Abstract
Climate change has been associated with intensified harmful algal blooms (HABs). Some harmful microalgae produce toxins that accumulate in food webs, adversely affecting the environment, public health and economy. Ocean acidification (OA) is a major consequence of high anthropogenic CO2 emissions. The carbon chemistry and pH of aquatic ecosystems have been significantly altered as a result. The impacts of climate change on the metabolisms of microalgae, especially toxin biosynthesis, remain largely unknown. This hinders the optimization of HAB mitigation for changed climate conditions. To bridge this knowledge gap, previous studies on the effects of ocean acidification on toxin biosynthesis in microalgae were reviewed. There was no solid conclusion for the toxicity change of saxitoxin-producing dinoflagellates from the genus Alexandrium after high CO2 treatment. Increased domoic acid content was observed in the diatom Pseudo-nitzschia. The brevetoxin content of Karenia brevis remained largely unchanged. The underlying regulatory mechanisms that account for the different toxicity levels observed have not been elucidated. Metabolic flux analysis is useful for investigating the carbon allocations of toxic microalgae under OA and revealing related metabolic pathways for toxin biosynthesis. Gaining knowledge of the responses of microalgae in high CO2 conditions will allow the better risk assessment of HABs in the future.
1. Introduction
Harmful algal blooms (HABs) refer to the rapid growth of phytoplankton, including cyanobacteria, dinoflagellates, raphidophytes, haptophytes, and macroalgae, which exerts harmful effects on the environment, human health, and the economy [1]. High microalgal biomass accumulation during HABs can cause hypoxia, suffocating surrounding aquatic organisms [2]. Some HAB species can produce algal toxins that accumulate in aquatic food webs [3]. Apart from causing the massive death of marine mammals and fish [4], algal toxins can lead to intoxication in humans. Approximately 50,000 to 500,000 cases of intoxication with a 1.5% mortality rate have been reported annually due to the consumption of contaminated shellfish or fish [5]. The health costs associated with HABs are substantial, ranging from around USD 90 to USD 12,000 for digestive and respiratory illnesses with different severities [6]. The aquacultural industry is the most vulnerable when considering the direct economic losses caused by HABs. From the 1980s to the 2010s, the aquacultural industry in Korea lost a total of USD 121 million due to HAB-induced fish and shellfish deaths [7]. In addition to the aquacultural industry, tourism is adversely affected by HABs. It was reported that the monthly revenue of coastal lodgings and restaurants decreased by USD 2.8 and 3.7 million, respectively, in Fort Walton Beach and Destin in Florida when HABs occurred [8]. To mitigate the negative effects of HABs, costly monitoring programs and mitigation measures are conducted in different regions, such as the US, Europe, and Australia, as well as by intergovernmental organizations [9].
The direct link between HABs and climate change was first affirmed in the Special Report on the Ocean and Cryosphere in a Changing Climate, published by the Intergovernmental Panel on Climate Change in 2019 [1]. Climate change has profoundly changed the aquatic environment over the years by increasing the surface temperature, acidifying water bodies, shifting nutrient availability, altering salinity, etc. Several studies have reviewed the association between climate change and intensified HABs [10,11,12,13]. In parallel with the spatial expansions and frequencies of HABs, the frequency and distribution of human intoxication by algal toxins have also increased globally [4]. According to the Harmful Algae Event Database (HAEDAT), 1598 HAB-related seafood poisoning cases were reported during the 20th century. A nearly five-fold increase in the number of cases (7843) was observed from 2001 to 2022. Intensified HABs can also further alter the food webs and disturb the ecosystem. The physiology, mortality, and toxicity of marine organisms at higher trophic levels were found to have changed when co-exposed to algal toxins and climate change stressors, as summarized by Griffith and Gobler [14]. Given the above-mentioned scenario, it is believed that the adverse effects of HABs will be aggravated in the future.
2. Algal Toxins
Intensified HABs of toxic microalgae may increase the algal toxins in aquacultures, subsequently raising the risks of seafood poisoning [15]. Consumption of contaminated seafood can lead to different poisoning syndromes, depending on the types of algal toxins accumulated in shellfish during HABs. As of December 2019, the incidence of paralytic shellfish poisoning (PSP) and diarrhetic shellfish poisoning (DSP) accounted for approximately one third of the reported cases of human intoxication, with PSP contributing 35% and DSP contributing 30% [16]. According to the data, the incidence of amnesic shellfish poisoning (ASP) was relatively low, at 9%, whereas both neurotoxic shellfish poisoning (NSP) and azaspiracid shellfish poisoning (AZP) each accounted for only 1% of cases.
2.1. Paralytic Shellfish Poisoning
The causative agents of PSP, saxitoxin (STX) and its 57 analogues, are collectively known as paralytic shellfish toxins (PSTs) [17]. STX is composed of a tricyclic 3,4-propinoperhydropurine backbone with two guanidine groups [18]. The analogues have been classified into different groups according to the side group moieties. PSTs are mainly produced by eukaryotic marine dinoflagellates from the genera Alexandrium, Gymnodinium, and Pyrodinium [19,20]. Prokaryotic freshwater cyanobacteria from the genera Anabaena, Cylindrospermopsis, Aphanizomenon, Planktothrix, and Lyngbya are producers of PSTs as well [21,22,23,24]. The toxicity levels of these analogues vary depending on the structures. Generally, the toxicity levels of STXs are inversely proportional to the degree of sulfation [17]. PSTs selectively and reversibly bind to receptor site 1 of the voltage-gated sodium channels in the nerves and muscles [25]. Due to the blockage of the channels, the propagation of action potential is terminated, leading to symptoms such as paralysis, burning sensations, and numbness [26]. In serious cases, death can result due to respiratory failure [27]. Most reported PSP cases were contributed by the toxic Alexandrium species, which are mainly present in Northern Europe, the Mediterranean, Northern Asia, East Asia, and North America [28,29,30,31]. Recently, the geographical distribution of Alexandrium has been expanded towards the poles [32].
2.2. Diarrhetic Shellfish Poisoning
Okadaic acid and its derivatives, the dinophysistoxins (DTXs), are lipophilic polyketides predominately produced by toxic dinoflagellates from the genera Prorocentrum and Dynophysis [33]. For human adults, DSP symptoms occur when a minimum dosage of 40 µg okadaic acid equivalents is consumed [34]. According to the Food and Agriculture Organization (FAO), the recommended okadaic equivalents for okadaic acid, DTX1, and DTX2 are 1.0, 1.0, and 0.5, respectively [35]. Diarrhea, nausea, vomiting, and abdominal pain are the common symptoms of DSP [36]. DSP is generally not life-threatening, and hospitalization is not required [37]. As potent inhibitors of serine/threonine phosphatases [38], okadaic acids show specifically high binding affinity to protein phosphatase 1 (PP1) and protein phosphatase 2 (PP2) [39]. The gastrointestinal symptoms of DSP may be a result of inhibited intestinal PP activities by okadaic acids [40]. In addition, the inhibiting effect of okadaic acids may induce the hyperphosphorylation of proteins that regulate the sodium secretion of intestinal cells [41]. The release of sodium from cells disturbs the osmotic gradient balance and subsequently causes the passive loss of fluids, leading to diarrhea. Both Prorocentrum and Dynophysis have expanded their niches in recent years. P. minimum is the most studied Prorocentrum species and is widespread in the Black Sea, the Baltic Sea, Lake Nakanoumi, the Mexican coast, and the Philippines [42]. The expansion of Dynophysis was observed in the coastal areas of the United States, Canada, and South Africa [13]. Moreover, increased abundances of Prorocentrum and Dynophysis in the Northeast Atlantic were reported [43].
2.3. Amnesic Shellfish Poisoning
ASP is caused by the consumption of domoic acid (DA)-accumulated shellfish. Red algae such as Chondria armata and diatoms from the genera Pseudo-nitzschia and Nitzchia (N. navis-varingica and N. bizertensis) are the primary producers of DA [44,45]. DA, a tricarboxylic amino acid, is analogous to glutamic acid and kainic acid [46]. Several DA isomers (isodomic acid A-H, 5′ epi-DA) have been identified [47]. With such structural similarity, Das can bind and activate three ionotropic glutamate receptor subtypes (N-methyl-D-aspartate (NMDA), kainate, and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors) located on the dendrites of postsynaptic cells [48,49]. An influx of calcium ions (Ca2+) into neurons is induced upon the activation of kainate and AMPA receptors [50], while an influx of both Ca2+ and sodium ions (Na+) is induced upon the activation of the NMDA receptor [51]. Desensitization is prevented by the low conformational mobility of DAs when docked to the receptors, leading to a continuous flow of cations to the postsynaptic cells [52]. DAs exert excitotoxic effects primarily through excess intracellular Ca2+, which triggers the mobilization of glutamate-containing vesicles towards the membrane surface, followed by the release of glutamate (Glu) into the synaptic cleft [53]. Excess Glu results in neurodegeneration and apoptosis [54,55]. Since neurons in the hippocampus, where the consolidation of memories takes place, are affected by DAs [56], symptoms such as short-term memory loss and anterograde amnesia may result [57]. Generally, the toxicity of DA isomers is lower than that of DA because of the lower binding affinity of DA isomers to glutamate receptors [58,59,60]. One of the major DA producers, Pseudo-nitzschia, has been detected in the Pacific Ocean and the Southern Ocean [61]. Expanded distributions of Pseudo-nitzschia and N. navis-varingica have been observed recently [44]. Several locations, including the Arctic, Angola, Singapore, Ukraine, and Pakistan, have recorded the presence of Pseudo-nitzschia for the first time, while N. navis-varingica has expanded to Malaysia, Australia, Indonesia, and the Philippines.
2.4. Neurotoxic Shellfish Poisoning
NSP is caused by the consumption of shellfish contaminated with brevetoxins (PbTx). PbTx are a set of cyclic polyether neurotoxins primarily produced by dinoflagellate Karenia brevis [62]. Besides K. brevis, recent research indicated PbTx production by raphidophyte Chattonella cf. verruculosa [63]. PbTx-1 and PbTx-2 are regarded as the parent molecules for other derivatives based on their different structural backbones. The toxicity of all identified natural derivatives is lower than that of the parent molecules [64]. PbTx bind specifically to receptor site five of the voltage-gated sodium channels in the nerves and muscles, with a preference for those located in the nerves [65,66]. The influx of Na+ ions into the cells is induced upon the activation of the sodium channels. Prolonged membrane depolarization persistently initiates the generation of action potentials in nerves and muscles, causing symptoms such as paresthesia, vertigo, and malaise [67]. Blooms of K. brevis occur nearly annually along the southwestern coast of Florida, which is notoriously known as the “Florida red tide” [68]. The low incident rate of NSP may be credited to the successful monitoring program of constantly occurring red tides. Apart from Florida, where regular red tides take place, a few large outbreaks of NSP have been reported in New Zealand and North Carolina [69].
2.5. Azaspiracid Shellfish Poisoning
Azaspiracids (AZAs) are polyether phytotoxins produced by some dinoflagellate species from the genera Azadinium and Amphidoma and the causative toxin of AZP [70]. AZA was named based on its unique spiro ring assemblies [71]. To date, over 60 AZA analogues have been identified [72]. They are different in the degrees of methylation and/or the number of hydroxyl groups and carboxyl groups that they possess. However, among the numerous analogues, only AZA1, AZA2, and AZA3 levels are monitored for regulatory purposes [33]. The symptoms of AZP are similar to those of DSP, including diarrhea, vomiting, nausea, and stomach cramps [73]. A minimum dosage ranging from 23 to 86 µg/person of AZA can have observable adverse health effects [74]. Unlike other phytotoxins, the molecular target(s) of AZAs has not been fully understood. Although the blocking of the hERG (human ether-à-go-go related gene) potassium channel by AZA1-3 was demonstrated in a recent study, relatively high concentrations of AZAs were required [75]. Therefore, there may be other molecular targets that have not been identified. The presence of toxigenic Azadinium species has been reported worldwide, including in the North Atlantic [76,77], Eastern South Atlantic [78], Mediterranean [79], Western Pacific [80], Eastern North Pacific [81], and Eastern South Pacific [82]. On the other hand, the occurrence of toxigenic Amphidoma species was only recorded in the North Atlantic [77,83,84].
3. Ocean Acidification
Ocean acidification (OA) is one of the major aspects of climate change and has indeed been significantly influencing the aquatic environment, where toxigenic microalgae live. Carbon dioxide (CO2) emissions have increased dramatically since the Industrial Revolution, primarily due to the combustion of fossil fuels. At present, the recorded atmospheric CO2 level is 417 ppm [85], which has increased by nearly 50% compared to the pre-industrial level. The atmospheric CO2 concentrations will continue to rise until the end of this century unless a very stringent CO2 emissions trajectory is satisfied (Representative Concentration Pathway 2.6) [86]. A large amount of atmospheric CO2 traps and prevents heat from escaping the planet, driving global warming and subsequent climate change. As a natural carbon sink, the ocean has absorbed around 48% of anthropogenic CO2 since the Industrial Revolution [87]. Given the elevated atmospheric CO2 levels, more CO2 has dissolved in the ocean. The dissolution of atmospheric CO2 produces carbonic acid (H2CO3), which dissociates to form bicarbonate ions (HCO3−) and hydrogen ions (H+). HCO3− further dissociates into carbonate ions (CO32−) and H+. The rise in the concentration of H+ in turn has acidified the ocean, resulting in OA. OA has altered the carbonate chemistry of the aquatic system. Excess H+ released from the dissociation of dissolved CO2 creates an imbalance in the carbonate equilibrium. The equilibrium is attained by the natural buffering capacity of the ocean. The free CO32− in the ocean binds with the excess H+ to produce more HCO3−. As a result, the dissolved CO2 and HCO3− concentrations increase while the CO32− levels decrease under OA. Since the decrease in CO32− levels leads to a lower saturation state of CaCO3, OA is particularly detrimental to marine-calcifying organisms such as molluscs, coccolithophores, and corals [88].
OA (increased pCO2/decreased pH) alters the carbon chemistry of the aquatic environment and acidifies the water. Microalgae have shown great adaptability towards different abiotic stress factors [89,90]. Therefore, it is believed that microalgae will respond and acclimate to OA by regulating their metabolic activities, which may in turn affect their toxicity. However, the effects of OA on microalgal toxicity and the underlying mechanisms have not been well characterized. Apart from leading to shellfish poisoning in humans, several studies have indicated the bioaccumulation of algal toxins in predators of microalgae and the biomagnification of algal toxins through food webs, which cause disease or even death among marine organisms [91,92,93,94,95]. A recent study suggested that the bioavailability of STX would increase under global warming and ocean acidification [96]. Although the bioavailability of STX and other phycotoxins in high CO2 conditions has not been fully examined, the possibility that more toxins can be accumulated along the food chain and harm the health of marine organisms and humans should not be neglected [96]. Predicting the toxicity of microalgae under OA is therefore important to evaluate the public health concerns and ecological impacts of intensified HABs in future high atmospheric CO2 scenarios, which will provide insights for policymakers to improve the monitoring programs for HABs. Considering the above, this paper reviewed the effects of OA on the toxicity of microalgae, and the underlying mechanisms proposed.
3.1. Effects of OA on the Toxicity of Microalgae
No studies that investigated the effects of OA on the toxicity changes in microalgae that produce okadaic acid and azaspiracids could be found in the literature. Therefore, the scope of the review was limited to microalgae that synthesize STXs, DA, and PbTx. The experimental setups and significant findings of the reviewed studies are summarized in Table 1.

Table 1.
Summary of reviewed studies on the effects of OA on toxigenic microalgae.
3.1.1. STXs-Producing Microalgae
Dinoflagellates from the genus Alexandrium are the most studied STX producers. However, there is not yet any solid conclusion drawn for the toxicity change in Alexandrium at elevated pCO2. Increases in cellular toxin levels were detected in A. catenella, A. minutum, and A. fundyense after high CO2 treatment [97,98,99]. It is believed that elevated pCO2 will permit microalgae to perform photosynthesis when more substrates are available for their unsaturated carbon-fixing enzyme ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco) [108]. Dinoflagellates may be particularly sensitive to elevated pCO2 as they possess type II Rubisco, which has a higher affinity with O2, a competitive inhibitor of CO2 [109]. Since carbon is essential for the synthesis of biomolecules, including phycotoxins, increased production of fixed carbon at elevated pCO2 may thus contribute to the higher toxicity of microalgae [99]. However, the carbon fixation rates of A. catenella, A. minutum, and A. fundyense after high CO2 treatment were not measured to support this hypothesis.
Another plausible explanation for the enhanced production of STXs is the increased availability of STX precursors [98]. To initiate the synthesis of STX, arginine (Arg), methionine (Met), and acetate are required [110]. In the study performed by Lian, Li, He, Chen, and Yu [98], the concentrations of Arg and Met were quantified in A. minutum at elevated pCO2. It was found that the content of Arg and Met increased at the beginning of the experiment and then decreased gradually. The increase in the Arg level was related to the enhanced activity of argininosuccinate synthase, an enzyme responsible for a rate-limiting step in Arg synthesis. However, the mechanisms behind the rise in the Met level remained undetermined. Since the STX content increased after the decline in Arg and Met levels with a slight delay, it was suggested that elevated pCO2 had indirectly promoted STX production by increasing Arg and Met supplies.
On the other hand, significant decreases in STX content were detected in both A. tamarense and A. ostenfeldii at elevated pCO2, resulting in lower toxicity [100,101]. Van de Waal, Eberlein, John, Wohlrab, and Rost [101] isolated the RNA of A. tamarense cultured at different pCO2 (180, 380, 800, 1200 ppm) to perform microarray-based gene expression analysis. When comparing the 800 ppm group to the 380 ppm group (control), 1238 differentially expressed genes (DEGs) were found. Genes associated with amino acid transport and metabolism were downregulated, implying that fewer Arg and Met molecules were synthesized and available for STX biosynthesis at elevated pCO2.
The differences in the toxicity of Alexandrium species may be caused by genetic variations between species. The gene expression patterns of the toxic A. fundyense and A. tamarense strains revealed by Taroncher-Oldenburg and Anderson [111] indicated the high interspecific variations between them. In parallel with this previous finding, A. fundyense and A. tamarense responded differently under OA. While A. fundyense promoted STX production at elevated pCO2 [97], A. tamarense inhibited STX production by reducing the synthesis of STX precursors [101]. The Alexandrium species are likely to regulate their metabolism differently under OA due to the interspecific variations in genetic expression, thus contributing to the toxicity differences.
Regarding the STX composition of Alexandrium, increased GTX1/4 is a common change observed in A. catenella and A. tamarense, although the toxin profiles of Alexandrium are highly varied [99,101,112]. According to the gene expression profile of A. tamarense, sulfur metabolism was differentially regulated under high CO2 treatment [101]. The significant upregulation of a putative sxtN homologue encoding a sulfotransferase involved in synthesizing sulfated STXs was found [113,114,115,116]. In contrast, genes encoding sulfatases that are responsible for the hydrolysis of sulfate esters were downregulated. Together, they might promote the transformation of non-sulfated STXs to sulfated STXs while inhibiting the transformation of sulfated STXs to non-sulfated STXs. Moreover, the downregulation of genes encoding sulfite reductase was reported. Inhibition of assimilated sulfur into amino acids might result in more sulfur being allocated for synthesizing sulfated STXs [117,118]. In parallel with these, lower non-sulfated STX content but higher sulfated GTX1/4 and C1/2 content of A. tamarense at elevated pCO2 were observed. Nevertheless, increases in both non-sulfated and sulfated STX levels were exhibited in A. catenella after high CO2 treatment [99], suggesting that the toxin composition could be altered by different metabolic pathways besides sulfur metabolism.
3.1.2. DA-Producing Microalgae
The diatom Pseudo-nitzschia has been extensively studied compared to other DA producers. Generally, both laboratory and mesocosm studies revealed increased cellular DA content of Pseudo-nitzschia at elevated pCO2 [102,103,104,105]. In line with the assumption that excess fixed carbon may be shunted for toxin biosynthesis in microalgae under OA [99], an increased growth rate, carbon fixation rate, and cellular DA content were reported in P. fraudulenta at elevated pCO2 [103]. Nonetheless, there were no consistent findings of P. australis [104]. The cellular DA content of P. australis was largely unchanged despite the increased carbon fixation rate at elevated pCO2.
For DA-producing Pseudo-nitzchia, environmental pH may also play a role in toxin biosynthesis by affecting cellular enzymatic activities, metal speciation, and the composition of symbiotic bacteria [119]. The intracellular pH of diatoms may be modified or maintained by regulating the metabolism under acidification [120]. Although research on the intracellular pH change of Pseudo-nitzchia at elevated pCO2 is lacking, the effects of acidification on DA biosynthesis should not be neglected. Lundholm, Hansen, and Kotaki [119] proposed that there might be an optimal pH for DA biosynthesis; however, it remains to be determined as an increased cellular DA level was detected under both low and high pH conditions in Pseudo-nitzschia [102,103,119,121]. The DA biosynthetic pathway has been modeled recently based on the transcriptome sequencing of P. multiseries [122]. The intracellular pH of Pseudo-nitzschia may be modified under acidification, which possibly affects the activities of putative DA biosynthetic (Dab) enzymes including terpene cyclase (dabA), hypothetical protein (dabB), alpha-ketoglutarate-dependent dioxygenase (dabC), and CYP450 (dabD), causing changes in cellular DA content.
The toxicity and availability of metals are likely to be affected by a decreased pH. For example, a rise in the concentrations of free copper (Cu2+) and dissolved iron (Fe3+) was expected at lower pH levels due to increased solubility [123]. While the Cu2+ level is generally closely related to its toxicity in phytoplankton [124], Fe3+ is one of the essential micronutrients for microalgal growth. Although the ecological and physiological roles of DA have not been well established, it was suggested that DA might be a trace metal chelator involved in iron acquisition and copper detoxification, given its similar structure to phytosiderophores [125]. Therefore, increases in Cu2+ and Fe3+ levels under an acidic environment may stimulate the DA production of Pseudo-nitzschia. However, both the cellular and dissolved DA content of P. multiseries remained largely unchanged after exposure to different copper levels [126]. On the other hand, a positive relationship between the iron concentration and total DA content in P. multiseries was indicated [127]. Thus, enhanced DA production at elevated pCO2 may be mainly contributed by the increased concentration of Fe3+ rather than Cu2+ under acidification.
Symbiotic bacteria are related to DA biosynthesis in Pseudo-nitzschia. Xenic cultures of P. multiseries exhibited increased DA content when compared with axenic cultures [128]. While free-living bacteria were likely to be incapable of producing DA autonomously [129,130], it is difficult to obtain a conclusion for epiphytic bacteria due to the difficulties in isolating them from the diatoms. Research on the bacteria–phytoplankton interaction mechanisms for DA biosynthesis in Pseudo-nitzschia is lacking. However, it was proposed that the attached bacteria might exchange metabolites that are used for DA biosynthesis with Pseudo-nitzschia [130]. Since bacterial abundance and diversity were shown to be affected by a decreased environmental pH [131], symbiotic bacteria that promote DA biosynthesis may become more abundant at lower pH levels given the observation of increased cellular DA content in Pseudo-nitzschia.
3.1.3. PbTx-Producing Microalgae
Dinoflagellate K. brevis is the primary producer of PbTx and its response to OA has been studied. Elevated pCO2 had no significant effect on the cellular PbTx content of K. brevis [106,107]. Although K. brevis shifted its inorganic carbon preference from HCO3− to CO2 and the half-saturation constant (K1/2) for CO2 increased under high CO2 treatment, the growth rate, carbon fixation rate, and cellular carbon composition remained largely unchanged [106]. The relative insensitivity of K. brevis to elevated pCO2 was surprising as high CO2 availability had been thought to be especially beneficial to dinoflagellates that possess inefficient type II Rubisco [109].
3.2. OA May Affect the Central Carbon Metabolism of Toxic Microalgae
Based on the above, OA can affect the toxicity of some species of dinoflagellate Alexandrium and diatom Pseudo-nitzschia. On the other hand, dinoflagellate K. brevis was shown to be somewhat more resistant to OA and its toxicity was largely unchanged. The way in which they regulate central carbon metabolism under OA is likely to contribute to the changes in toxicity or to help to achieve homeostasis. OA was shown to be beneficial to P. fraudulenta as it promoted the carbon fixation rate by increasing inorganic carbon availability [103]. The increased growth rate and cellular toxin content gave rise to the hypothesis that excess carbon not used for growth might be shunted for toxin biosynthesis [99]. However, the positive relationship between the carbon fixation rate and toxin level does not always apply, as observed in P. multiseries [104]. All biomolecules are carbon-based, so the excess fixed carbon is not necessarily transported for toxin production. The detailed routes of carbon reallocation for toxin biosynthesis have not been elucidated either. Therefore, investigating the central carbon metabolism of Alexandrium and Pseudo-nitzschia is important to validate the hypothesis. For K. brevis, the acquisition of inorganic carbon was shown to be affected by OA despite the nearly unchanged growth rate, carbon fixation rate, and toxin content [106]. Since central carbon metabolism is highly conserved across phylogeny [132], comparing the central carbon metabolism of relatively resistant K. brevis to that of other sensitive microalgae at elevated pCO2 may help to explain the unique response of K. brevis to OA. A decreased pH was proposed to affect the toxicity of Pseudo-nitzschia, though the exact mechanisms had not been demonstrated [119]. Increased inorganic carbon availability and decreased pH may have combined effects on the regulation of central carbon metabolism in microalgae, hence affecting toxin biosynthesis. Considering the above, studying the carbon fluxes in Alexandrium, Pseudo-nitzschia, and K. brevis is essential to deepen our understanding of the effects of OA on microalgae.
4. Future Research Directions
Manipulating seawater to a range of increased pCO2 levels for OA experiments is not a straightforward task due to the interdependency of ocean carbon system components [133]. To predict the microalgal responses under OA accurately, the seawater used for microalgae cultivation should resemble the ocean carbon chemistry in future high CO2 scenarios as closely as possible. Multiple seawater manipulation methods with distinct advantages and disadvantages have been applied to OA experiments. Most of the reviewed studies adopted CO2 gas bubbling to model OA (Table 1). CO2 gas bubbling is an efficient seawater manipulation method that exactly mimics the carbon chemistry of the ocean in future high atmospheric CO2 scenarios (increased DIC without altering AT) [134]. In addition, it is easy to implement and it can maintain the initial conditions in the long term [135]. Thus, CO2 gas bubbling was adopted by most of the studies to investigate the effects of OA on microalgae. However, the turbulence induced by bubbling may affect the growth of phytoplankton to a different extent, especially Alexandrium spp. [136]. Hence, the effects of turbulence became an uncontrolled confounding variable, which increased the difficulties in generating reproducible results for the microalgal adaptive response to OA [137]. To minimize the effects of turbulence, a dialysis bag with a 3 kDa molecular weight cut-off should be used to enclose the microalgae, so as to reduce the mechanical disturbance introduced by the aeration [135]. Unfortunately, none of the reviewed studies mentioned the uses of dialysis bags in their methodology. Besides CO2 gas bubbling, mixing CO2-enriched water prior to cell inoculation, and the combined addition of HCl and Na2CO3/NaHCO3, are other seawater manipulation methods that also closely resemble the carbon chemistry of the ocean in future high atmospheric CO2 conditions [134,135]. These methods can be effective and reliable alternatives to CO2 gas bubbling without the mechanical disturbance to microalgae. Thus, they are especially useful for studying the effects of OA on microalgae with high sensitivity to turbulence. Regardless of the method used, it is noteworthy that the biological processes of microalgae, such as respiration and photosynthesis, will affect the carbon chemistry of seawater, especially when the biomass is high [138,139]. Thus, it would be preferable to monitor at least two carbon chemistry parameters of pH, AT, DIC, and pCO2, in addition to temperature and salinity, throughout the experiments [140].
With appropriate methods to model OA and monitor the carbon chemistry of the seawater, metabolomics is a useful technique to investigate the metabolic changes of the microalgae in response to OA. This will help to reveal the biological regulation of toxin biosynthesis in microalgae under OA. Metabolomics is the investigation of a complete set of small molecules with molecular weights lower than 1500 Da, also known as the metabolome, of biological samples [141]. It has been increasingly applied in algal toxin research with technological advancements [142,143]. Metabolic flux analysis (MFA) is an effective metabolomic tool that examines the turnover rate of metabolites by using stable isotope tracers such as 13C, 2H, and 15N [144]. With the help of nuclear magnetic resonance (NMR) or mass spectrometry (MS), the isotope labeling patterns of intracellular metabolites are determined, which helps to construct a flux map, including reverse fluxes [145]. Compared to the measurement of metabolite levels, analyzing metabolic fluxes is more informative as it reveals the production and consumption rates of metabolites, which illustrate the biochemical events behind the changes in metabolite levels [146]. Based on the reviewed studies, it is hypothesized that the central carbon metabolism of toxic microalgae may be regulated differently under OA. The altered central carbon metabolism may then affect the toxicity of microalgae or relieve the stress brought by OA. Therefore, further studies on the central carbon metabolism of toxic microalgae are warranted. MFA has been applied for the investigation of photosynthesis and central carbon metabolism in microalgae, C3, and C4 plants recently in 13CO2 pulse labeling, and the distinct carbon flux pattern of microalgae has been successfully identified [147]. In this light, carbon allocation in toxic microalgae can be investigated to reveal the metabolic pathways related to toxin biosynthesis and their regulation under OA by MFA in order to elucidate the underlying mechanisms of toxicity changes. When conducting MFA to study the effects of OA on the carbon allocations of microalgae, it is critical to note that CO2 gas bubbling without the continuous control of the pH would alter the pCO2, DIC, and pH of the seawater at the same time. As a result, it is difficult to determine which confounding variable causes the observed effects. Although CO2 gas bubbling successfully mimics the carbon chemistry of seawater under OA observed in reality, this method fails to decouple different variables in the carbon chemistry to study the individual effects of the variables on the carbon allocations of microalgae. Therefore, other methods or systems that can decouple different variables of the carbon chemistry of seawater under OA should also be adopted to investigate in detail the biological mechanisms of the changes in carbon allocation of microalgae under OA when using the metabolomic approach (Figure 1) [148].
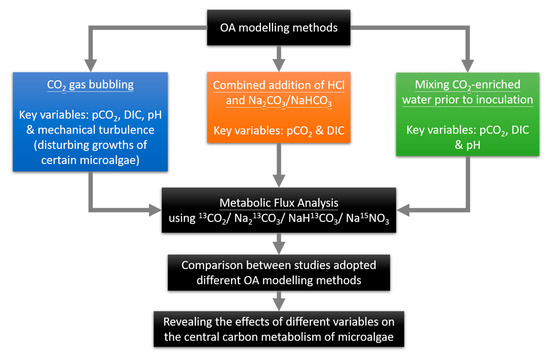
Figure 1.
Schematic diagram illustrating the use of metabolomics to elucidate the individual effects of confounding factors on the carbon allocation of microalgae.
Knowledge of the relationship between environmental drivers and HABs is of fundamental importance to optimize the current mitigation of HABs [149]. Under climate change, it is undoubted that the HAB dynamic has been altered [1]. However, the physiological responses of toxigenic microalgae in changed climate conditions have not been well documented. A precise linkage between environmental factors and microalgal toxicity has not been confirmed due to the diverse responses displayed by microalgae belonging to the same genus or even the same species, as in the case of the STX-producing dinoflagellate Alexandrium [97,98,99,100,101,150]. MFA of the central carbon metabolism of microalgae can reveal the metabolic pathways that are responsible for the toxicity changes under OA. The identification of differentially regulated pathways will facilitate the discovery of universal biomarkers for potentially more toxic microalgae in future elevated atmospheric CO2 scenarios. Given the diverse responses of microalgae to different environmental conditions, detecting universal biomarkers is a relatively efficient way to identify microalgae with increased toxicity under climate change. Monitoring and forecasting these microalgae can be prioritized to help establish early warning systems for the coastal tourism and aquaculture sectors, to minimize the economic losses caused by HABs [151,152,153]. Policymakers can guide the closure of beaches, fish farms, and shellfish-harvesting areas in an appropriate timeframe according to the early warning system, to protect public health while minimizing economic losses. Moreover, more research efforts can be implemented to understand the interactions between the potentially more toxic microalgae and other organisms in the food web under climate pressure. The findings can be used to assess the ecological risk of HABs in the future, which will aid policymakers to achieve a balance between environmental protection and economic development.
Author Contributions
Conceptualization: H.-K.K.; writing—original draft preparation, T.-K.V.T. and H.-K.K.; supervision: H.-K.K.; project administration: H.-K.K.; funding acquisition: H.-K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by research grants from the Hong Kong Polytechnic University (A/C: 1-BD8F) and the Research Grants Council (Ref. No.: 15102122).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019. [Google Scholar]
- Lopez, C.; Jewett, E.; Dortch, Q.; Walton, B.; Hudnell, H. Scientific Assessment of Freshwater Harmful Algal Blooms; Interagency Working Group on Harmful Algal Blooms, Hypoxia, and Human Health of the Joint Subcommittee on Ocean Science and Technology: Washington, DA, USA, 2008.
- Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.; van den Top, H.J.; de Boer, J. Marine toxins: Chemistry, toxicity, occurrence and detection, with special reference to the Dutch situation. Toxins 2010, 2, 878–904. [Google Scholar] [CrossRef] [PubMed]
- Van Dolah, F.M. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect. 2000, 108 (Suppl. S1), 133–141. [Google Scholar] [CrossRef]
- Wang, D.Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, C.R.C.; Poder, T.G. Economic impact of harmful algal blooms on human health: A systematic review. J. Water Health 2019, 17, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G.; Lim, W.A.; Park, Y.T.; Lee, C.K.; Jeong, H.J. Economic impact, management and mitigation of red tides in Korea. Harmful Algae 2013, 30, S131–S143. [Google Scholar] [CrossRef]
- Larkin, S.L.; Adams, C.M.; Ballyram, D.M.; Hodges, A. Red tides and coastal businesses: Measuring economic consequences in Florida. Soc. Nat. Resour. 2007, 20, 2007. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, T. Algal bloom and its economic impact; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate Change and Harmful Algal Blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. In Proceedings of the Centers for Oceans and Human Health Investigators Meeting, Woods Hole, MA, USA, 24–27 April 2007; p. S4. [Google Scholar]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Kazmi, S.S.U.H.; Yapa, N.; Karunarathna, S.C.; Suwannarach, N. Perceived Intensification in Harmful Algal Blooms Is a Wave of Cumulative Threat to the Aquatic Ecosystems. Biology 2022, 11, 852. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A.; Schweibold, L.; De Rijcke, M. GLOBAL Harmful Algal Bloom Status Report 2021; UNESCO: Paris, France, 2021. [Google Scholar]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Schantz, E.J.; Ghazarossian, V.E.; Schnoes, H.K.; Strong, F.M.; Springer, J.P.; Pezzanite, J.O.; Clardy, J. Letter: The structure of saxitoxin. J. Am. Chem. Soc. 1975, 97, 1238. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Blackburn, S.; Hallegraeff, G. Comparative study on paralytic shellfish toxin profiles of the dinoflagellate Gymnodinium catenatum from three different countries. Mar. Biol. 1993, 116, 471–476. [Google Scholar] [CrossRef]
- Usup, G.; Kulis, D.M.; Anderson, D.M. Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures. Nat. Toxins 1994, 2, 254–262. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Evans, W.R.; Yin, Q.Q.; Bell, P.; Moczydlowski, E. Evidence for paralytic shellfish poisons in the freshwater cyanobacterium Lyngbya wollei (Farlow ex Gomont) comb. nov. Appl. Environ. Microbiol. 1997, 63, 3104–3110. [Google Scholar] [CrossRef]
- Hoeger, S.J.; Shaw, G.; Hitzfeld, B.C.; Dietrich, D.R. Occurrence and elimination of cyanobacterial toxins in two Australian drinking water treatment plants. Toxicon 2004, 43, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, W.; Li, D.; Shen, Y.; Li, G.; Liu, Y. First report of aphantoxins in China--waterblooms of toxigenic Aphanizomenon flos-aquae in Lake Dianchi. Ecotoxicol. Environ. Saf. 2006, 65, 84–92. [Google Scholar] [CrossRef]
- Pomati, F.; Sacchi, S.; Rossetti, C.; Giovannardi, S.; Onodera, H.; Oshima, Y.; Neilan, B.A. The Freshwater Cyanobacterium Planktothrix Sp. Fp1: Molecular Identification and Detection of Paralytic Shellfish Poisoning Toxins. J. Phycol. 2000, 36, 553–562. [Google Scholar] [CrossRef]
- Cestele, S.; Catterall, W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 2000, 82, 883–892. [Google Scholar] [CrossRef]
- Llewellyn, L.E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 2006, 23, 200–222. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef] [PubMed]
- Klemm, K.; Cembella, A.; Clarke, D.; Cusack, C.; Arneborg, L.; Karlson, B.; Liu, Y.; Naustvoll, L.; Siano, R.; Gran-Stadniczenko, S.; et al. Apparent biogeographical trends in Alexandrium blooms for northern Europe: Identifying links to climate change and effective adaptive actions. Harmful Algae 2022, 119, 102335. [Google Scholar] [CrossRef] [PubMed]
- Schweibold, L. Global Status of Harmful Algal Blooms: First Steps of a Metadata Approach; ResearchGate: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, J.; Krock, B.; Ding, G.; MacKenzie, L.; Smith, K.F.; Gu, H. Dynamics of the Toxic Dinoflagellate Alexandrium pacificum in the Taiwan Strait and Its Linkages to Surrounding Populations. Water 2021, 13, 2681. [Google Scholar] [CrossRef]
- Selina, M.; Konovalova, G.; Morozova, T.; Orlova, T.Y. Genus Alexandrium Halim, 1960 (Dinophyta) from the Pacific coast of Russia: Species composition, distribution, and dynamics. Russ. J. Mar. Biol. 2006, 32, 321–332. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fachon, E.; Pickart, R.S.; Lin, P.; Fischer, A.D.; Richlen, M.L.; Uva, V.; Brosnahan, M.L.; McRaven, L.; Bahr, F.; et al. Evidence for massive and recurrent toxic blooms of Alexandrium catenella in the Alaskan Arctic. Proc. Natl. Acad. Sci. USA 2021, 118, e2107387118. [Google Scholar] [CrossRef] [PubMed]
- Egmond, H.P.; Van Apeldoorn, M. Marine Biotoxins; Food & Agriculture Organization: Rome, Italy, 2004. [Google Scholar]
- Hamano, Y.; Kinoshita, Y.; Yasumoto, T. Enteropathogenicity of Diarrhetic Shellfish Toxins in Intestinal Models Studies on Diarrhetic Shellfish Toxins. I. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1986, 27, 375–379_1. [Google Scholar] [CrossRef]
- FAO. Toxicity Equivalence Factors for Marine Biotoxins Associated with Bivalve Molluscs; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- WHO. Aquatic (Marine and Freshwater) Biotoxins-Environmental Health Criteria 37; WHO: Geneva, Switzerland, 1984. [Google Scholar]
- Reboreda, A.; Lago, J.; Chapela, M.J.; Vieites, J.M.; Botana, L.M.; Alfonso, A.; Cabado, A.G. Decrease of marine toxin content in bivalves by industrial processes. Toxicon 2010, 55, 235–243. [Google Scholar] [CrossRef]
- Dounay, A.B.; Forsyth, C.J. Okadaic acid: The archetypal serine/threonine protein phosphatase inhibitor. Curr. Med. Chem. 2002, 9, 1939–1980. [Google Scholar] [CrossRef]
- Takai, A.; Murata, M.; Torigoe, K.; Isobe, M.; Mieskes, G.; Yasumoto, T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem. J. 1992, 284 Pt 2, 539–544. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.Y.; Lin, L.; Gao, Y.; Hong, H.S.; Wang, D.Z. Quantitative proteomic analysis of okadaic acid treated mouse small intestines reveals differentially expressed proteins involved in diarrhetic shellfish poisoning. J. Proteomics 2012, 75, 2038–2052. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R. Algal toxins in seafood and drinking water; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Heil, C.A.; Glibert, P.M.; Fan, C. Prorocentrum minimum (Pavillard) Schiller. Harmful Algae 2005, 4, 449–470. [Google Scholar] [CrossRef]
- Edwards, M.; Johns, D.; Leterme, S.; Svendsen, E.; Richardson, A. Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol. Oceanogr. 2006, 51, 820–829. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.S.; Brunson, J.K.; Maeno, Y.; Terada, R.; Allen, A.E.; Yotsu-Yamashita, M.; Chekan, J.R.; Moore, B.S. Domoic acid biosynthesis in the red alga Chondria armata suggests a complex evolutionary history for toxin production. Proc. Natl. Acad. Sci. USA 2022, 119, e2117407119. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Boyd, R.; Freitas, A.d.; Falk, M.; Foxall, R.; Jamieson, W.; Laycock, M.; McCulloch, A.; McInnes, A.; Odense, P. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar] [CrossRef]
- de la Iglesia, P.; Gimenez, G.; Diogene, J. Determination of dissolved domoic acid in seawater with reversed-phase extraction disks and rapid resolution liquid chromatography tandem mass spectrometry with head-column trapping. J. Chromatogr. A 2008, 1215, 116–124. [Google Scholar] [CrossRef]
- Berman, F.W.; Murray, T.F. Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. J. Neurochem. 1997, 69, 693–703. [Google Scholar] [CrossRef]
- Larm, J.A.; Beart, P.M.; Cheung, N.S. Neurotoxin domoic acid produces cytotoxicity via kainate- and AMPA-sensitive receptors in cultured cortical neurones. Neurochem. Int. 1997, 31, 677–682. [Google Scholar] [CrossRef]
- Pinheiro, P.; Mulle, C. Kainate receptors. Cell Tissue Res. 2006, 326, 457–482. [Google Scholar] [CrossRef]
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef]
- Carcache, L.M.; Rodriguez, J.; Rein, K.S. The structural basis for kainoid selectivity at AMPA receptors revealed by low-mode docking calculations. Bioorganic Med. Chem. 2003, 11, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.R.; Manalo, J.L. The activation of glutamate receptors by kainic acid and domoic acid. Nat. Toxins 1998, 6, 153–158. [Google Scholar] [CrossRef]
- Colman, J.R.; Nowocin, K.J.; Switzer, R.C.; Trusk, T.C.; Ramsdell, J.S. Mapping and reconstruction of domoic acid-induced neurodegeneration in the mouse brain. Neurotoxicol. Teratol. 2005, 27, 753–767. [Google Scholar] [CrossRef]
- Giordano, G.; Klintworth, H.M.; Kavanagh, T.J.; Costa, L.G. Apoptosis induced by domoic acid in mouse cerebellar granule neurons involves activation of p38 and JNK MAP kinases. Neurochem. Int. 2008, 52, 1100–1105. [Google Scholar] [CrossRef]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Perl, T.M.; Bedard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.; Remis, R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med 1990, 322, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Holland, P.T.; McNabb, P.; Selwood, A.I.; Rhodes, L.L. Comparative toxicity to mice of domoic acid and isodomoic acids A, B and C. Toxicon 2008, 52, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Sawant, P.M.; Tyndall, J.D.; Holland, P.T.; Peake, B.M.; Mountfort, D.O.; Kerr, D.S. In vivo seizure induction and affinity studies of domoic acid and isodomoic acids-D, -E and -F. Neuropharmacology 2010, 59, 129–138. [Google Scholar] [CrossRef]
- Sawant, P.M.; Weare, B.A.; Holland, P.T.; Selwood, A.I.; King, K.L.; Mikulski, C.M.; Doucette, G.J.; Mountfort, D.O.; Kerr, D.S. Isodomoic acids A and C exhibit low KA receptor affinity and reduced in vitro potency relative to domoic acid in region CA1 of rat hippocampus. Toxicon 2007, 50, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.W.; Bargu, S.; Coale, S.L.; Benitez-Nelson, C.R.; Garcia, A.C.; Roberts, K.J.; Sekula-Wood, E.; Bruland, K.W.; Coale, K.H. Toxic diatoms and domoic acid in natural and iron enriched waters of the oceanic Pacific. Proc. Natl. Acad. Sci. USA 2010, 107, 20762–20767. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Flewelling, L.J.; Naar, J. Karenia brevis red tides, brevetoxins in the food web, and impacts on natural resources: Decadal advancements. Harmful Algae 2009, 8, 598–607. [Google Scholar] [CrossRef]
- Bourdelais, A.J.; Tomas, C.R.; Naar, J.; Kubanek, J.; Baden, D.G. New fish-killing alga in coastal Delaware produces neurotoxins. Environ. Health Perspect. 2002, 110, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef]
- Ramsdell, J. The Molecular and Integrative Basis to Brevetoxin Toxicity. In Seafood and Freshwater Toxins; Food Science and Technology; Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 519–550. [Google Scholar]
- Konoki, K.; Baden, D.G.; Scheuer, T.; Catterall, W.A. Molecular Determinants of Brevetoxin Binding to Voltage-Gated Sodium Channels. Toxins 2019, 11, 513. [Google Scholar] [CrossRef]
- Morris, P.D.; Campbell, D.S.; Taylor, T.J.; Freeman, J.I. Clinical and epidemiological features of neurotoxic shellfish poisoning in North Carolina. Am. J. Public Health 1991, 81, 471–474. [Google Scholar] [CrossRef]
- Steidinger, K.A. Historical perspective on Karenia brevis red tide research in the Gulf of Mexico. Harmful Algae 2009, 8, 549–561. [Google Scholar] [CrossRef]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef]
- Tillmann, U. Amphidomataceae. In Harmful Algae Blooms, a Compendium Desk Reference; Wiley-Blackwell Publishing: Chichester, West Sussex, 2018; pp. 575–582. [Google Scholar]
- Satake, M.; Ofuji, K.; Naoki, H.; James, K.J.; Furey, A.; McMahon, T.; Silke, J.; Yasumoto, T. Azaspiracid, a new marine toxin having unique spiro ring assemblies, isolated from Irish mussels, Mytilus edulis. J. Am. Chem. Soc. 1998, 120, 9967–9968. [Google Scholar] [CrossRef]
- Krock, B.; Tillmann, U.; Tebben, J.; Trefault, N.; Gu, H. Two novel azaspiracids from Azadinium poporum, and a comprehensive compilation of azaspiracids produced by Amphidomataceae, (Dinophyceae). Harmful Algae 2019, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Twiner, M.J.; Rehmann, N.; Hess, P.; Doucette, G.J. Azaspiracid shellfish poisoning: A review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs 2008, 6, 39–72. [Google Scholar] [CrossRef] [PubMed]
- Furey, A.; O’Doherty, S.; O’Callaghan, K.; Lehane, M.; James, K.J. Azaspiracid poisoning (AZP) toxins in shellfish: Toxicological and health considerations. Toxicon 2010, 56, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Twiner, M.J.; Doucette, G.J.; Rasky, A.; Huang, X.P.; Roth, B.L.; Sanguinetti, M.C. Marine algal toxin azaspiracid is an open-state blocker of hERG potassium channels. Chem. Res. Toxicol. 2012, 25, 1975–1984. [Google Scholar] [CrossRef]
- Tillmann, U.; Edvardsen, B.; Krock, B.; Smith, K.F.; Paterson, R.F.; Voss, D. Diversity, distribution, and azaspiracids of Amphidomataceae (Dinophyceae) along the Norwegian coast. Harmful Algae 2018, 80, 15–34. [Google Scholar] [CrossRef]
- McGirr, S.; Clarke, D.; Kilcoyne, J.; Silke, J.; Touzet, N. Co-localisation of Azaspiracid Analogs with the Dinoflagellate Species Azadinium spinosum and Amphidoma languida in the Southwest of Ireland. Microb. Ecol. 2022, 83, 635–646. [Google Scholar] [CrossRef]
- Akselman, R.; Negri, R.M. Blooms of Azadinium cf. spinosum Elbrächter et Tillmann (Dinophyceae) in northern shelf waters of Argentina, Southwestern Atlantic. Harmful Algae 2012, 19, 30–38. [Google Scholar] [CrossRef]
- Percopo, I.; Siano, R.; Rossi, R.; Soprano, V.; Sarno, D.; Zingone, A. A new potentially toxic Azadinium species (Dinophyceae) from the Mediterranean Sea, A. dexteroporum sp. nov. J. Phycol. 2013, 49, 950–966. [Google Scholar] [CrossRef]
- Gu, H.; Luo, Z.; Krock, B.; Witt, M.; Tillmann, U. Morphology, phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the China Sea. Harmful Algae 2013, 21–22, 64–75. [Google Scholar] [CrossRef]
- Kim, J.H.; Tillmann, U.; Adams, N.G.; Krock, B.; Stutts, W.L.; Deeds, J.R.; Han, M.S.; Trainer, V.L. Identification of Azadinium species and a new azaspiracid from Azadinium poporum in Puget Sound, Washington State, USA. Harmful Algae 2017, 68, 152–167. [Google Scholar] [CrossRef]
- Tillmann, U.; Trefault, N.; Krock, B.; Parada-Pozo, G.; De la Iglesia, R.; Vásquez, M. Identification ofAzadinium poporum(Dinophyceae) in the Southeast Pacific: Morphology, molecular phylogeny, and azaspiracid profile characterization. J. Plankton Res. 2017, 39, 350–367. [Google Scholar] [CrossRef]
- Tillmann, U.; Jaen, D.; Fernandez, L.; Gottschling, M.; Witt, M.; Blanco, J.; Krock, B. Amphidoma languida (Amphidomatacea, Dinophyceae) with a novel azaspiracid toxin profile identified as the cause of molluscan contamination at the Atlantic coast of southern Spain. Harmful Algae 2017, 62, 113–126. [Google Scholar] [CrossRef]
- Wietkamp, S.; Tillmann, U.; Clarke, D.; Toebe, K. Molecular detection and quantification of the azaspiracid-producing dinoflagellate Amphidoma languida (Amphidomataceae, Dinophyceae). J. Plankton Res. 2019, 41, 101–113. [Google Scholar] [CrossRef]
- Keeling, C.D.; Piper, S.C.; Bacastow, R.B.; Wahlen, M.; Whorf, T.P.; Heimann, M.; Meijer, H.A. Exchanges of Atmospheric CO2 and 13CO2 with the Terrestrial Biosphere and Oceans from 1978 to 2000. I. Global Aspects; Springer: New York, NY, USA, 2001. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.; Tilbrook, B.; et al. The oceanic sink for anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef]
- Al Jabri, H.; Taleb, A.; Touchard, R.; Saadaoui, I.; Goetz, V.; Pruvost, J. Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130. Appl. Sci. 2021, 11, 3799. [Google Scholar] [CrossRef]
- Procházková, G.; Brányiková, I.; Zachleder, V.; Brányik, T. Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J. Appl. Phycol. 2013, 26, 1359–1377. [Google Scholar] [CrossRef]
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The biology and ecology of a toxic genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef]
- Shearn-Bochsler, V.; Lance, E.W.; Corcoran, R.; Piatt, J.; Bodenstein, B.; Frame, E.; Lawonn, J. Fatal paralytic shellfish poisoning in Kittlitz’s Murrelet (Brachyramphus brevirostris) nestlings, Alaska, USA. J. Wildl. Dis. 2014, 50, 933–937. [Google Scholar] [CrossRef]
- Garcia, C.; Perez, F.; Contreras, C.; Figueroa, D.; Barriga, A.; Lopez-Rivera, A.; Araneda, O.F.; Contreras, H.R. Saxitoxins and okadaic acid group: Accumulation and distribution in invertebrate marine vectors from Southern Chile. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2015, 32, 984–1002. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, A.C.; VanDola, F.M.; Gulland, F.M.; Rowles, T.K.; Schwacke, L.H. Production and toxicity of the marine biotoxin domoic acid and its effects on wildlife: A review. Hum. Ecol. Risk Assess. 2008, 14, 544–567. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Shumway, S.E.; Glibert, P.M. Food web and ecosystem impacts of harmful algae. Harmful Algal Bloom. A Compend. Desk Ref. 2018, 243–336. [Google Scholar]
- Roggatz, C.C.; Fletcher, N.; Benoit, D.M.; Algar, A.C.; Doroff, A.; Wright, B.; Wollenberg Valero, K.C.; Hardege, J.D. Saxitoxin and tetrodotoxin bioavailability increases in future oceans. Nat. Clim. Change 2019, 9, 840–844. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.K.; Smith, J.L.; Wallace, R.B.; Merlo, L.; Koch, F.; Mittelsdorf, H.; Goleski, J.A.; Anderson, D.M.; Gobler, C.J. The effects of elevated CO(2) on the growth and toxicity of field populations and cultures of the saxitoxin-producing dinoflagellate, Alexandrium fundyense. Limnol Oceanogr 2015, 60, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Li, F.; He, X.; Chen, J.; Yu, R.-C. Rising CO2 will increase toxicity of marine dinoflagellate Alexandrium minutum. J. Hazard. Mater. 2022, 431, 128627. [Google Scholar] [CrossRef]
- Tatters, A.O.; Flewelling, L.J.; Fu, F.; Granholm, A.A.; Hutchins, D.A. High CO2 promotes the production of paralytic shellfish poisoning toxins by Alexandrium catenella from Southern California waters. Harmful Algae 2013, 30, 37–43. [Google Scholar] [CrossRef]
- Brandenburg, K.M.; Krock, B.; Klip, H.C.L.; Sluijs, A.; Garbeva, P.; Van de Waal, D.B. Intraspecific variation in multiple trait responses of Alexandrium ostenfeldii towards elevated pCO(2). Harmful Algae 2021, 101, 101970. [Google Scholar] [CrossRef] [PubMed]
- Van de Waal, D.B.; Eberlein, T.; John, U.; Wohlrab, S.; Rost, B. Impact of elevated pCO(2) on paralytic shellfish poisoning toxin content and composition in Alexandrium tamarense. Toxicon 2014, 78, 58–67. [Google Scholar] [CrossRef]
- Sun, J.; Hutchins, D.A.; Feng, Y.; Seubert, E.L.; Caron, D.A.; Fu, F.-X. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnol. Oceanogr. 2011, 56, 829–840. [Google Scholar] [CrossRef]
- Tatters, A.O.; Fu, F.X.; Hutchins, D.A. High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzschia fraudulenta. PLoS ONE 2012, 7, e32116. [Google Scholar] [CrossRef]
- Wingert, C.J.; Cochlan, W.P. Effects of ocean acidification on the growth, photosynthetic performance, and domoic acid production of the diatom Pseudo-nitzschia australis from the California Current System. Harmful Algae 2021, 107, 102030. [Google Scholar] [CrossRef]
- Wohlrab, S.; John, U.; Klemm, K.; Eberlein, T.; Forsberg Grivogiannis, A.M.; Krock, B.; Frickenhaus, S.; Bach, L.T.; Rost, B.; Riebesell, U.; et al. Ocean acidification increases domoic acid contents during a spring to summer succession of coastal phytoplankton. Harmful Algae 2020, 92, 101697. [Google Scholar] [CrossRef] [PubMed]
- Bercel, T.L.; Kranz, S.A. Insights into carbon acquisition and photosynthesis in Karenia brevis under a range of CO2 concentrations. Prog. Oceanogr. 2019, 172, 65–76. [Google Scholar] [CrossRef]
- Errera, R.M.; Yvon-Lewis, S.; Kessler, J.D.; Campbell, L. Reponses of the dinoflagellate Karenia brevis to climate change: pCO2 and sea surface temperatures. Harmful Algae 2014, 37, 110–116. [Google Scholar] [CrossRef]
- Raven, J.A.; Cockell, C.S.; De La Rocha, C.L. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos. Trans. R. Soc. Lond B. Biol. Sci. 2008, 363, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Tortell, P.D. Evolutionary and ecological perspectives on carbon acquisition in phytoplankton. Limnol. Oceanogr. 2000, 45, 744–750. [Google Scholar] [CrossRef]
- Kellmann, R.; Mihali, T.K.; Jeon, Y.J.; Pickford, R.; Pomati, F.; Neilan, B.A. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2008, 74, 4044–4053. [Google Scholar] [CrossRef]
- Taroncher-Oldenburg, G.; Anderson, D.M. Identification and characterization of three differentially expressed genes, encoding S-adenosylhomocysteine hydrolase, methionine aminopeptidase, and a histone-like protein, in the toxic dinoflagellate Alexandrium fundyense. Appl. Environ. Microbiol. 2000, 66, 2105–2112. [Google Scholar] [CrossRef]
- Pang, M.; Xu, J.; Qu, P.; Mao, X.; Wu, Z.; Xin, M.; Sun, P.; Wang, Z.; Zhang, X.; Chen, H. Effect of CO(2) on growth and toxicity of Alexandrium tamarense from the East China Sea, a major producer of paralytic shellfish toxins. Harmful Algae 2017, 68, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.D.; Wisecaver, J.H.; Brosnahan, M.L.; Kulis, D.M.; Anderson, D.M.; Bhattacharya, D.; Plumley, F.G.; Erdner, D.L. Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol. Biol. Evol. 2013, 30, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Loram, J.E.; Hackett, J.D.; Anderson, D.M.; Plumley, F.G.; Bhattacharya, D. Origin of saxitoxin biosynthetic genes in cyanobacteria. PLoS ONE 2009, 4, e5758. [Google Scholar] [CrossRef] [PubMed]
- Sako, Y.; Yoshida, T.; Uchida, A.; Arakawa, O.; Noguchi, T.; Ishida, Y. Purification and characterization of a sulfotransferase specific to N-21 of saxitoxin and gonyautoxin 2+ 3 from the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). J. Phycol. 2001, 37, 1044–1051. [Google Scholar] [CrossRef]
- Soto-Liebe, K.; Murillo, A.A.; Krock, B.; Stucken, K.; Fuentes-Valdes, J.J.; Trefault, N.; Cembella, A.; Vasquez, M. Reassessment of the toxin profile of Cylindrospermopsis raciborskii T3 and function of putative sulfotransferases in synthesis of sulfated and sulfonated PSP toxins. Toxicon 2010, 56, 1350–1361. [Google Scholar] [CrossRef]
- Giordano, M.; Norici, A.; Hell, R. Sulfur and phytoplankton: Acquisition, metabolism and impact on the environment. New Phytol. 2005, 166, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Shibagaki, N.; Grossman, A. The state of sulfur metabolism in algae: From ecology to genomics. In Sulfur Metabolism in Phototrophic Organisms; Springer: Berlin/Heidelberg, Germany, 2008; pp. 231–267. [Google Scholar]
- Lundholm, N.; Hansen, P.J.; Kotaki, Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar. Ecol. Prog. Ser. 2004, 273, 1–15. [Google Scholar] [CrossRef]
- Shi, D.; Hong, H.; Su, X.; Liao, L.; Chang, S.; Lin, W. The physiological response of marine diatoms to ocean acidification: Differential roles of seawater pCO(2) and pH. J. Phycol. 2019, 55, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Trimborn, S.; Lundholm, N.; Thoms, S.; Richter, K.U.; Krock, B.; Hansen, P.J.; Rost, B. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: The effect of pH-induced changes in seawater carbonate chemistry. Physiol. Plant. 2008, 133, 92–105. [Google Scholar] [CrossRef]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Obornik, M.; Smith, G.J.; et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef]
- MillerO, F.J.; Woosley, R.; DiTrolio, B.; Waters, J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography 2009, 22, 72–85. [Google Scholar] [CrossRef]
- Perez, P.; Beiras, R.; Fernandez, E. Monitoring copper toxicity in natural phytoplankton assemblages: Application of Fast Repetition Rate fluorometry. Ecotoxicol. Environ. Saf. 2010, 73, 1292–1303. [Google Scholar] [CrossRef]
- Rue, E.; Bruland, K. Domoic acid binds iron and copper: A possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Mar. Chem. 2001, 76, 127–134. [Google Scholar] [CrossRef]
- Lelong, A.; Jolley, D.F.; Soudant, P.; Hegaret, H. Impact of copper exposure on Pseudo-nitzschia spp. physiology and domoic acid production. Aquat. Toxicol. 2012, 118–119, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.S.; Léger, C.; Satchwell, M.; Boyer, G.L. The effects of iron on domoic acid production by Pseudo-nitzschia multiseries. In Proceedings of the 9th International Conference on Harmful Algal Blooms, Hobart, Tasmania, 7–11 February 2000; pp. 320–323. [Google Scholar]
- Kobayashi, K.; Takata, Y.; Kodama, M. Direct contact between Pseudo-nitzschia multiseries and bacteria is necessary for the diatom to produce a high level of domoic acid. Fish. Sci. 2009, 75, 771–776. [Google Scholar] [CrossRef]
- Bates, S.S.; Douglas, D.J.; Doucette, G.J.; Leger, C. Enhancement of domoic acid production by reintroducing bacteria to axenic cultures of the diatom Pseudo-nitzschia multiseries. Nat. Toxins 1995, 3, 428–435. [Google Scholar] [CrossRef]
- Bates, S.S.; Gaudet, J.; Kaczmarska, I.; Ehrman, J.M. Interaction between bacteria and the domoic-acid-producing diatom Pseudo-nitzschia multiseries (Hasle) Hasle; can bacteria produce domoic acid autonomously? Harmful Algae 2004, 3, 11–20. [Google Scholar] [CrossRef]
- Maas, E.W.; Law, C.S.; Hall, J.A.; Pickmere, S.; Currie, K.I.; Chang, F.; Voyles, K.M.; Caird, D. Effect of ocean acidification on bacterial abundance, activity and diversity in the Ross Sea, Antarctica. Aquat. Microb. Ecol. 2013, 70, 1–15. [Google Scholar] [CrossRef]
- Schada von Borzyskowski, L.; Bernhardsgrutter, I.; Erb, T.J. Biochemical unity revisited: Microbial central carbon metabolism holds new discoveries, multi-tasking pathways, and redundancies with a reason. Biol. Chem. 2020, 401, 1429–1441. [Google Scholar] [CrossRef]
- Dickson, A.G. The carbon dioxide system in seawater: Equilibrium chemistry and measurements. Guide Best Pract. Ocean. Acidif. Res. Data Report. 2010, 1, 17–40. [Google Scholar]
- Gattuso, J.-P.; Lavigne, H. Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 2009, 6, 2121–2133. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Lee, K.; Rost, B.; Schulz, K. Approaches and Tools to Manipulate the Carbonate Chemistry. Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Llaveria, G.; Figueroa, R.I.; Garces, E.; Berdalet, E. Cell Cycle and Cell Mortality of Alexandrium Minutum (Dinophyceae) under Small-Scale Turbulence Conditions 1. J. Phycol. 2009, 45, 1106–1115. [Google Scholar] [CrossRef]
- Shi, D.; Xu, Y.; Morel, F. Effects of the pH/pCO 2 control method on medium chemistry and phytoplankton growth. Biogeosciences 2009, 6, 1199–1207. [Google Scholar] [CrossRef]
- Hurd, C.L.; Hepburn, C.D.; Currie, K.I.; Raven, J.A.; Hunter, K.A. Testing the effects of ocean acidification on algal metabolism: Considerations for experimental designs1. J. Phycol. 2009, 45, 1236–1251. [Google Scholar] [CrossRef]
- Rost, B.; Zondervan, I.; Wolf-Gladrow, D. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: Current knowledge, contradictions and research directions. Mar. Ecol. Prog. Ser. 2008, 373, 227–237. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Hurd, C.L. Experimental design in ocean acidification research: Problems and solutions. ICES J. Mar. Sci. 2016, 73, 572–581. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Tsuchiya, S.; Omura, T.; Koike, K.; Oikawa, H.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. Metabolomic study of saxitoxin analogues and biosynthetic intermediates in dinoflagellates using (15)N-labelled sodium nitrate as a nitrogen source. Sci. Rep. 2019, 9, 3460. [Google Scholar] [CrossRef]
- Brown, E.R.; Moore, S.G.; Gaul, D.A.; Kubanek, J. Predator cues target signaling pathways in toxic algal metabolome. Limnol. Oceanogr. 2022, 67, 1227–1237. [Google Scholar] [CrossRef]
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and Isotope Tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef]
- Rahim, M.; Ragavan, M.; Deja, S.; Merritt, M.E.; Burgess, S.C.; Young, J.D. INCA 2.0: A tool for integrated, dynamic modeling of NMR- and MS-based isotopomer measurements and rigorous metabolic flux analysis. Metab. Eng. 2022, 69, 275–285. [Google Scholar] [CrossRef]
- Wang, Y.; Wondisford, F.E.; Song, C.; Zhang, T.; Su, X. Metabolic Flux Analysis-Linking Isotope Labeling and Metabolic Fluxes. Metabolites 2020, 10, 447. [Google Scholar] [CrossRef]
- Treves, H.; Kuken, A.; Arrivault, S.; Ishihara, H.; Hoppe, I.; Erban, A.; Hohne, M.; Moraes, T.A.; Kopka, J.; Szymanski, J.; et al. Carbon flux through photosynthesis and central carbon metabolism show distinct patterns between algae, C(3) and C(4) plants. Nat. Plants 2022, 8, 78–91. [Google Scholar] [CrossRef]
- Gimenez, I.; Waldbusser, G.G.; Langdon, C.J.; Hales, B.R. The dynamic ocean acidification manipulation experimental system: Separating carbonate variables and simulating natural variability in laboratory flow-through experiments. Limnol. Oceanogr. Methods 2019, 17, 343–361. [Google Scholar] [CrossRef]
- West, J.J.; Järnberg, L.; Berdalet, E.; Cusack, C. Understanding and Managing Harmful Algal Bloom Risks in a Changing Climate: Lessons From the European CoCliME Project. Front. Clim. 2021, 3. [Google Scholar] [CrossRef]
- Kremp, A.; Godhe, A.; Egardt, J.; Dupont, S.; Suikkanen, S.; Casabianca, S.; Penna, A. Intraspecific variability in the response of bloom-forming marine microalgae to changed climate conditions. Ecol. Evol. 2012, 2, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Andersen, P.; Bricelj, V.M.; Cullen, J.J.; Rensel, J.J. Monitoring and Management Strategies for Harmful Algal Blooms in Coastal Waters; UNESCO: Paris, France, 2001. [Google Scholar]
- Anderson, C.R.; Kudela, R.M.; Kahru, M.; Chao, Y.; Rosenfeld, L.K.; Bahr, F.L.; Anderson, D.M.; Norris, T.A. Initial skill assessment of the California Harmful Algae Risk Mapping (C-HARM) system. Harmful Algae 2016, 59, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.; Anderson, D.M.; Mateus, M.; Reguera, B.; Silke, J.; Sourisseau, M.; Maguire, J. Forecasting the risk of harmful algal blooms. Harmful Algae 2016, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).