Exploring the Role of Water Molecules in Acetylsalicylic Acid Adsorption Energy on HY Zeolite: A Density Functional Theory Approach
Abstract
1. Introduction
2. Methods and Computational Details
Intramolecular Interactions
3. Results and Discussion
3.1. DFT Calculations for the 3T Zeolite Model Cluster-Preliminary Investigation
3.2. Two-Layer ONIOM-Method on the 38T Model Cluster and Water Molecule Effects
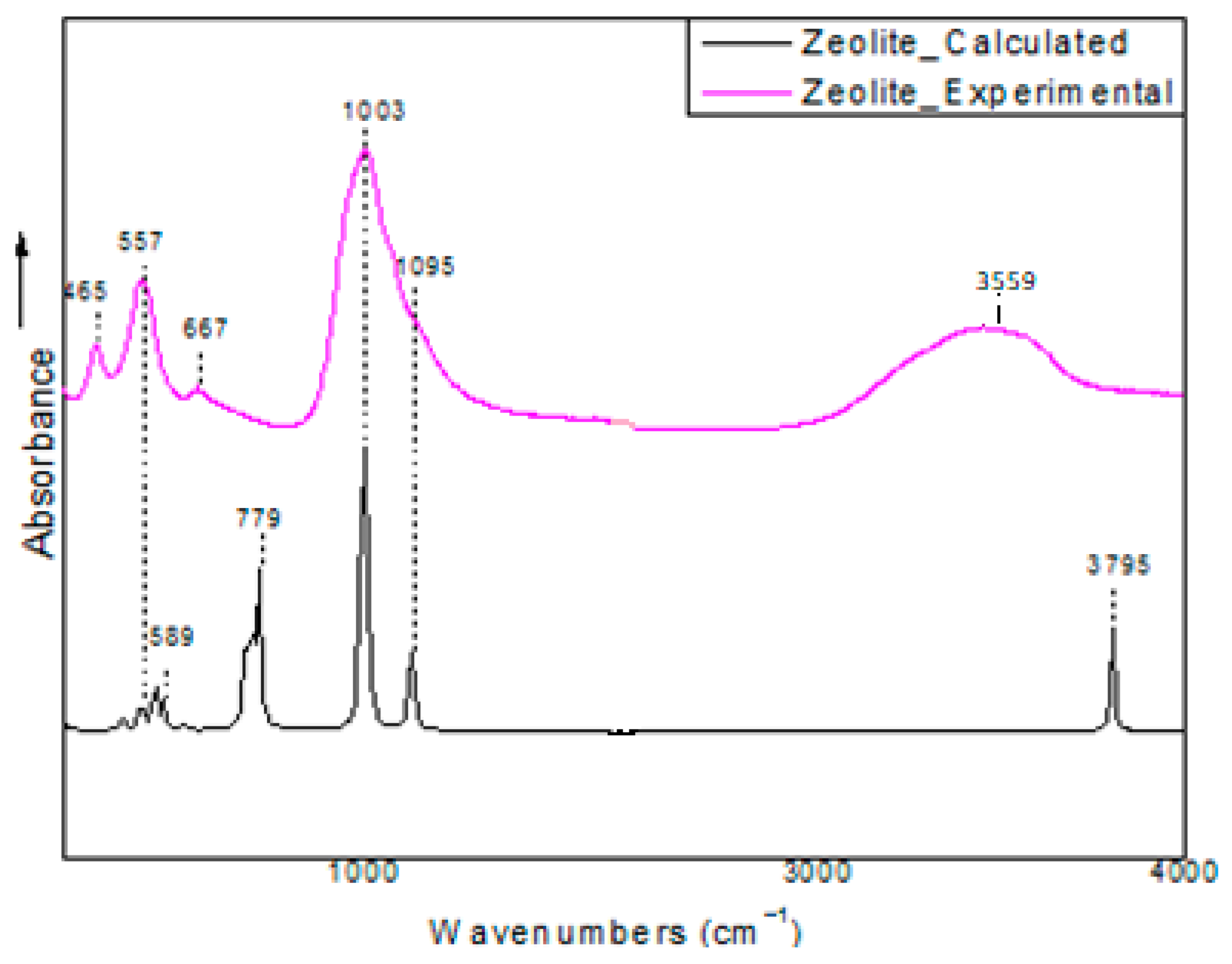
Vibrational Frequencies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Q.; Shi, J. Mesoporous silicananoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, J.; Li, Y.; Chen, H.; Shen, W.; Dong, X. Storage and release of ibuprofen drug molecules in hollow mesoporous silica spheres with modified pore surface. Microporous Mesoporous Mater. 2005, 85, 75–81. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.E.; Lee, J.; Yu, J.H.; Kim, B.C.; An, K.; Hwang, Y.; Shin, C.-H.; Park, J.G.; Kim, J.; et al. Magnetic Fluorescent Delivery Vehicle Using Uniform Mesoporous Silica Spheres Embedded with Monodisperse Magnetic and Semiconductor Nanocrystals. J.Am. Chem. Soc. 2005, 128, 688–689. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, Y.; Wu, D.; Shen, W.; Deng, F. Synthesis, Characterization, and in Vitro pH-Controllable Drug Release from Mesoporous Silica Spheres with Switchable Gates. Langmuir 2010, 26, 17133–17138. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef]
- Zheng, J.P.; Luan, L.; Wang, H.Y.; Xi, L.F.; Yao, K.D. Study on ibuprofen/montmorillonite intercalation composites as drug release system. Appl. Clay Sci. 2007, 36, 297–301. [Google Scholar] [CrossRef]
- Tielens, F.; Folliet, N.; Bondaz, L.; Etemovic, S.; Babonneau, F.; Gervais, C.; Azaïs, T. Molecular Picture of the Adsorption of Ibuprofen and Benzoic Acid on Hydrated Amorphous Silica through DFT-D Calculations Combined with Solid-State NMR Experiments. J. Phys. Chem. C 2017, 121, 17339–17347. [Google Scholar] [CrossRef]
- Gackowski, M.; Ruggiero-Mikołajczyk, M.; Zimowska, M. Unassisted Solventless Mesopore Filling of SBA-15 with Ibuprofen: A Solid-State Study and Long-Term Characterization. J. Phys. Chem. C 2025, 129, 3735–3744. [Google Scholar] [CrossRef]
- Eldeeb, A.; Serag, E.; Elmowafy, M.; Khouly, M. pH-responsive zeolite-A/chitosan nanocarrier for enhanced ibuprofen drug delivery in gastrointestinal systems. Int. J. Biol. Macromol. 2025, 289, 138879. [Google Scholar] [CrossRef]
- Kryuchkova, M.; Batasheva, S.; Akhatova, F.; Babaev, V.; Buzyurova, D.; Vikulina, A.; Volodkin, D.; Fakhrullin, R.; Rozhina, E. Pharmaceuticals Removal by Adsorption with Montmorillonite Nanoclay. Int. J. Mol. Sci. 2021, 22, 9670. [Google Scholar] [CrossRef] [PubMed]
- Krajišnik, D.; Daković, A.; Malenović, A.; Kragović, M.; Milić, J. Ibuprofen sorption and release by modified natural zeolites as prospective drug carriers. Clay Miner. 2015, 50, 11–15. [Google Scholar] [CrossRef]
- Li, Z.; Gómez-Avilés, A.; Sellaoui, L.; Bedia, J.; Petriciolet, P.; Belver, C. Adsorption of ibuprofen on organo-sepiolite and on zeolite/sepiolite heterostructure: Synthesis, characterization and statistical physics modeling. Chem. Eng. J. 2019, 371, 868–875. [Google Scholar] [CrossRef]
- Lam, A.; Sierra, L.R.; Rojas-Lorenzo, G.; Rivera, A.; Rodriguez-Fuentes, G.; Montero, L. Theoeretical study of the physical adsorption of acetylsalicylic acid on natural clinoptilolite. Microporous Mesoporous Mater. 1998, 23, 247–252. [Google Scholar] [CrossRef]
- Petushkov, A.; Ndiege, N.; Salem, A.K.; Larsen, S.C. Toxicity of Silica Nanomaterials: Zeolites, Mesoporous Silica, and Amorphous Silica Nanoparticles. Adv. Mol. Toxicol. 2010, 4, 223–266. [Google Scholar] [CrossRef]
- Petushko, A.; Intra, J.; Graham, J.B.; Larsen, S.C.; Salem, A.K. Effect of Crystal Size and Surface Functionalization on the Cytotoxicity of Silicalite-1 Nanoparticles. Chem. Res. Toxicol. 2009, 22, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, I.; Croce, A.; Di Renzo, F.; Tiozzo, R.; Fubini, B. Pure-Silica Zeolites (Porosils) as Model Solids for the Evaluation of the Physicochemical Features Determining Silica Toxicity to Macrophages. Chem. Res. Toxicol. 2000, 13, 489–500. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Chen, T.; Bao, X. Zeolites: A series of promising biomaterials in bone tissueengineering. Front. Bioeng. Biotechnol. 2022, 10, 1066552. [Google Scholar] [CrossRef]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef] [PubMed]
- Csajbók, É.; Bányai, I.; Vander Elst, L.; Muller, R.N.; Zhou, W.; Peters, J.A. Gadolinium(III)-Loaded Nanoparticulate Zeolites as Potential High-Field MRI Contrast Agents: Relationship Between Structure and Relaxivity. Chem.–A Eur. J. 2005, 11, 4799–4807. [Google Scholar] [CrossRef]
- Tsotsalas Manuel, M.; Kopka, K.; Luppi, G.; Wagner, S.; Law, M.P.; Schäfers, M.; De Cola, L. Encapsulating 111In in Nanocontainers for Scintigraphic Imaging: Synthesis, Characterization, and In Vivo Biodistribution. ACS Nano 2010, 4, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ndiege, N.; Raidoo, R.; Schultz, M.K.; Larsen, S. Preparation of a Versatile Bifunctional Zeolite for Targeted Imaging Application. Langmuir 2011, 27, 2904–2909. [Google Scholar] [CrossRef]
- Bresinska, I.; Balkus, K.J.J. Studies of Gd(III)-Exchanged Y-Type Zeolites Relevant to Magnetic Resonance Imaging. J. Phys. Chem. 1994, 98, 12989–12994. [Google Scholar] [CrossRef]
- Galownia, J.; Martin, J.; Davis, M.E. Aluminophosphate-based, microporous materials for blood clotting. Microporous Mesoporous Mater. 2006, 92, 61–63. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, Y.; Dutta, P.K. Controlled release of paraquat from surface-modified zeolite Y. Microporous Mesoporous Mater. 2006, 88, 312–318. [Google Scholar] [CrossRef]
- Braschi, I.; Gatti, G.; Paul, G.; Gessa, C.E.; Cossi, M.; Marchese, L. Sulfonamide Antibiotics Embedded in High Silica Zeolite Y: A Combined Experimental and Theoretical Study of Host−Guest and Guest−Guest Interactions. Langmuir 2010, 26, 9524–9532. [Google Scholar] [CrossRef]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, C.E.; Alberti, A.; Martucci, A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. J. Hazard. Mater. 2010, 178, 218–225. [Google Scholar] [CrossRef]
- Dyer, A.; Morgan, S.; Wells, P.; Williams, C. The use of zeolites as slow release anthelmintic carriers. J. Helminthol. 2000, 74, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Remington, J.P.; Gennaro, A.R. Remington’s Pharmaceutical Sciences, 18th ed.; Mack Publishing Co.: Easton, PA, USA, 1990. [Google Scholar]
- Lanas, A.I.; Sousa, F.L.; Ortego, J.; Esteva, F.; Blas, J.M.; Soria, J.; Sáinz, R. Acetylsalicylic acid renders the oesophageal mucosa more permeable to acid and pepsin. Eur. J. Gastroenterol. Hepatol. 1995, 7, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Datt, A.; Fields, D.; Larsen, S.C. An Experimental and Computational Study of the Loading and Release of Aspirin from Zeolite HY. J. Phys. Chem. C 2012, 116, 21382–21390. [Google Scholar] [CrossRef]
- Izmaylov, A.F.; Scuseria, G.E.; Frisch, M.J. Efficient evaluation of short-range Hartree-Fock exchange in large molecules and periodic systems. J. Chem. Phys. 2006, 125, 104103. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.M.; Izmaylov, A.F.; Scalmani, G.; Scuseria, G.E. Can short-range hybrids describe long-range-dependent properties? J. Chem. Phys. 2009, 131, 44108. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Davidson, E.R. Comment on “Comment on Dunning’s correlation-consistent basis sets”. Chem. Phys. Lett. 1996, 260, 514–518. [Google Scholar] [CrossRef]
- Heyd, J.; Peralta, J.E.; Scuseria, G.E.; Martin, R.L. Energy band gaps and lattice parameters evaluated with the Heyd-Scuseria-Ernzerhof screened hybrid functional. J. Chem. Phys. 2005, 123, 174101. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E. Efficient hybrid density functional calculations in solids: Assessment of the Heyd–Scuseria–Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 2004, 121, 1187–1192. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E. Assessment and validation of a screened Coulomb hybrid density functional. J. Chem. Phys. 2004, 120, 7274–7280. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Burke, K.; Perdew, J.P.; Wang, Y. Derivation of a Generalized Gradient Approximation: The PW91 Density Functional. In Book cover Electronic Density Functional Theory; Springer: New York, NY, USA, 1998. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, J.; Lynch, B.J.; Truhlar, D.G. Tests of second-generation and third-generation density functionals for thermochemical kinetics. Phys. Chem. Chem. Phys. 2004, 6, 673–676. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16; Gaussian Inc: Wallingford, CT, USA, 2016. [Google Scholar]
- Boronat, M.; Viruela, P.M.; Corma, A. Reaction Intermediates in Acid Catalysis by Zeolites: Prediction of the Relative Tendency To Form Alkoxides or Carbocations as a Function of Hydrocarbon Nature and Active Site Structure. J. Am. Chem. Soc. 2004, 126, 3300–3309. [Google Scholar] [CrossRef]
- Noronha, L.A.; Souza-Aguiar, E.F.; Mota, C.J.A. Conversion of chloromethane to light olefins catalyzed by ZSM-5 zeolites. Catal. Today 2005, 101, 9–13. [Google Scholar] [CrossRef]
- Zheng, X.; Blowers, P. An ab initio study of ethane conversion reactions on zeolites using the complete basis set composite energy method. J. Mol. Catal. A Chem. 2005, 229, 77–85. [Google Scholar] [CrossRef]
- Zheng, X.; Blowers, P. Reactivity of Isobutane on Zeolites: A First Principles Study. J. Phys. Chem. A 2006, 110, 2455–2460. [Google Scholar] [CrossRef]
- Frash, M.V.; Van Santen, R.A. Quantum-chemical modeling of the hydrocarbon transformations in acid zeolite catalysts. Top. Catal. 1999, 9, 191–205. [Google Scholar] [CrossRef]
- Zhou, D.; Bao, Y.; Yang, M.; He, N.; Yang, G. DFT studies on the location and acid strength of Brönsted acid sites in MCM-22 zeolite. J. Mol. Catal. A Chem. 2006, 244, 11–19. [Google Scholar] [CrossRef]
- Soscún, H.; Castellano, O.; Hernandez, J.; Arrieta, F.; Bermúdez, Y.; Hinchliffe, A.; Brussin, M.R.; Sanchez, M.; Sierraalta, A.; Ruette, F. An ab initio and DFT study of the interaction between ethanethiol and zeolites. J. Mol. Catal. A Chem. 2007, 278, 165–172. [Google Scholar] [CrossRef]
- Olson, D.H.; Dempsey, E. The crystal structure of the zeolite hydrogen faujasite. J. Catal. 1969, 13, 221–231. [Google Scholar] [CrossRef]
- Svensson, M.; Humbel, S.; Froese, R.D.J.; Matsubara, T.; Sieber, S.; Morokuma, K. ONIOM: A Multilayered Integrated MO+MM Method for Geometry Optimizations and Single Point Energy Predictions. A Test for Diels−Alder Reactions and Pt(P(t-Bu)3)2 + H2 Oxidative Addition. J. Phys. Chem. 1996, 100, 19357–19363. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Freude, D.; Klinowski, J.; Hamdan, H. Solid-state NMR studies of the geometry of brønsted acid sites in zeolitic catalysts. Chem. Phys. Lett. 1988, 149, 355–362. [Google Scholar] [CrossRef]
- Jiang, Y.; Hunger, M.; Wang, W. On the Reactivity of Surface Methoxy Species in Acidic Zeolites. J. Am. Chem. Soc. 2006, 128, 11679–11692. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalyzed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Aloulou, H.; Bouhamed, H.; Ghorbel, A.; Ben Amar, R.; Khemakhem, S. Elaboration and characterization of ceramic microfiltration membranes from natural zeolite: Application to the treatment of cuttlefish effluents. Desalin Water Treat 2017, 59, 9–17. [Google Scholar] [CrossRef]
- Pechar, F.; Rykl, D. Infrared spectra of natural zeolites of the stilbite group. Chem. Zvesti 1981, 35, 189–202. [Google Scholar]
- Boroglu, M.S.; Gurkaynak, M.A. Fabrication and characterization of silica modified polyimide–zeolite mixed matrix membranes for gas separation properties. Polym. Bull. 2011, 66, 463–478. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Von-Kiti, E.; Nkrumah, I.; Erdoo Ikyreve, R.; Radecka, I.; Williams, C. Parametric, equilibrium, and kinetic study of the removal of salt ions from Ghanaian seawater by adsorption onto zeolite X. Desalination Water Treat. 2016, 57, 21654–21663. [Google Scholar] [CrossRef]
- Trombetta, M.; Armaroli, T.; Alejandre, A.G.; Solis, J.R.; Busca, G. An FT-IR study of the internal and external surfaces of HZSM5 zeolite. Appl. Catal. A 2000, 192, 125–136. [Google Scholar] [CrossRef]
- Renganayaki, V.; Srinivasan, S.; Suriya, S. Vibrational Spectroscopy Investigation on Acetylsalicylic acid Using Semi-Empirical Calculations. Int. J. ChemTech Res. 2012, 4, 983–990. [Google Scholar]
- RTheophanides, T. Infrared Spectroscopy; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1960. [Google Scholar]
- Krishnakumar, V.; Arivazhagan, M. Vibrational and normal coordinate analysis of thymine hydrochloride. Indian J. Pure Appl. Phys. 2003, 41, 341–345. [Google Scholar]
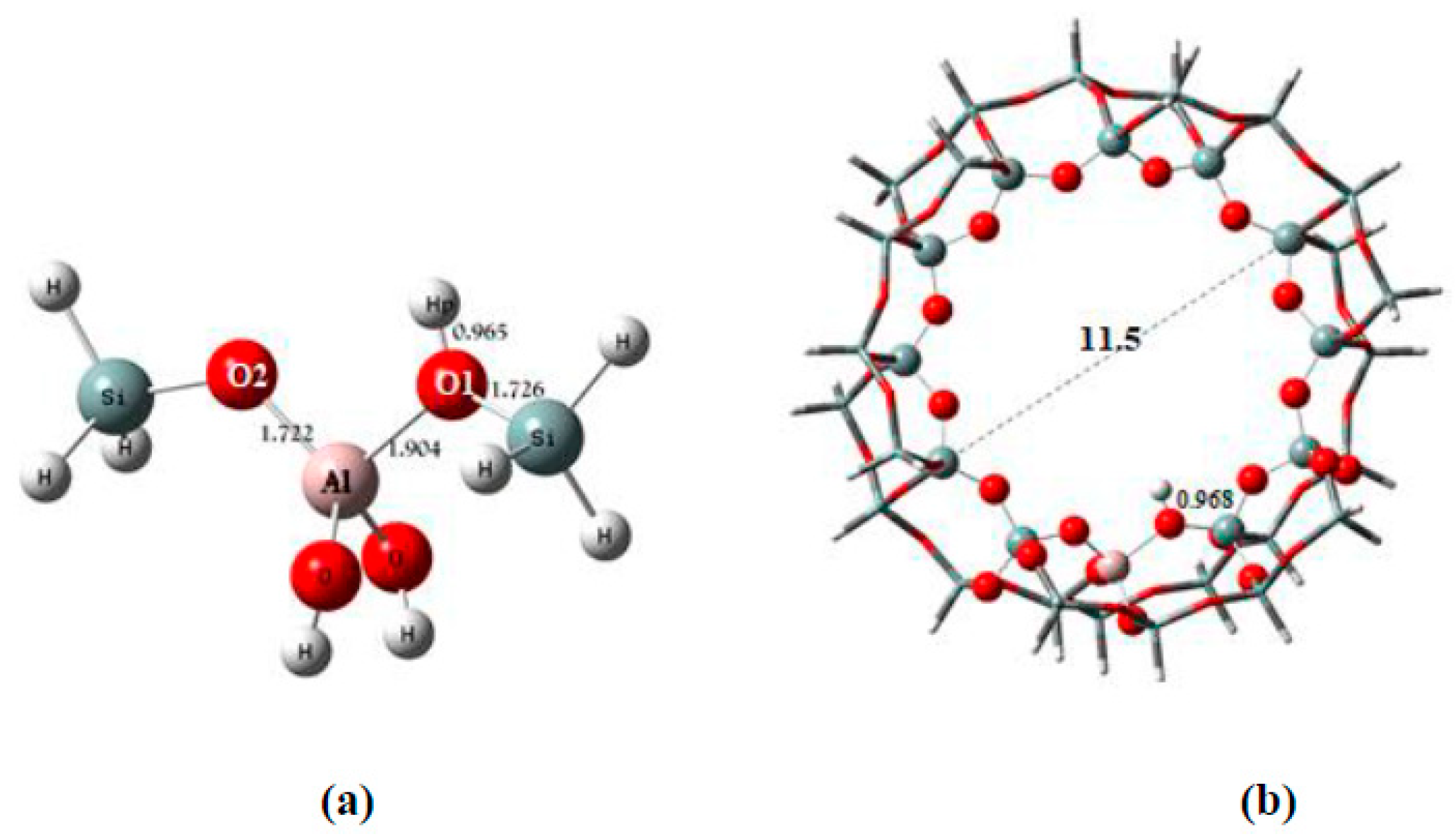

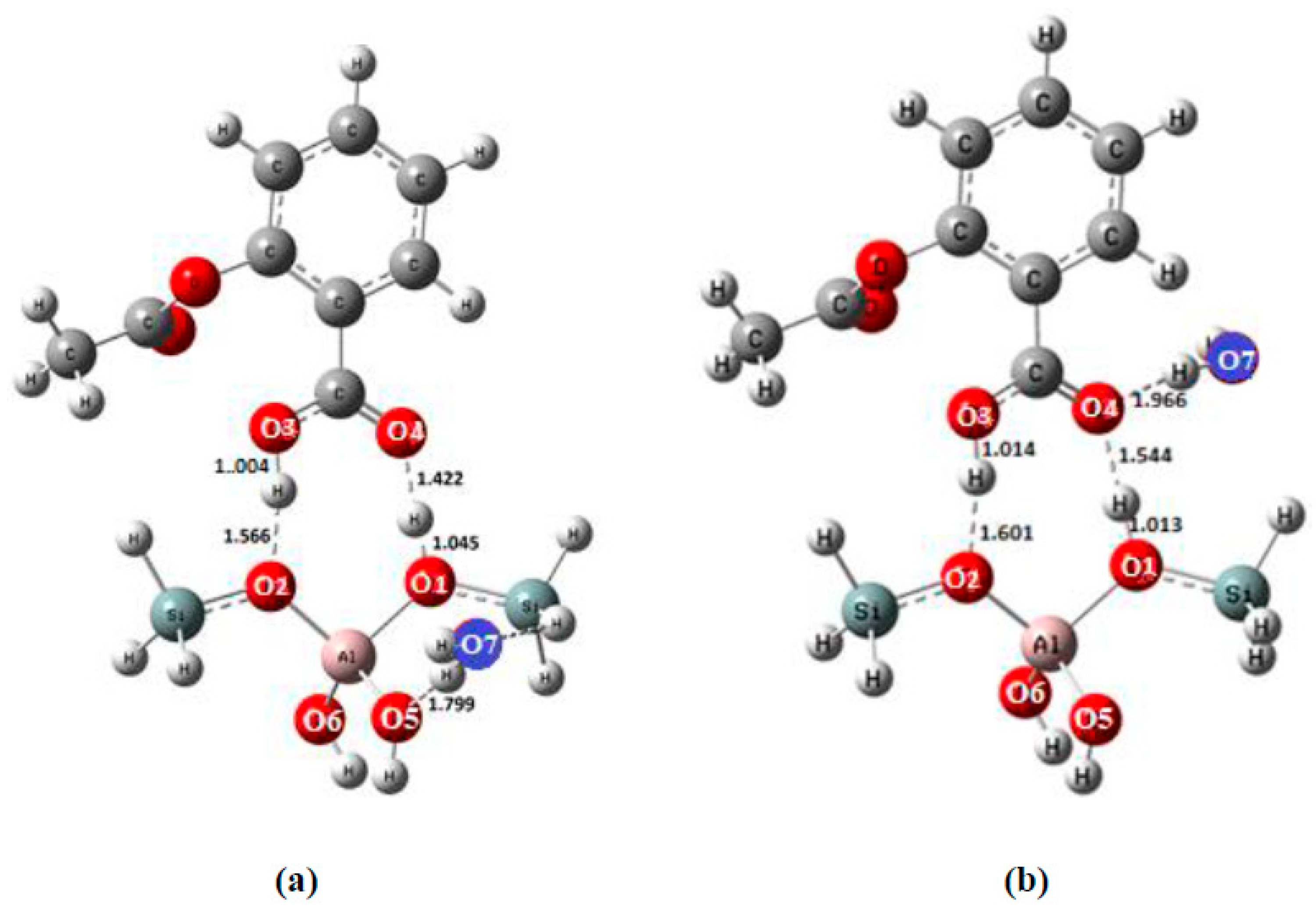
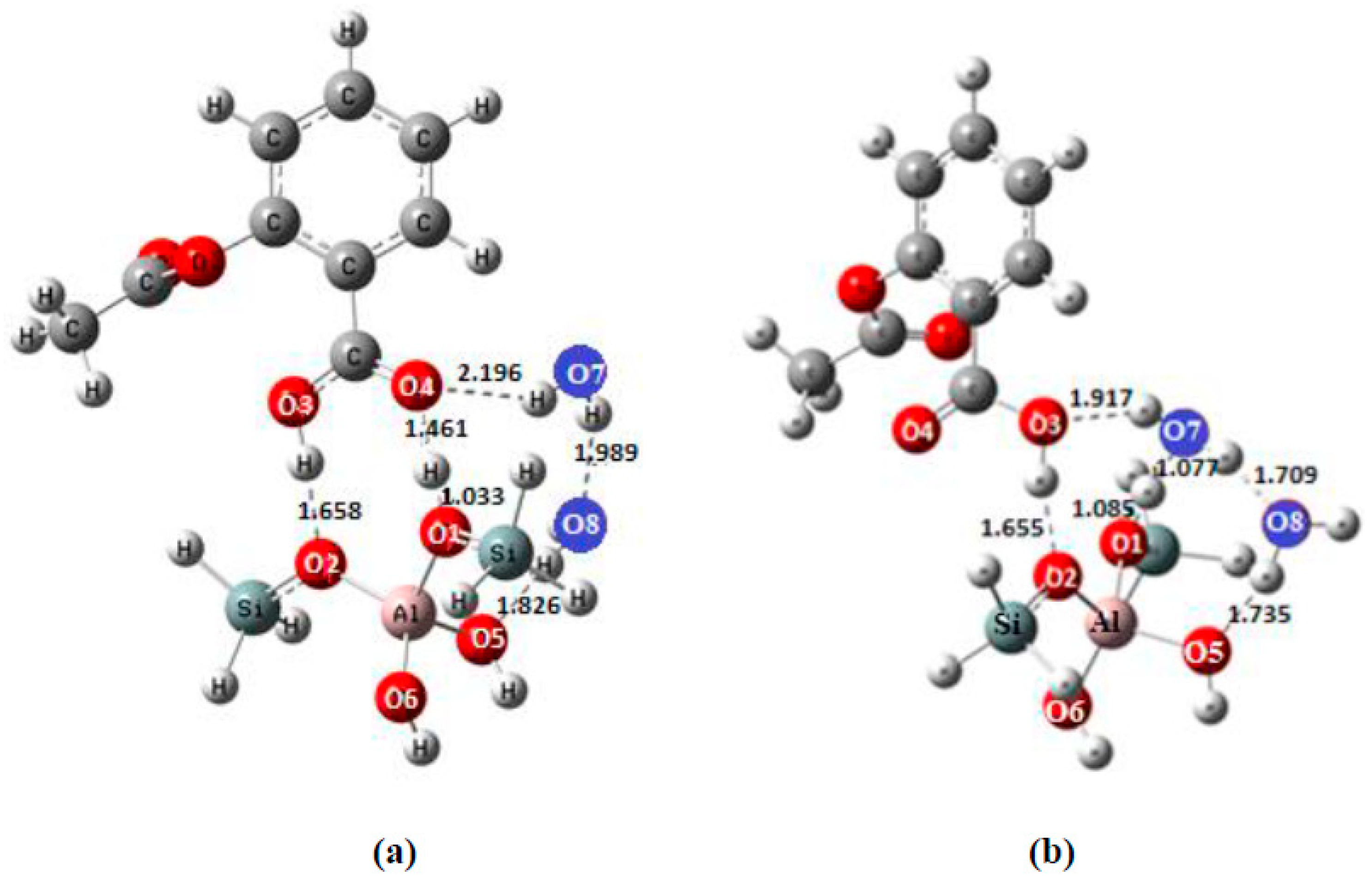
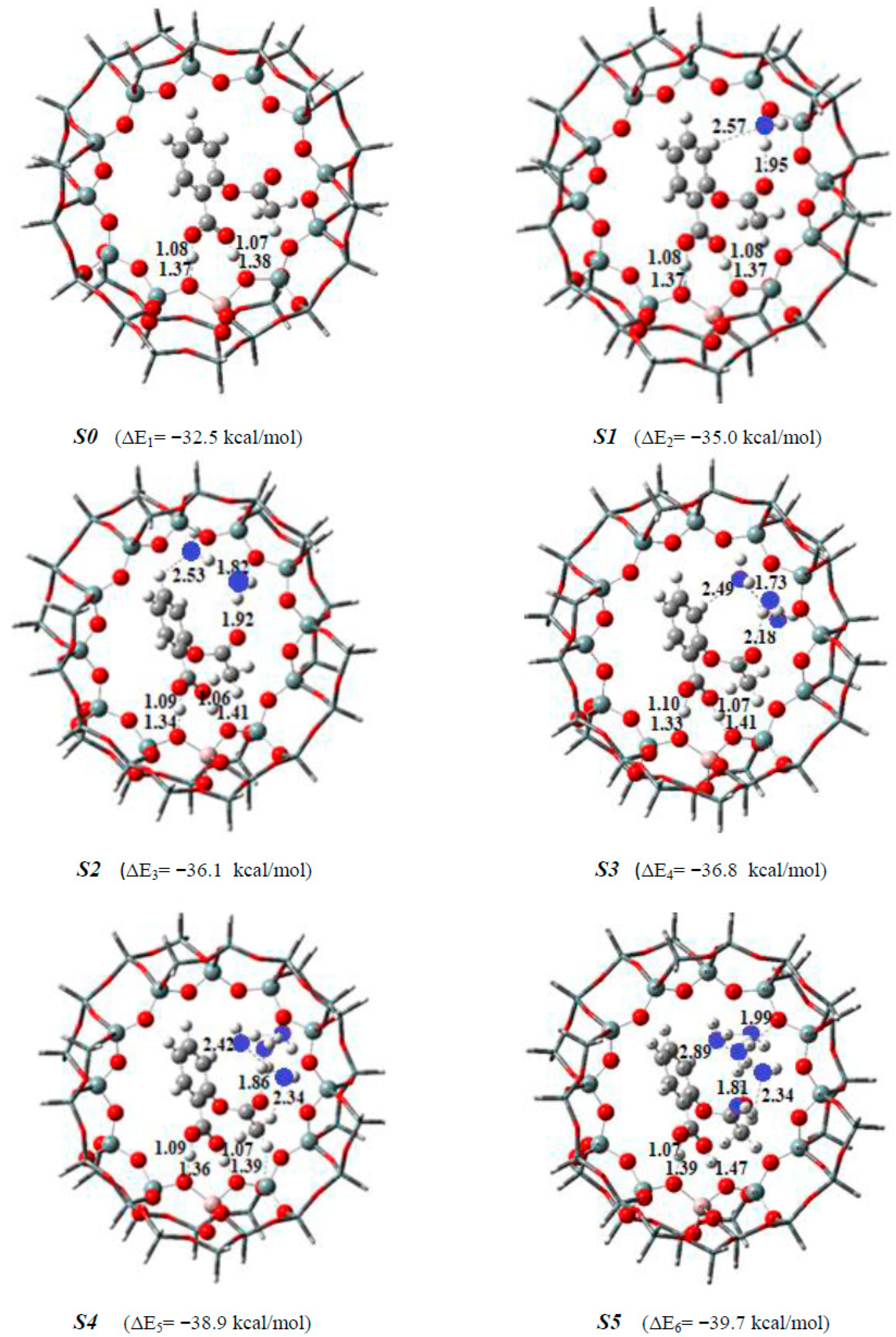
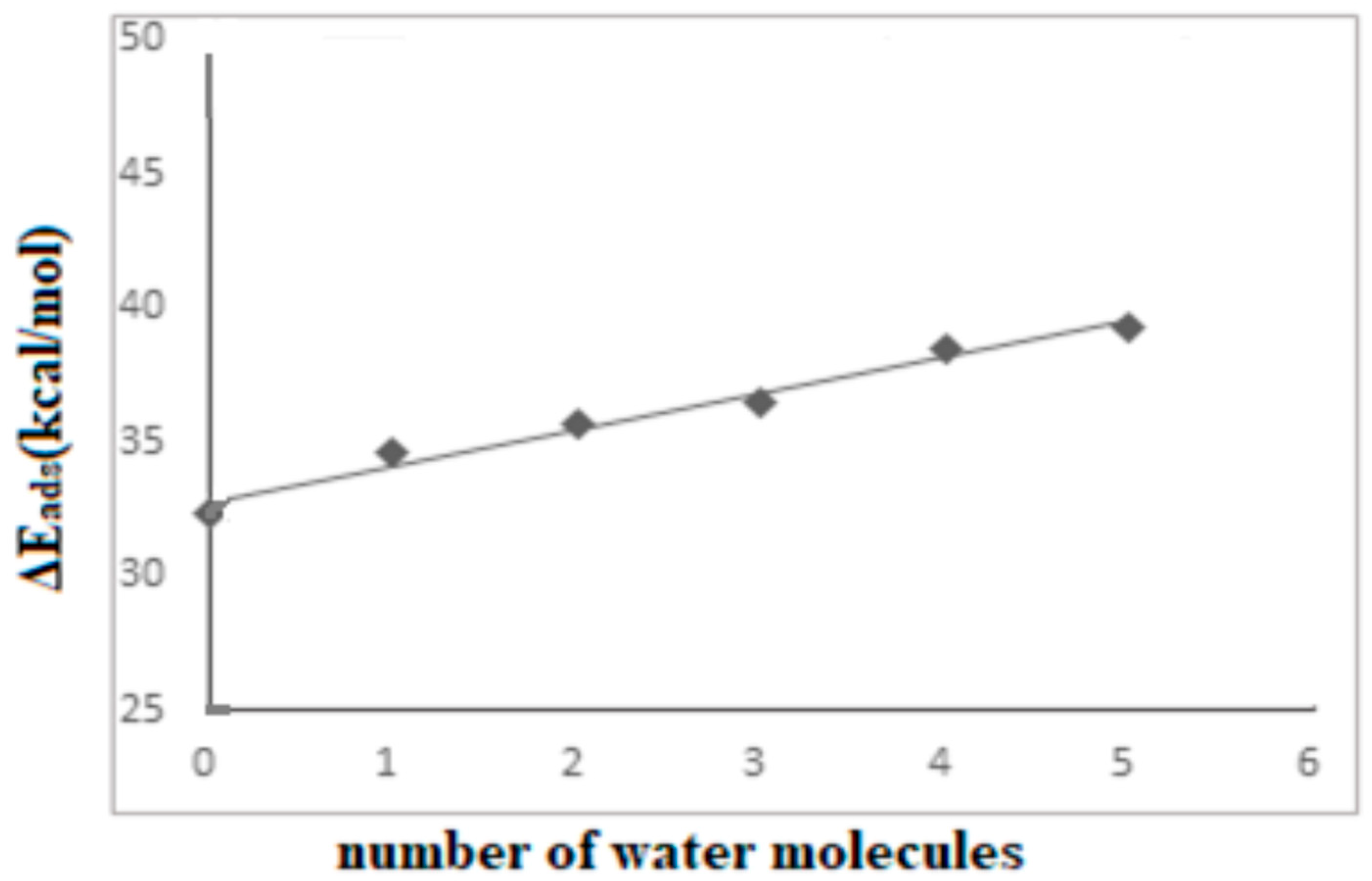
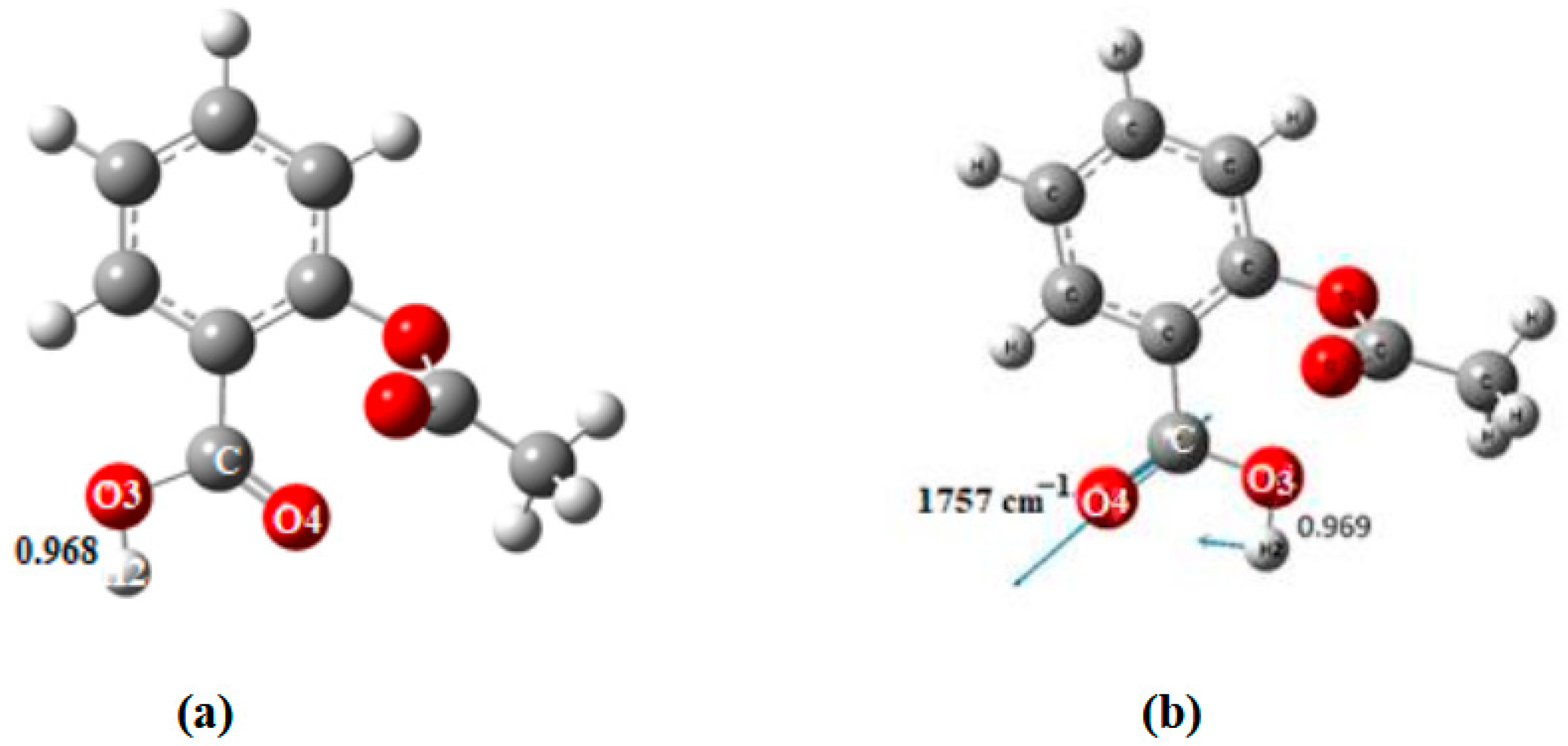
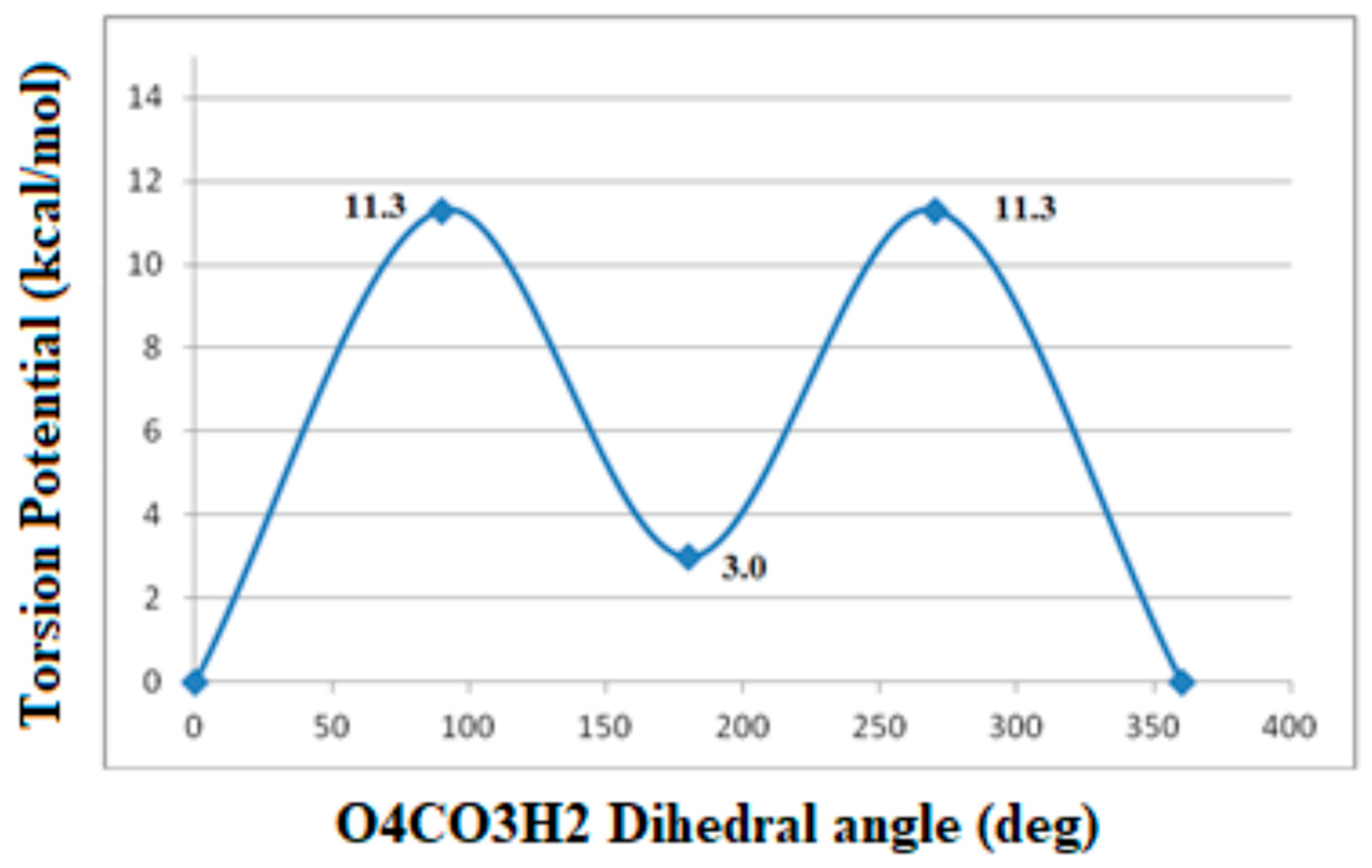
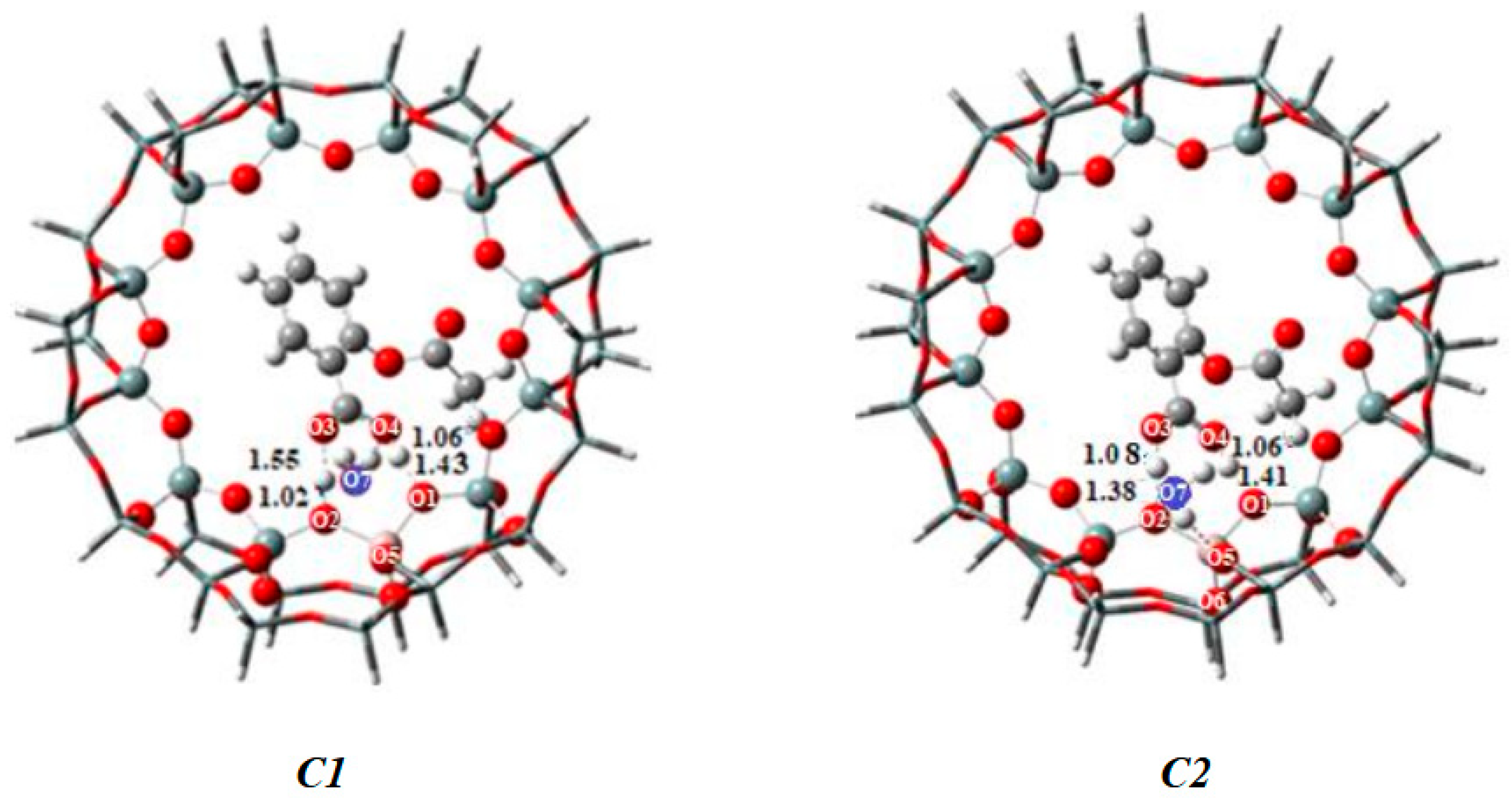
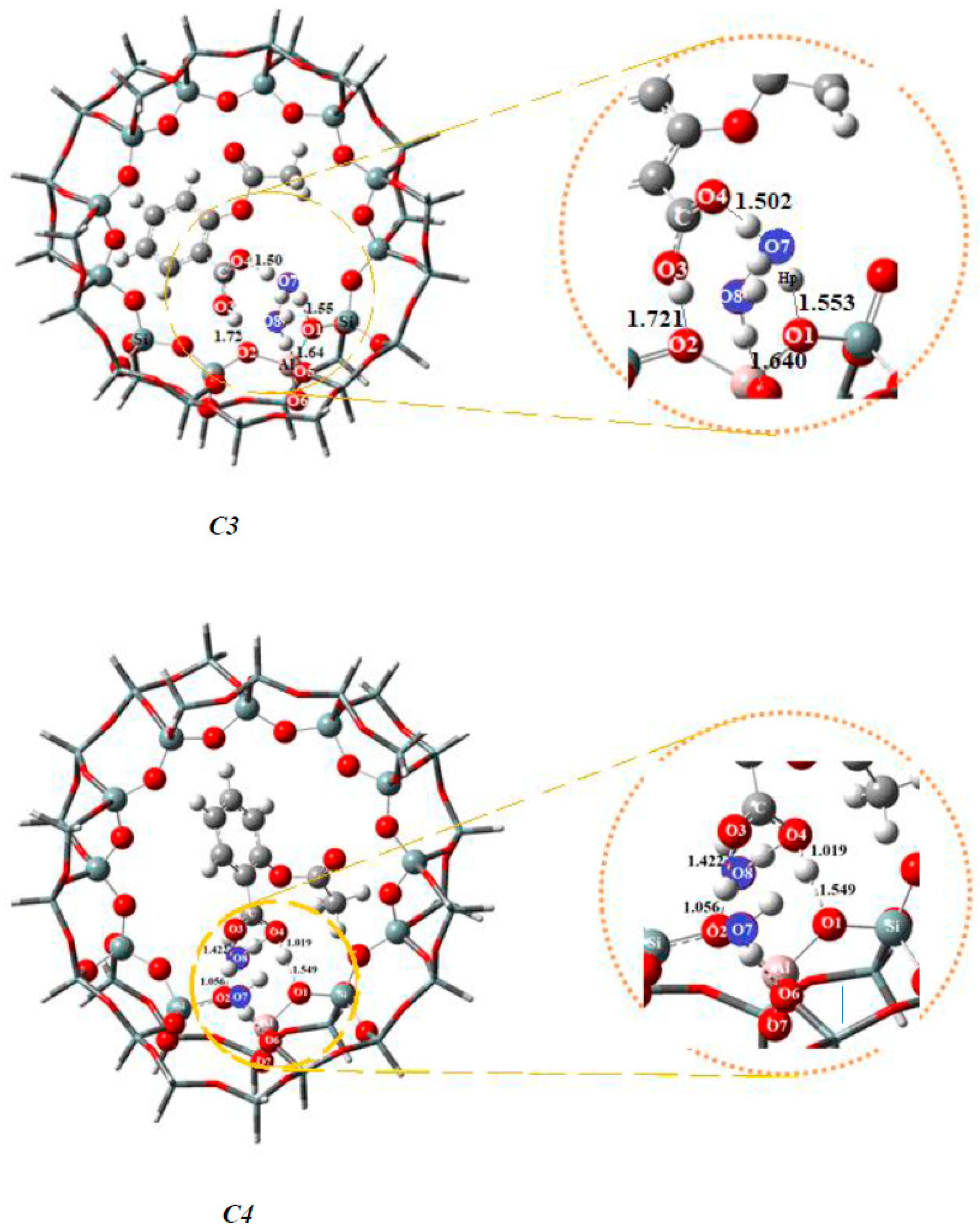


| Bond Lengths | Cluster 3T | Acetylsalicylic Acid (ASA) | Complex1 3T-ASA | Complex2 3T-ASA-1H2O | Complex3 3T-ASA-2H2O |
|---|---|---|---|---|---|
| O1-Hp | 0.965 | ------ | 1.043 | 1.045 a 1.013 b | 1.033 c 1.085 d |
| O2-H2 | ------ | ------ | 1.549 | 1.566 a 1.601 b | 1.658 c 1.655 d |
| O4-Hp | ------ | ------ | 1.427 | 1.422 a 1.544 b | 1.461 c 4.653 d |
| O3-H2 | ------ | 0.969 | 1.020 | 1.004 a 1.014 b | 1.005 c 1.004 d |
| Al-Hp | 2.464 2.38 ± 0.04 e | ------- | ------- | ------- | -------- |
| Frequencies νasCOO- | 1757 | 1654 |
| Species | Complex1 3T-ASA | Complex2 3T-ASA-1H2O | Complex3 3T-ASA-2H2O |
|---|---|---|---|
| ΔEads | −26.35 | −19.40 a, −22.15 b | −18.71 c, −14.09 d |
| ΔEads | −14.05 e | ------- | ------- |
| Bond Lengths | Cluster 38T | Acetylsalicylic Acid (ASA) | Complex1 3T-ASA | Complex2 3T-ASA-1H2O | Complex3 3T-ASA-2H2O | Complex4 3T-ASA-3H2O |
|---|---|---|---|---|---|---|
| O1-Hp | 0.968 | 1.380 | 1.427 a, 1.411 b | 1.553 c, 1.549 d | 1.533 | |
| O2-H1 | ------ | 1.372 | 1.020 a, 1.381 b | 1.721 c, 1.056 d | 1.717 | |
| O3-H1 | ------ | 0.968 | 1.081 | 1.553 a, 1.076 b | 1.006 c, 1.422 d | |
| O4-Hp | ------ | ------- | 1.073 | 1.061a, 1.061 b | 2.886 c, 1.019 d | 2.923 |
| Al-Hp | 2.401 2.38 ± 0.04 e 2.48 ± 0.04 e 2.43 ± 0.03 e | ------- | ------- | ------- | ------- | ------- |
| Frequencies νasCOO | 1757 | 1552 |
| MAP (e) | |||||
|---|---|---|---|---|---|
| Atoms | Fragments | Complex S0 38T-ASA | Complex C1 38T-ASA-1H2O | ComplexC2 38T-ASA-1H2O | |
| 38T | ASA | ||||
| O1 | −0.935 | −0.449 | −0.434 | −0.448 | |
| O2 | −0.982 | −0.49 | −0.387 | −0.486 | |
| O3 | −0.504 | −0.392 | −0.411 | −0.394 | |
| O4 | −0.458 | −0.502 | −0.522 | −0.503 | |
| Hp | +0.524 | +0.531 | +0.526 | +0.524 | |
| H1 | +0.407 | +0.536 | +0.490 | +0.532 | |
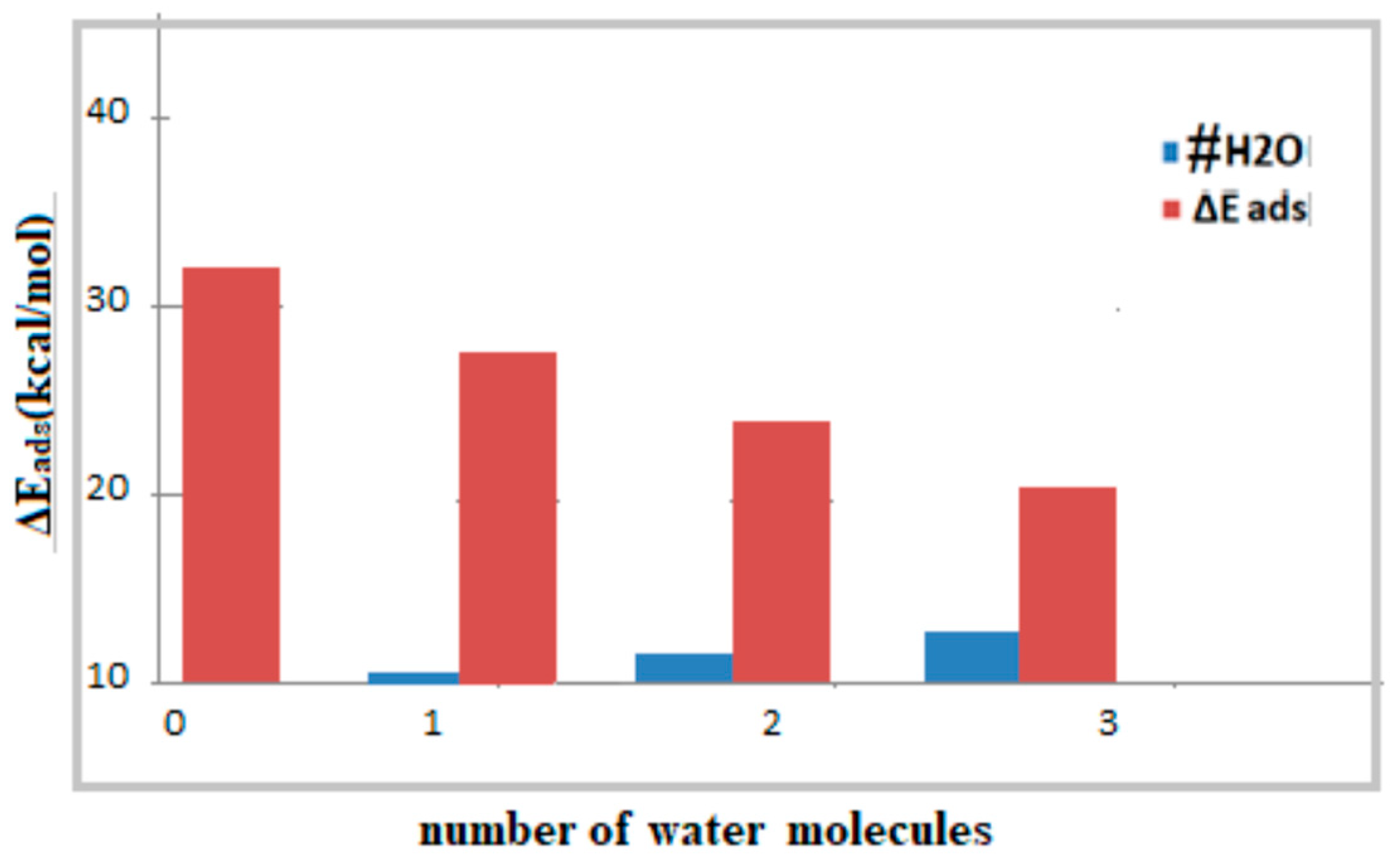
| Species | Complex1 38T-ASA | Complex2 38T-ASA-1H2O | Complex3 38T-ASA-2H2O | Complex4 38T-ASA-3H2O |
|---|---|---|---|---|
| ΔEads | −32.55 | −28.52 a | −26.01 c | −22.10 |
| ΔEads | ------- | −30.83 b | −28.09 d | ------- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioti, C.; Papayannis, D.K.; Melissas, V.S. Exploring the Role of Water Molecules in Acetylsalicylic Acid Adsorption Energy on HY Zeolite: A Density Functional Theory Approach. AppliedChem 2025, 5, 22. https://doi.org/10.3390/appliedchem5030022
Gioti C, Papayannis DK, Melissas VS. Exploring the Role of Water Molecules in Acetylsalicylic Acid Adsorption Energy on HY Zeolite: A Density Functional Theory Approach. AppliedChem. 2025; 5(3):22. https://doi.org/10.3390/appliedchem5030022
Chicago/Turabian StyleGioti, Christina, Dimitrios K. Papayannis, and Vasilios S. Melissas. 2025. "Exploring the Role of Water Molecules in Acetylsalicylic Acid Adsorption Energy on HY Zeolite: A Density Functional Theory Approach" AppliedChem 5, no. 3: 22. https://doi.org/10.3390/appliedchem5030022
APA StyleGioti, C., Papayannis, D. K., & Melissas, V. S. (2025). Exploring the Role of Water Molecules in Acetylsalicylic Acid Adsorption Energy on HY Zeolite: A Density Functional Theory Approach. AppliedChem, 5(3), 22. https://doi.org/10.3390/appliedchem5030022






