolute Configurations of Marine Terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Oli, S.; Abdelmohsen, U.; Hentschel, U.; Schirmeister, T. Identification of Plakortide E from the Caribbean Sponge Plakortis Halichondroides as a Trypanocidal Protease Inhibitor Using Bioactivity-Guided Fractionation. Mar. Drugs 2014, 12, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Schirmeister, T.; Oli, S.; Wu, H.; Della Sala, G.; Costantino, V.; Seo, E.-J.; Efferth, T. Cytotoxicity of Endoperoxides from the Caribbean Sponge Plakortis Halichondrioides towards Sensitive and Multidrug-Resistant Leukemia Cells: Acids vs. Esters Activity Evaluation. Mar. Drugs 2017, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Munawar, S.; Zahoor, A.F.; Hussain, S.M.; Ahmad, S.; Mansha, A.; Parveen, B.; Ali, K.G.; Irfan, A. Steglich Esterification: A Versatile Synthetic Approach toward the Synthesis of Natural Products, Their Analogues/Derivatives. Heliyon 2024, 10, e23416. [Google Scholar] [CrossRef] [PubMed]
- del Sol Jiménez, M.; Garzón, S.P.; Rodríguez, A.D. Plakortides M and N, Bioactive Polyketide Endoperoxides from the Caribbean Marine Sponge Plakortis h Alichondrioides. J. Nat. Prod. 2003, 66, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.A.; Quintela, A.L.; Ferreira, E.G.; Sousa, T.S.; Pinto, F.d.C.L.; Hajdu, E.; Carvalho, M.S.; Salani, S.; Rocha, D.D.; Wilke, D.V.; et al. Cytotoxic Plakortides from the Brazilian Marine Sponge Plakortis Angulospiculatus. J. Nat. Prod. 2015, 78, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Alarif, W.M.; Basaif, S.S.; Abo-Elkarm, M.; Hamann, M.T.; Wahba, A.E.; Ayyad, S.-E.N. New Cytotoxic Cyclic Peroxide Acids from Plakortis Sp. Marine Sponge. ARKIVOC Free. Online J. Org. Chem. 2015, 2015, 164. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Persico, M.; Yang, F.; Lin, H.-W.; Guo, Y.-W.; Basilico, N.; Parapini, S.; Taramelli, D.; Taglialatela-Scafati, O.; Fattorusso, C. Endoperoxide Polyketides from a Chinese Plakortis Simplex: Further Evidence of the Impact of Stereochemistry on Antimalarial Activity of Simple 1, 2-Dioxanes. Bioorg. Med. Chem. 2014, 22, 4572–4580. [Google Scholar] [CrossRef]
- Phillipson, D.W.; Rinehart, K.L., Jr. Antifungal Peroxide-Containing Acids from Two Caribbean Sponges. J. Am. Chem. Soc. 1983, 105, 7735–7736. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Quach, T.; Nicholas, G.N.; Molinski, T.F. Amplification of the Cotton Effect of a Single Chromophore through Liposomal Ordering—Stereochemical Assignment of Plakinic Acids I and J. Angew. Chem. 2009, 121, 4431–4435. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Quach, T.; Molinski, T.F. Liposomal Circular Dichroism. Assignment of Remote Stereocenters in Plakinic Acids K and L from a Plakortis− Xestospongia Sponge Association. Org. Lett. 2010, 12, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Quach, T.; Molinski, T.F. Liposomal Circular Dichroism. Assignment of Remote Stereocenters in Plakinic Acids K and L from a Plakortis-Xestospongia Sponge Association. Org. Lett. 2011, 13, 4152. [Google Scholar] [CrossRef][Green Version]
- Jamison, M.T.; Dalisay, D.S.; Molinski, T.F. Peroxide Natural Products from Plakortis Zyggompha and the Sponge Association Plakortis Halichondrioides–Xestospongia Deweerdtae: Antifungal Activity against Cryptococcus Gattii. J. Nat. Prod. 2016, 79, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.A.; Mosher, H.S. Nuclear Magnetic Resonance Enantiomer Regents. Configurational Correlations via Nuclear Magnetic Resonance Chemical Shifts of Diastereomeric Mandelate, O-Methylmandelate, and. Alpha.-Methoxy-. Alpha.-Trifluoromethylphenylacetate (MTPA) Esters. J. Am. Chem. Soc. 1973, 95, 512–519. [Google Scholar] [CrossRef]
- Ankisetty, S.; Gochfeld, D.J.; Diaz, M.C.; Khan, S.I.; Slattery, M. Chemical Constituents of the Deep Reef Caribbean Sponges Plakortis Angulospiculatus and Plakortis Halichondrioides and Their Anti-Inflammatory Activities. J. Nat. Prod. 2010, 73, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-H.; van Soest, R.; Roberge, M.; Andersen, R.J. Spiculoic Acids A and B, New Polyketides Isolated from the Caribbean Marine Sponge Plakortis Angulospiculatus. Org. Lett. 2004, 6, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Brunt, M.A.; Davies, J.E. The Cayman Islands: Natural History and Biogeography; Springer: Dordrecht, The Netherlands, 1994; ISBN 978-94-011-0904-8. [Google Scholar]
- Bolland, O.N. Belize: A New Nation in Central America, 1st ed.; Routledge: London, UK, 2019; ISBN 978-0-429-03508-1. [Google Scholar]
- Ravi, B.N.; Armstrong, R.W.; Faulkner, D.J. Some Aromatic Compounds from the Marine Sponge Plakortis Halichondrioides. J. Org. Chem. 1979, 44, 3109–3113. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Faulkner, D.J. Absolute Configuration of Methyl (2Z, 6R, 8R, 9E)-3, 6-Epoxy-4, 6, 8-Triethyl-2, 4, 9-Dodecatrienoate from the Sponge Plakortis Halichondrioides. Tetrahedron Lett. 1996, 37, 6681–6684. [Google Scholar] [CrossRef]
- Slinger-Friedman, V. Dominica. In Landscapes and Landforms of the Lesser Antilles; Allen, C.D., Ed.; World Geomorphological Landscapes; Springer International Publishing: Cham, Switzerland, 2017; pp. 153–171. ISBN 978-3-319-55785-4. [Google Scholar]
- Williams, D.E.; Allen, T.M.; Van Soest, R.; Behrisch, H.W.; Andersen, R.J. Glánvillic Acids A and B and Methyl Capucinoate A, New Metabolites Isolated from the Caribbean Sponges Plakortis h Alichondrioides and Plakinastrella o Nkodes. J. Nat. Prod. 2001, 64, 281–285. [Google Scholar] [CrossRef]
- Lalor, G.C. Geochemical Mapping in Jamaica. Environ. Geochem. Health 1996, 18, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rudi, A.; Kashman, Y. Three New Cytotoxic Metabolites from the Marine Sponge Plakortis Halichondrioides. J. Nat. Prod. 1993, 56, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, D.J.; Hamann, M.T. Isolation and Biological Evaluation of Filiformin, Plakortide F, and Plakortone G from the Caribbean Sponge Plakortis Sp. J. Nat. Prod. 2001, 64, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-F.; Gao, H.-F.; Kelly, M.; Hamann, M.T. Plakortides I–L, Four New Cyclic Peroxides from an Undescribed Jamaican Sponge Plakortis Sp. (Homosclerophorida, Plakinidae). Tetrahedron 2001, 57, 9379–9383, Corrigendum in Tetrahedron 2002, 6, 1233. [Google Scholar] [CrossRef]
- Mohammed, R.; Peng, J.; Kelly, M.; Yousaf, M.; Winn, E.; Odde, S.; Bie, Z.; Xie, A.; Doerksen, R.J.; Hamann, M.T. Polyketide-Peroxides from a Species of Jamaican Plakortis (Porifera: Demospongiae). Aust. J. Chem. 2010, 63, 877–885. [Google Scholar] [CrossRef]
- Compagnone, R.S.; Piña, I.C.; Rangel, H.R.; Dagger, F.; Suárez, A.I.; Reddy, M.V.R.; Faulkner, D.J. Antileishmanial cyclic peroxides from the Palauan sponge Plakortis aff. angulospiculatus. Tetrahedron 1998, 54, 3057–3068. [Google Scholar] [CrossRef]
- Modlin, E.A.; Allen, C.D. Martinique. In Landscapes and Landforms of the Lesser Antilles; Allen, C.D., Ed.; World Geomorphological Landscapes; Springer International Publishing: Cham, Switzerland, 2017; pp. 173–189. ISBN 978-3-319-55785-4. [Google Scholar]
- Berrué, F.; Thomas, O.P.; Fernández, R.; Amade, P. Iso-, nor-, and Dinor-Spiculoic Acids A, Polyketides from the Marine Sponge Plakortis Zyggompha. J. Nat. Prod. 2005, 68, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Berrué, F.; Thomas, O.P.; Bon, C.F.-L.; Reyes, F.; Amade, P. New Bioactive Cyclic Peroxides from the Caribbean Marine Sponge Plakortis Zyggompha. Tetrahedron 2005, 61, 11843–11849. [Google Scholar] [CrossRef]
- Palka, E.J. A Geographic Overview of Panama. In The Río Chagres, Panama; Harmon, R.S., Ed.; Water Science and Technology Library; Springer: Berlin/Heidelberg, Germany, 2005; Volume 52, pp. 3–18. ISBN 978-1-4020-3298-1. [Google Scholar]
- Appeldoorn, R.S.; Alfaro, M.; Ballantine, D.L.; Bejarano, I.; Ruíz, H.J.; Schizas, N.V.; Schmidt, W.E.; Sherman, C.E.; Weil, E. Puerto Rico. In Mesophotic Coral Ecosystems; Loya, Y., Puglise, K.A., Bridge, T.C.L., Eds.; Coral Reefs of the World; Springer International Publishing: Cham, Switzerland, 2019; Volume 12, pp. 111–129. ISBN 978-3-319-92734-3. [Google Scholar]
- Jiménez-Romero, C.; Ortiz, I.; Vicente, J.; Vera, B.; Rodríguez, A.D.; Nam, S.; Jove, R. Bioactive Cycloperoxides Isolated from the Puerto Rican Sponge Plakortis Halichondrioides. J. Nat. Prod. 2010, 73, 1694–1700. [Google Scholar] [CrossRef]
- Perry, T.L.; Dickerson, A.; Khan, A.A.; Kondru, R.K.; Beratan, D.N.; Wipf, P.; Kelly, M.; Hamann, M.T. New Peroxylactones from the Jamaican Sponge Plakinastrella Onkodes, with Inhibitory Activity against the AIDS Opportunistic Parasitic Infection Toxoplasma Gondii. Tetrahedron 2001, 57, 1483–1487. [Google Scholar] [CrossRef]
- Chen, Y.; Killday, K.B.; McCarthy, P.J.; Schimoler, R.; Chilson, K.; Selitrennikoff, C.; Pomponi, S.A.; Wright, A.E. Three New Peroxides from the Sponge Plakinastrella Species. J. Nat. Prod. 2001, 64, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Romero, C.; Rodríguez, A.D.; Nam, S. Plakortinic Acids A and B: Cytotoxic Cycloperoxides with a Bicyclo[4.2.0]Octene Unit from Sponges of the Genera Plakortis and Xestospongia. Org. Lett. 2017, 19, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Romero, C.; Amador, L.A.; Rodríguez, A.D. Plakortinic Acids C and D: A Pair of Peroxide-Polyketides Possessing a Rare 7,8-Dioxatricyclo[4.2.2.02,5]Dec-9-Ene Core from a Two-Sponge Association of Plakortis Symbiotica–Xestospongia Deweerdtae. Tetrahedron Lett. 2021, 66, 152833. [Google Scholar] [CrossRef] [PubMed]
- Amador, L.A.; Colón-Lorenzo, E.E.; Rodríguez, A.D.; Serrano, A.E. Probing the Antiplasmodial Properties of Plakortinic Acids C and D: An Uncommon Pair of Marine Peroxide-Polyketides Isolated from a Two-Sponge Association of Plakortis Symbiotica and Xetospongia Deweerdtae Collected near Puerto Rico. Life 2024, 14, 684. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.C. Geology of Tobago, British West Indies. Geol. Soc. Am. Bull. 1948, 59, 801. [Google Scholar] [CrossRef]
- Kushlan, D.M.; Faulkner, D.J. A Novel Perlactone from the Caribbean Sponge Plakortis Angulospiculatus. J. Nat. Prod. 1991, 54, 1451–1454. [Google Scholar] [CrossRef]




| Polyketides | Location | Plakorstis sp. | Biological Activity | Reference |
|---|---|---|---|---|
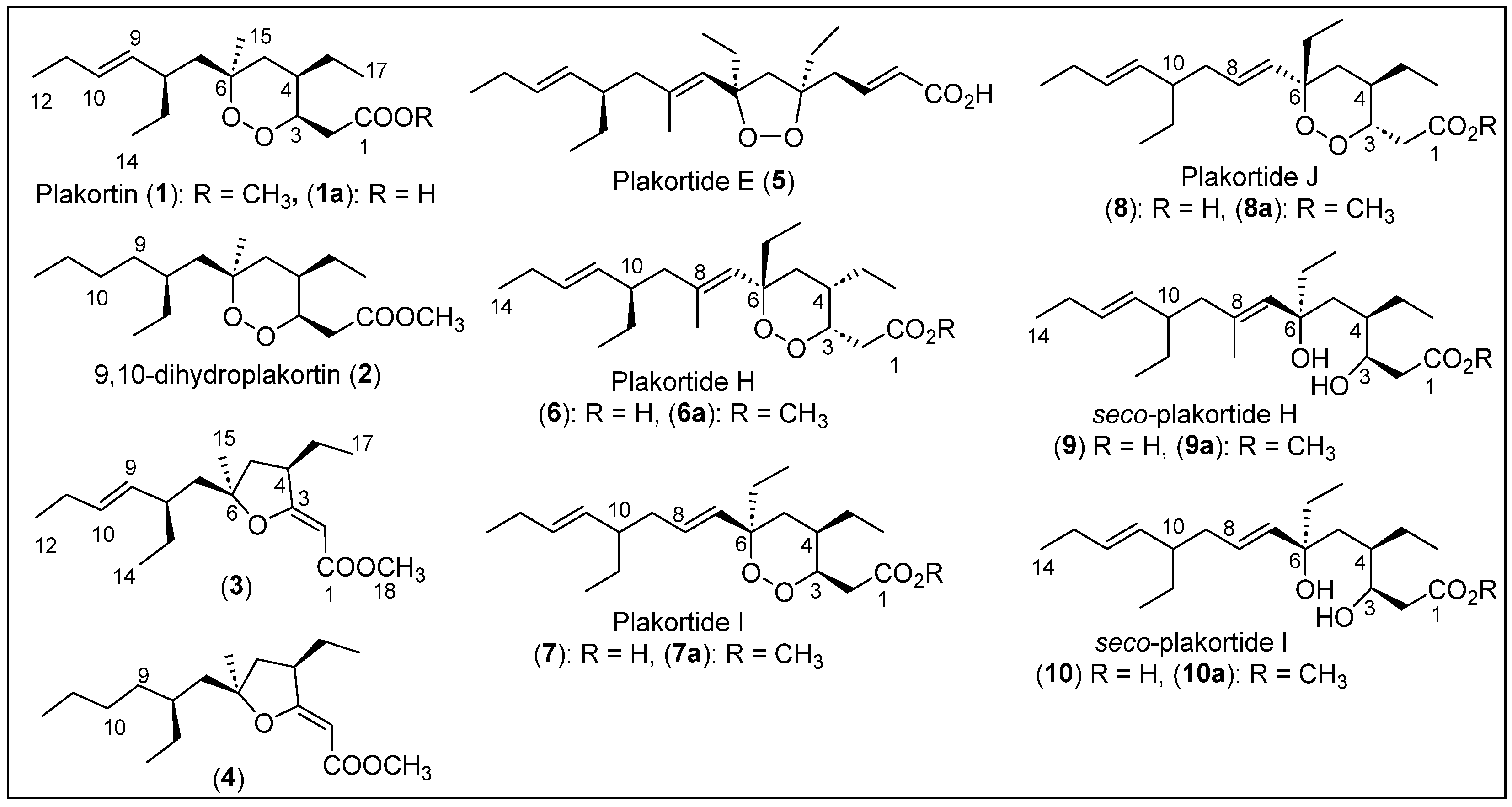
| 1 | The Bahamas, and Panamá | P. simplex and P. halocondroides | Cytotoxic activity against WEHI 164, antimalarial activity, activity against the drug-sensitive leukemia CCRF-CEM cell line and multi-drug-resistant subline CEM/ADR5000 | [32,33,34,38,39,41,42,46,47,48,59] |
| 2 | The Bahamas | P. simplex and P. halocondroides | Cytotoxic activity against WEHI 164, antimalarial activity, activity against the drug-sensitive leukemia CCRF-CEM cell line and multi-drug-resistant subline CEM/ADR5000 | [32,34,38,41,42] |
| 3,4 | The Bahamas | P. simplex | Cytotoxic activity against WEHI 164 | [32] |
| 5 | Bahamas and Jamaica | P. simplex and P. halocondroides | Antimalarial activity and antiparasitic | [16,34,35,41,42] |
| 6 | Bahamas and Jamaica | P. simplex and P. halocondroides | Cytotoxic activity against WEHI 164 and activators of cardiac SR-Ca2+-pumping ATPase | [36,37,42] |
| 7–10 | The Bahamas, Panamá, and Jamaica | P. simplex | Cytotoxic activity against WEHI 164 | [33,36] |
| 11–15 | Belize, Jamaica, and The Bahamas | P. simplex and P. halichondrioides | Antimalarial activity | [32,33,38,39,40] |
| 16 | The Bahamas | P. halichondrioides | Cytotoxic activity against leukemia | [41,42,43,45] |
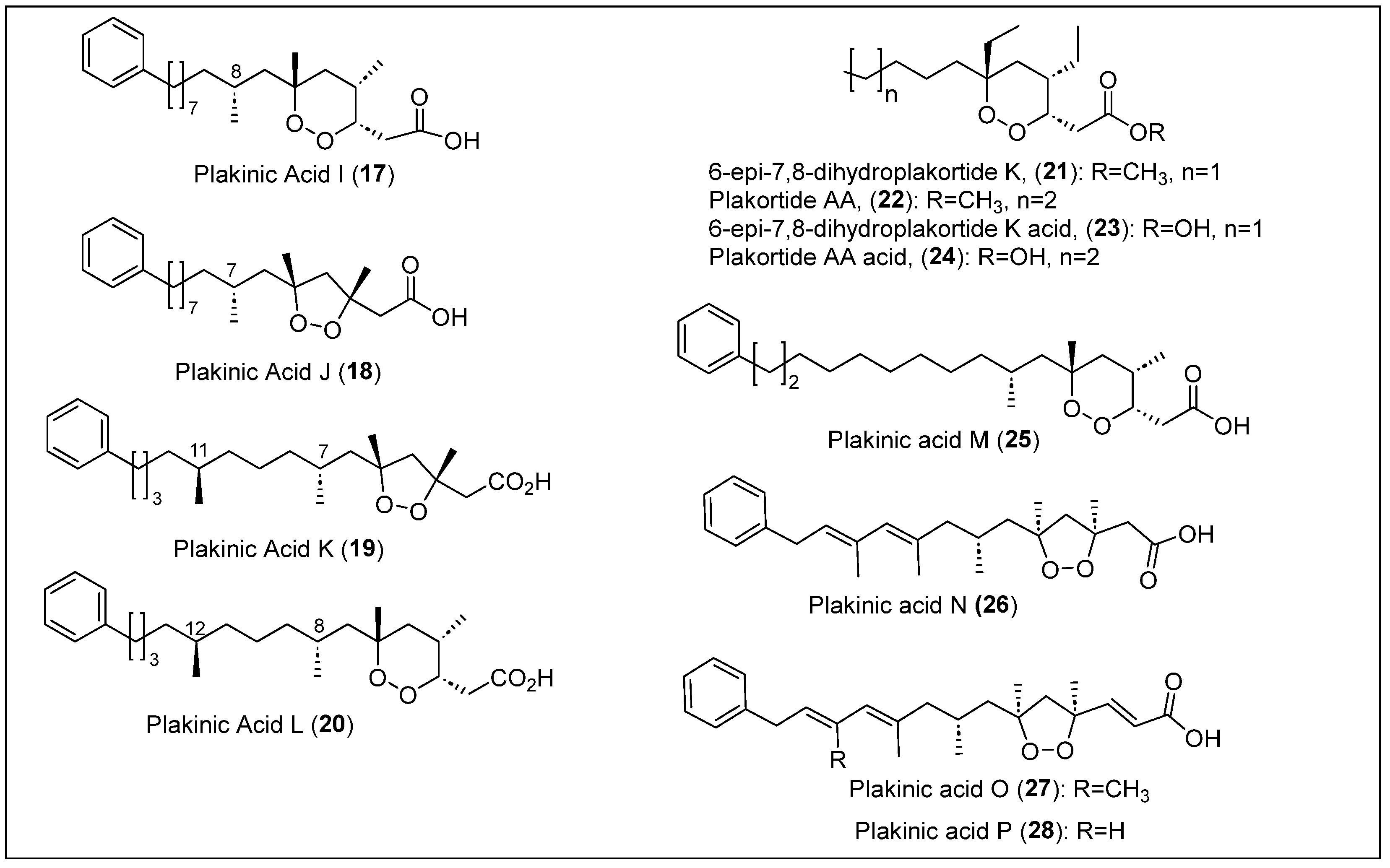
| 17–20 | The Bahamas | P. halichondrioides | Antifungal activity | [49,50,51] |
| 21–22 | The Bahamas | P. halichondrioides-X. deweerdtae and P. zyggompha | No antifungal activity | [52,53,65] |
| 23–24 | The Bahamas | P. halichondrioides-X. deweerdtae and P. zyggompha | Antifungal activity against Candida and Cryptococcus | [16,50,51] |
| 25–28 | The Bahamas | P. halichondrioides-X. deweerdtae and P. zyggompha | Compound 25 has antifungal activity | [16,50,51] |
| 29 | The Bahamas | P. angulospiculatus | Inhibition of NFκB’s activity | [54,55] |
| 30–32 | The Bahamas | P. halichondrioides | Was not reported | [55] |
| 33–36 | Cayman Islands | P. angulospiculatus | Compound 34 shows inhibition of NFκB’s activity | [54] |
| 37–43 | Jamaica | P. halichondrioides | Was not reported | [58] |
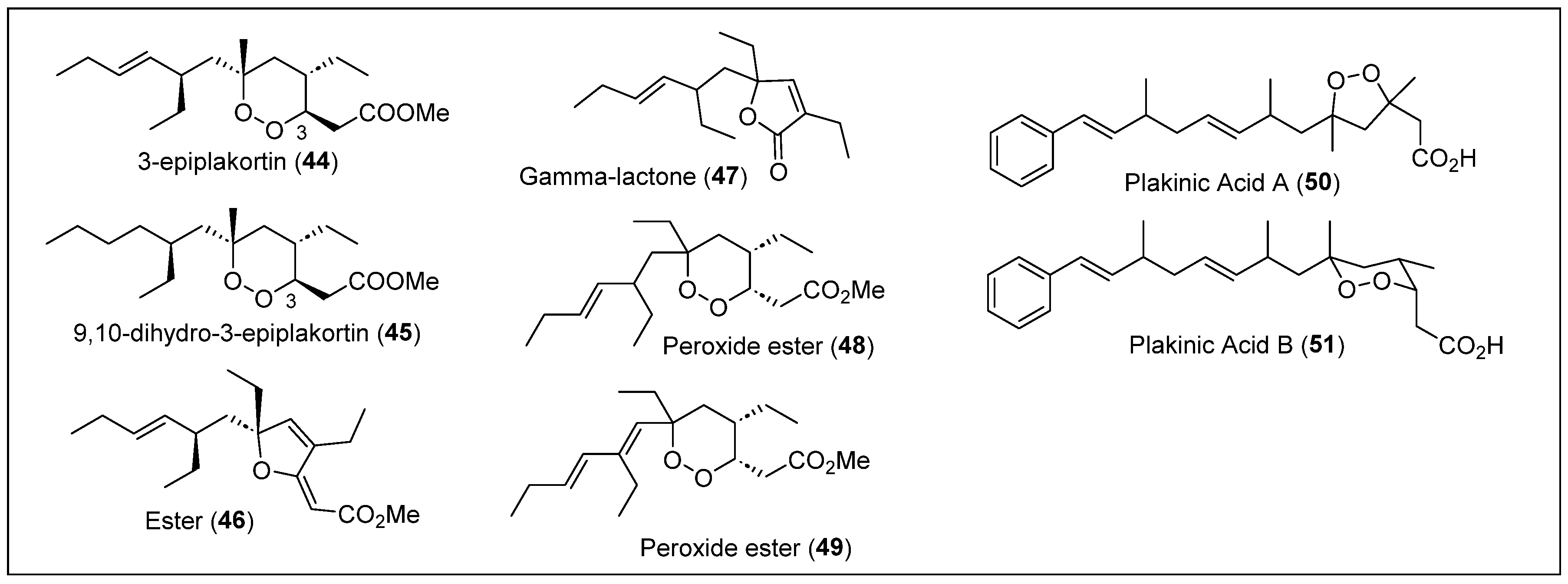
| 44–49 | Jamaica, Belize, and Puerto Rico | P. halichondrioides | Compound 44 is an activator of cardiac SR-Ca2+-pumping ATPase | [35,37,39,59,63] |
| 50–51 | Jamaica | P. zyggompha | Antifungal activity against S. cerevisiae (yeast) and Penicillium atrovenetum (filamentous fungus), also inhibited leukemia cell line | [48] |
| 52–53 | Dominica | P. halichondrioides | In vitro cytotoxicity against leukemia | [33] |
| 54–55 | Coasts of Dominica | P. angulospiculatus | Compound 55 shows cytotoxicity activity against human breast cancer | [55] |
| 56–58 | Jamaica | P. halichondrioides | In vitro cytotoxicity against leukemia. | [54,65] |
| 59–62 | Jamaica | P. halichondrioides | Activator of cardiac SR-Ca2+-pumping ATPase | [35] |
| 63–64 | Puerto Rico | P. halichondrioides | Compound 63 is moderately active against Mtb inhibitions and shows anticancer activity. Activators of cardiac SR-Ca2+-pumping ATPase and biological activity against L. donovani. | [35,37,39,63,64] |
| 65 | Jamaica | Plakortis sp. | Antimalarial activity | [64] |
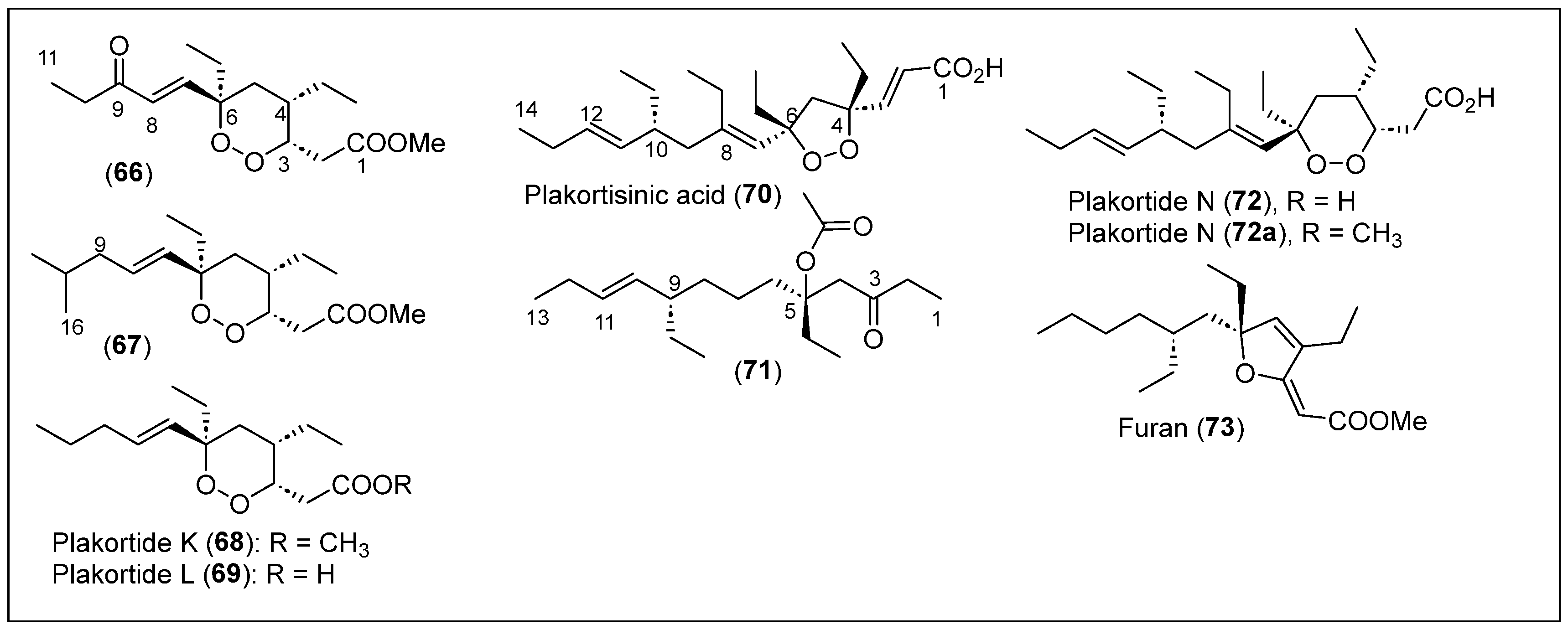
| 66–69 | Jamaica | Plakortis sp. | Compound 66 has antimalarial activity | [65] |
| 70–73 | Jamaica, and Puerto Rico | Plakortis sp. | Compounds 70 and 71 show biological activity against L. donovani. Compounds 70 and 72 are moderately active against Mtb inhibitions and have anti-HIV activity in humans. Compound 72 has anticancer activity. | [37,44,67] |
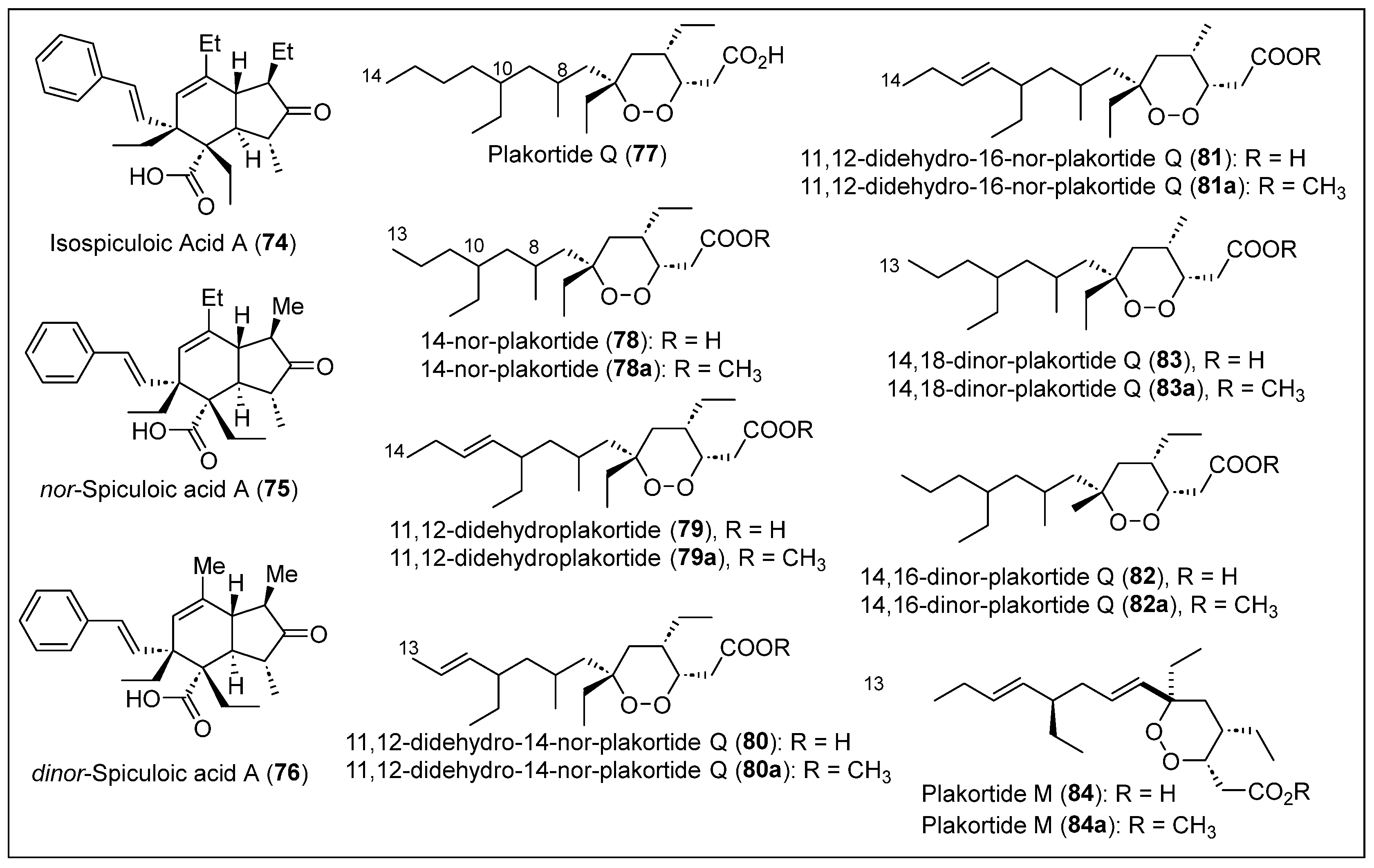
| 74–76 | Martinique Island | P. zyggompha | Compounds 74 and 76 are cytotoxic against tumor cell lines of lung and colon carcinoma | [45,55,69] |
| 77–83 | Martinique Island | P. zyggompha | Compounds are cytotoxic against tumor cell lines of lung and colon carcinoma | [65,69] |
| 84 | Puerto Rico | P. halichondrioides | Cytotoxic activity against tumor cell lines and antimalarial activity | [44] |
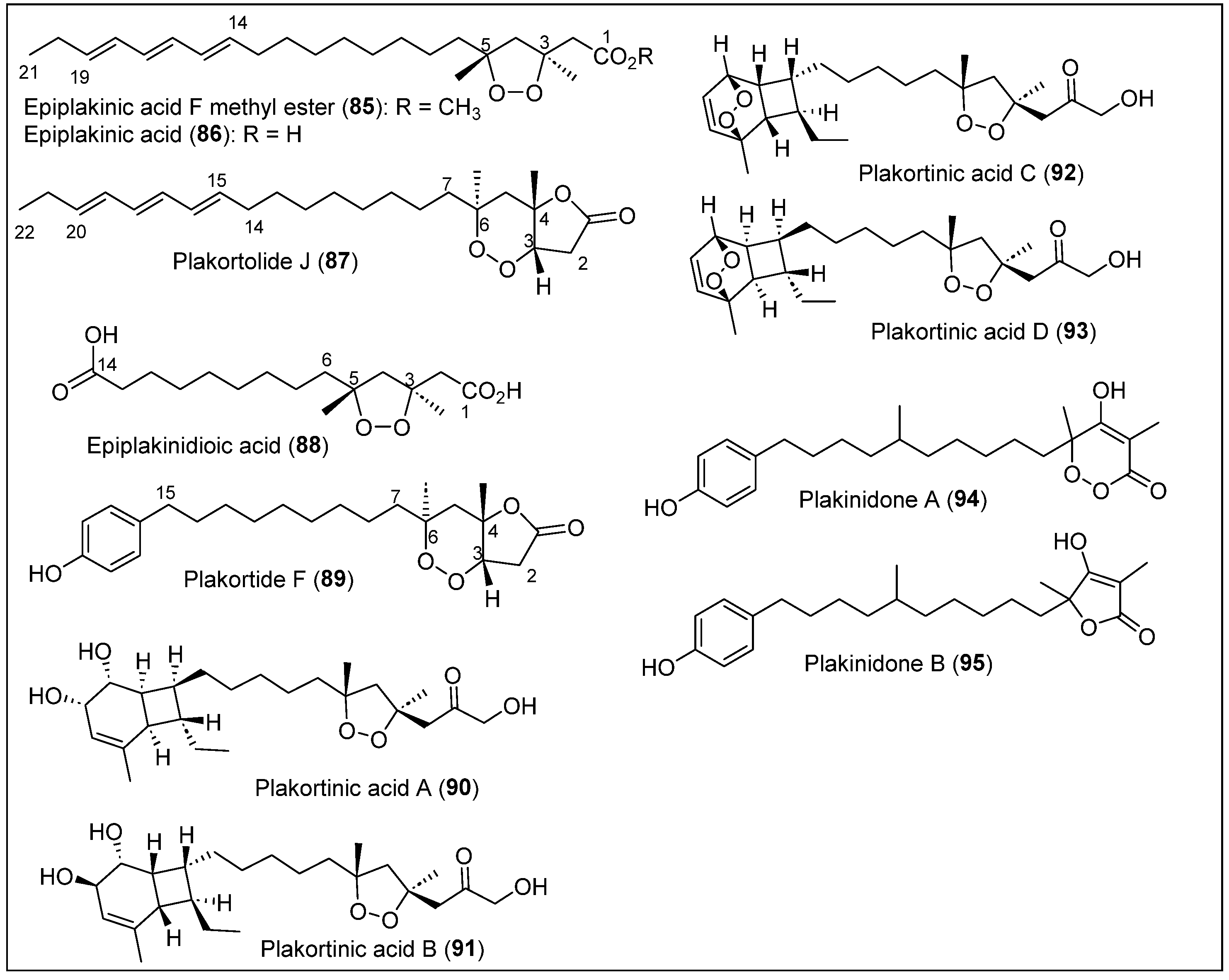
| 85–89 | Puerto Rico | P. halichondrioides | Antimalarial, anticancer, and antituberculosis activity | [73,74,75] |
| 90–91 | Puerto Rico | P. halichondrioides-X. deweerdtae | Cytotoxic activity against melanoma and prostate cancer cells | [76] |
| 92–93 | Puerto Rico | P. halichondrioides-X. deweerdtae | In vitro toxicity against a panel of 60 tumor cell lines and antiparasitic | [77,78] |
| 94–95 | Tobago Island | P. angulospiculatus | Antimicrobial activity against S. aureus and Bacillus subtilis | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Berríos, R.R.; Rodríguez-Vargas, J.A.; Colón-Cartagena, F.; Maldonado-Silva, U.; Ortiz-Colón, Y.W.; Escalante-Castaneda, A.; Conde-González, A.; Álamo-Diverse, K.Y. Polyketides from Plakortis Sponges around Caribbean Coastal Regions: Collection, Isolation, Characterization, and Bioactivity. Coasts 2024, 4, 568-593. https://doi.org/10.3390/coasts4030029
Rodríguez-Berríos RR, Rodríguez-Vargas JA, Colón-Cartagena F, Maldonado-Silva U, Ortiz-Colón YW, Escalante-Castaneda A, Conde-González A, Álamo-Diverse KY. Polyketides from Plakortis Sponges around Caribbean Coastal Regions: Collection, Isolation, Characterization, and Bioactivity. Coasts. 2024; 4(3):568-593. https://doi.org/10.3390/coasts4030029
Chicago/Turabian StyleRodríguez-Berríos, Raúl R., Jeremy A. Rodríguez-Vargas, Francisco Colón-Cartagena, Ulises Maldonado-Silva, Yermarie W. Ortiz-Colón, Alejandro Escalante-Castaneda, Arianthony Conde-González, and Keiry Y. Álamo-Diverse. 2024. "Polyketides from Plakortis Sponges around Caribbean Coastal Regions: Collection, Isolation, Characterization, and Bioactivity" Coasts 4, no. 3: 568-593. https://doi.org/10.3390/coasts4030029
APA StyleRodríguez-Berríos, R. R., Rodríguez-Vargas, J. A., Colón-Cartagena, F., Maldonado-Silva, U., Ortiz-Colón, Y. W., Escalante-Castaneda, A., Conde-González, A., & Álamo-Diverse, K. Y. (2024). Polyketides from Plakortis Sponges around Caribbean Coastal Regions: Collection, Isolation, Characterization, and Bioactivity. Coasts, 4(3), 568-593. https://doi.org/10.3390/coasts4030029






